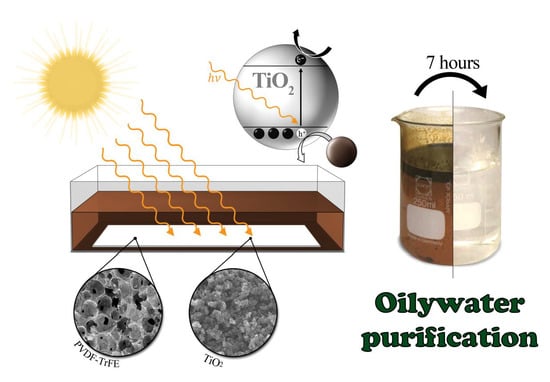
Polymer-Based Membranes for Oily Wastewater Remediation
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Wastewater Sample
2.5. Oily Wastewater Parameters
2.6. Photocatalytic Degradation Measurement
3. Results and Discussion
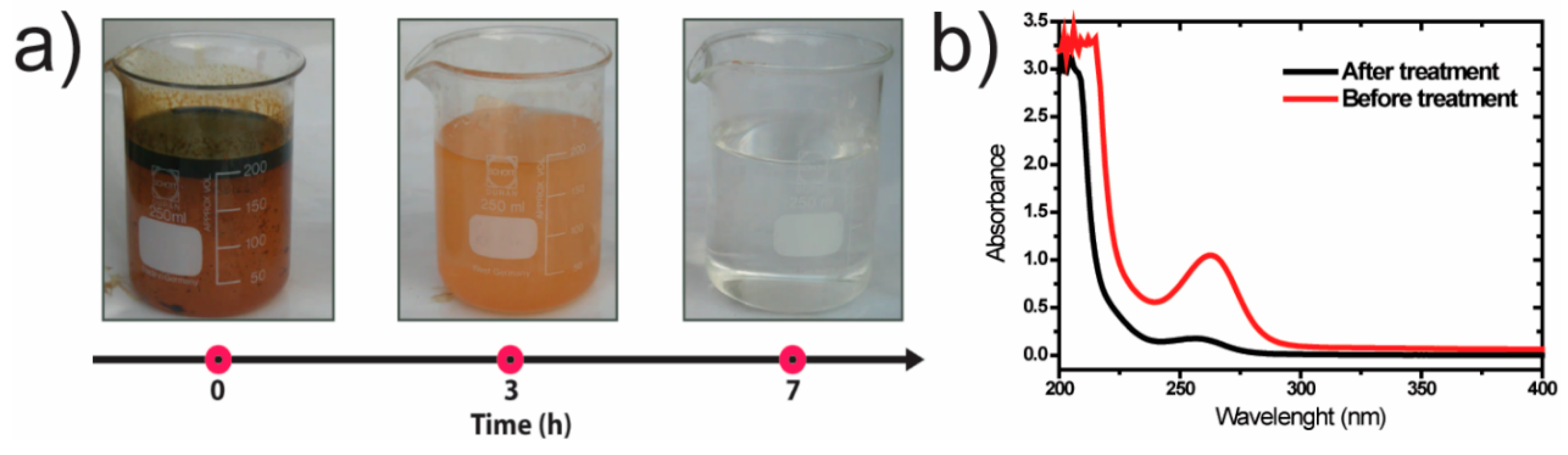
3.1. Photocatalytic Degradation of Oily Wastewater
3.1.1. Effect of Initial Chemical Oxygen Demand
3.1.2. Effect of Oily Water pH
3.1.3. Wastewater Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rusli, U.N.; Alias, N.H.; Shahruddin, M.Z.; Othman, N.H. Photocatalytic Degradation of Oil using Polyvinylidene Fluoride/Titanium Dioxide Composite Membrane for Oily Wastewater Treatment. In Proceedings of the MATEC Web Conference, Shah Alam, Selangor, Malaysia, 2 August 2016; Volume 69, p. 05003. [Google Scholar]
- Sun, S.; Xiao, Q.R.; Zhou, X.; Wei, Y.Y.; Shi, L.; Jiang, Y. A bio-based environment-friendly membrane with facile preparation process for oil-water separation. Colloids Surf. Physicochem. Eng. Asp. 2018, 559, 18–22. [Google Scholar] [CrossRef]
- Stack, L.J.; Carney, P.A.; Malone, H.B.; Wessels, T.K. Factors influencing the ultrasonic separation of oil-in-water emulsions. Ultrason. Sonochem. 2005, 12, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.F.; Rodrigues, M.I.; Trindade, T.; Daniel-da-Silva, A.L. Chitosan-silica hybrid nanosorbents for oil removal from water. Colloids Surf. Physicochem. Eng. Asp. 2017, 532, 305–313. [Google Scholar] [CrossRef]
- Cañizares, P.; Martínez, F.; Jiménez, C.; Sáez, C.; Rodrigo, M.A. Coagulation and electrocoagulation of oil-in-water emulsions. J. Hazard. Mater. 2008, 151, 44–51. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Investigation of submerged membrane photocatalytic reactor (sMPR) operating parameters during oily wastewater treatment process. Desalination 2014, 353, 48–56. [Google Scholar] [CrossRef]
- Sun, Y.; Zong, Y.; Yang, N.; Zhang, N.; Jiang, B.; Zhang, L.; Xiao, X. Surface hydrophilic modification of PVDF membranes based on tannin and zwitterionic substance towards effective oil-in-water emulsion separation. Sep. Purif. Technol. 2020, 234, 116015. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, Y.; Li, P.; Yan, L.; Fan, X.; Wang, Z.; Zhang, W.; Liu, H. Ampholytic Chitosan/Alginate Composite Nanofibrous Membranes with Super Anti-Crude Oil-Fouling Behavior and Multifunctional Oil/Water Separation Properties. ACS Sustain. Chem. Eng. 2019, 7, 15463–15470. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Qu, R.; Liu, Y.; Weia, Y.; Feng, L. Janus membrane decorated: Via a versatile immersion-spray route: Controllable stabilized oil/water emulsion separation satisfying industrial emission and purification criteria. J. Mater. Chem. A 2019, 7, 4941–4949. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Xiao, X.; Liu, G.; Xu, Z.; Wang, B.; Yu, C.; Ras, R.H.A.; Jiang, L. Efficient separation of immiscible oil/water mixtures using a perforated lotus leaf. Green Chem. 2019, 21, 6579–6584. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, S.; Zhu, Y.; Shen, Y.; Gao, X.; Shi, W.; Tay, J.H. Adsorption mechanisms of crude oil onto polytetrafluoroethylene membrane: Kinetics and isotherm, and strategies for adsorption fouling control. Sep. Purif. Technol. 2020, 235, 116212. [Google Scholar] [CrossRef]
- Yagoub, H.; Zhu, L.; Shibraen, M.H.M.A.; Altam, A.A.; Babiker, D.M.D.; Rehan, K.; Mukwaya, V.; Xu, J.; Yang, S. Manipulating the surface wettability of polysaccharide based complex membrane for oil/water separation. Carbohydr. Polym. 2019, 225, 115231. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, W.; Wang, C.; Sun, H.; Zhang, J.; Yu, Y.; Dong, W.; Zhu, Z.; Li, A. Fabrication of palygorskite coated membrane for multifunctional oil-in-water emulsions separation. Appl. Clay Sci. 2019, 182, 105295. [Google Scholar] [CrossRef]
- Saththasivam, J.; Wubulikasimu, Y.; Ogunbiyi, O.; Liu, Z. Fast and efficient separation of oil/saltwater emulsions with anti-fouling ZnO microsphere/carbon nanotube membranes. J. Water Process Eng. 2019, 32, 100901. [Google Scholar] [CrossRef]
- Wang, Z.X.; Lau, C.H.; Zhang, N.Q.; Bai, Y.P.; Shao, L. Mussel-inspired tailoring of membrane wettability for harsh water treatment. J. Mater. Chem. A 2015, 3, 2650–2657. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, Y.; Xu, J.; Han, Y.; Xu, X. Preparation, performances of PVDF/ZnO hybrid membranes and their applications in the removal of copper ions. Appl. Surf. Sci. 2014, 316, 333–340. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; Wang, J.; Zhong, X. The removal of natural organic matter with LiCl–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. Desalination 2014, 344, 412–421. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Cheng, L.-H.; Xu, K.; Xu, Q.-P.; Chen, H.-L.; Lai, J.-Y.; Tung, K.-L. Extracorporeal endotoxin removal by novel l-serine grafted PVDF membrane modules. J. Membr. Sci. 2012, 405, 104–112. [Google Scholar] [CrossRef]
- Gao, L.; Alberto, M.; Gorgojo, P.; Szekely, G.; Budd, P.M. High-flux PIM-1/PVDF thin film composite membranes for 1-butanol/water pervaporation. J. Membr. Sci. 2017, 529, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Ramaiah, K.P.; Satyasri, D.; Sridhar, S.; Krishnaiah, A. Removal of hazardous chlorinated VOCs from aqueous solutions using novel ZSM-5 loaded PDMS/PVDF composite membrane consisting of three hydrophobic layers. J. Hazard. Mater. 2013, 261, 362–371. [Google Scholar] [CrossRef]
- Zuo, X.; Shi, W.; Tian, Z.; Yu, S.; Wang, S.; He, J. Desalination of water with a high degree of mineralization using SiO2/PVDF membranes. Desalination 2013, 311, 150–155. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Faki, B.; Luque-Alled, J.M.; Alberto, M.; Vijayaraghavan, A.; Holmes, S.M.; Szekely, G.; Badawy, M.I.; Shokri, N.; et al. Flux-enhanced PVDF mixed matrix membranes incorporating APTS-functionalized graphene oxide for membrane distillation. J. Membr. Sci. 2018, 554, 309–323. [Google Scholar] [CrossRef]
- Salazar, H.; Nunes-Pereira, J.; Correia, D.M.; Cardoso, V.F.; Gonçalves, R.; Martins, P.M.; Ferdov, S.; Martins, M.D.; Botelho, G.; Lanceros-Méndez, S. Poly(vinylidene fluoride-hexafluoropropylene)/bayerite composite membranes for efficient arsenic removal from water. Mater. Chem. Phys. 2016, 183, 430–438. [Google Scholar] [CrossRef]
- Aoudjit, L.; Martins, P.M.; Madjene, F.; Petrovykh, D.Y.; Lanceros-Mendez, S. Photocatalytic reusable membranes for the effective degradation of tartrazine with a solar photoreactor. J. Hazard. Mater. 2018, 344, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.M.; Miranda, R.; Marques, J.; Tavares, C.J.; Botelho, G.; Lanceros-Méndez, S. Comparative efficiency of TiO2 nanoparticles in suspension vs. immobilization into P(VDF-TrFE) porous membranes. RSC Adv. 2016, 6, 12708–12716. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Gonçalves, R.; Cardoso, V.F.; Lanceros-Méndez, S. Electroactive poly(vinylidene fluoride)-based structures for advanced applications. Nat. Protoc. 2018, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Lanceros-Méndez, S. Fluorinated polymers as smart materials for advanced biomedical applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Zorba, S.; He, L.; Hu, Y.; Maxwell, R.T.; Farah, C.; Zhanga, Q.; Yin, Y. Self-assembly of superparamagnetic magnetite particles into peapod-like structures and their application in optical modulation. J. Mater. Chem. 2010, 20, 7965–7969. [Google Scholar] [CrossRef]
- Wu, S.-H.; Wu, J.L.; Jia, S.Y.; Chang, Q.W.; Ren, H.; Liu, Y. Cobalt (II) phthalocyanine-sensitized hollow Fe3O4@ SiO2@ TiO2 hierarchical nanostructures: Fabrication and enhanced photocatalytic properties. Appl. Surf. Sci. 2013, 287, 389–396. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Song, S.; Zhang, H. Fe3O4@ SiO2@ TiO2@ Pt Hierarchical Core–Shell Microspheres: Controlled Synthesis, Enhanced Degradation System, and Rapid Magnetic Separation to Recycle. Cryst. Growth Des. 2014, 14, 5506–5511. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase characterization of TiO2 powder by XRD and TEM. Kasetsart J. Nat. Sci. 2008, 42, 357–361. [Google Scholar]
- Levlin, E. Conductivity measurements for controlling municipal waste-water treatment. In Proceedings of the Polish-Swedish-Ukrainian Seminar, Ustron, Poland, 23–24 November 2007. [Google Scholar]
- Li, G.; An, T.; Chen, J.; Sheng, G.; Fu, J.; Chen, F.; Zhang, S.; Zhao, H. Photoelectrocatalytic decontamination of oilfield produced wastewater containing refractory organic pollutants in the presence of high concentration of chloride ions. J. Hazard. Mater. 2006, 138, 392–400. [Google Scholar] [CrossRef] [PubMed]




| COD (mg/L) | Removal Rate (%) |
|---|---|
| 51,758 | 49 |
| 25,879 | 99.6 |
| Parameter | Before Treatment | After Treatment | Removed (%) | Limit Values 1 |
|---|---|---|---|---|
| pH | 4.3 | 5.3 | - | 6.5–8.5 |
| Temperature (°C) | 21.1 | 19.1 | 9.47 | 30 |
| Turbidity (NTU) | 205 | 7 | 96.58 | 10 |
| COD (mg L−1) | 25879 | 105 | 99.59 | 120 |
| Conductivity (ms cm−1) | 236 | 167.4 | 29.06 | 2 |
| Chloride (mg L−1) | 1047.4 | 804.2 | 25.82 | 500 |
| Suspended solids (mg L−1) | 73.9 | 17.9 | 75.77 | 35 |
| Total hydrocarbons (mg L−1) | 48.69 | 0.1492 | 99.69 | 10 |
| TOC (mg L−1) | 872 | 19.81 | 97.72 | 20 |
| Nitrate (mg L−1) | 440 | 137 | 68.86 | 50 |
| Nitrite (mg L−1) | 0.99 | 0 | 100 | 1 |
| Phosphate (mg L−1) | 0.17 | 0 | 100 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zioui, D.; Salazar, H.; Aoudjit, L.; Martins, P.M.; Lanceros-Méndez, S. Polymer-Based Membranes for Oily Wastewater Remediation. Polymers 2020, 12, 42. https://doi.org/10.3390/polym12010042
Zioui D, Salazar H, Aoudjit L, Martins PM, Lanceros-Méndez S. Polymer-Based Membranes for Oily Wastewater Remediation. Polymers. 2020; 12(1):42. https://doi.org/10.3390/polym12010042
Chicago/Turabian StyleZioui, Djamila, Hugo Salazar, Lamine Aoudjit, Pedro M. Martins, and Senentxu Lanceros-Méndez. 2020. "Polymer-Based Membranes for Oily Wastewater Remediation" Polymers 12, no. 1: 42. https://doi.org/10.3390/polym12010042
APA StyleZioui, D., Salazar, H., Aoudjit, L., Martins, P. M., & Lanceros-Méndez, S. (2020). Polymer-Based Membranes for Oily Wastewater Remediation. Polymers, 12(1), 42. https://doi.org/10.3390/polym12010042