Structural Transformation of Polyacrylonitrile (PAN) Fibers during Rapid Thermal Pretreatment in Nitrogen Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Diefendorf, R.J.; Tokarsky, E. High-performance carbon fibers. Polym. Eng. Sci. 1975, 15, 150–159. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Sporl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef] [PubMed]
- Fitzer, E. PAN-based carbon fibers—Present state and trend of the technology from the viewpoint of possibilities and limits to influence and to control the fiber properties by the process parameters. Carbon 1989, 27, 621–645. [Google Scholar] [CrossRef]
- Chand, S. Review carbon fibers for composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Morgan, P. Carbon Fibers and Their Composites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Morris, E.A.; Weisenberger, M.C.; Bradley, S.B.; Abdallah, M.G.; Mecham, S.J.; Pisipati, P.; McGrath, J.E. Synthesis, spinning, and properties of very high molecular weight poly(acrylonitrile-co-methyl acrylate) for high performance precursors for carbon fiber. Polymer 2014, 55, 6471–6482. [Google Scholar] [CrossRef] [Green Version]
- Warner, S.B.; Peebles, L.H., Jr.; Uhlmann, D.R. Oxidative stabilization of acrylic fibres. J. Mater. Sci. 1979, 14, 556–564. [Google Scholar] [CrossRef]
- Bajaj, P.; Roopanwal, A. Thermal stabilization of acrylic precursors for the production of carbon fibers: An overview. J. Macromol. Sci. Part C 1997, 37, 97–147. [Google Scholar] [CrossRef]
- Fitzer, E.; Frohs, W.; Heine, M. Optimization of stabilization and carbonization treatment of PAN fibres and structural characterization of the resulting carbon fibres. Carbon 1986, 24, 387–395. [Google Scholar] [CrossRef]
- Fitzer, E.; Müller, D.J. The influence of oxygen on the chemical reactions during stabilization of PAN as carbon fiber precursor. Carbon 1975, 13, 63–69. [Google Scholar] [CrossRef]
- Gupta, A.; Harrison, I.R. New aspects in the oxidative stabilization of PAN-based carbon fibers. Carbon 1996, 34, 1427–1445. [Google Scholar] [CrossRef]
- Gupta, A.; Harrison, I.R. New aspects in the oxidative stabilization of PAN-based carbon fibers: II. Carbon 1997, 35, 809–818. [Google Scholar] [CrossRef]
- Dalton, S.; Heatley, F.; Budd, P.M. Thermal stabilization of polyacrylonitrile fibres. Polymer 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- Edie, D.D. The effect of processing on the structure and properties of carbon fibers. Carbon 1998, 36, 345–362. [Google Scholar] [CrossRef]
- Ko, T.-H.; Chiranairadul, P.; Ting, H.-Y.; Lin, C.-H. The effect of modification on structure and dynamic mechanical behavior during the processing of acrylic fiber to stabilized fiber. J. Appl. Polym. Sci. 1989, 37, 541–552. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, W. Chemical modification for PAN fibers during heat-treatment process. Phys. Procedia 2011, 18, 202–205. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, R.; Liu, J.; Chen, G.; Guo, S.; Xu, L.; Xiao, S.; Shen, Z. Effect of KMnO4 on chemical, crystal and microscopic structure of polyacrylonitrile fibers. Ceram. Int. 2019, 45, 17669–17674. [Google Scholar] [CrossRef]
- Park, M.; Choi, Y.; Lee, S.-Y.; Kim, H.-Y.; Park, S.-J. Influence of electron-beam irradiation on thermal stabilization process of polyacrylonitrile fibers. J. Ind. Eng. Chem. 2014, 20, 1875–1878. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, Y.; Wang, M.; Qin, X.; Zhao, W.; Luan, J. Effect of gamma-irradiation on the mechanical properties of polyacrylonitrile-based carbon fiber. Carbon 2013, 52, 427–439. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, Y.; Zhao, W.; Yang, C.; Jiang, J. Effects of gamma ray irradiation on poly(acrylonitrile-co-methyl acrylate) fibers. Polym. Degrad. Stab. 2016, 128, 149–157. [Google Scholar] [CrossRef]
- Son, S.-Y.; Jo, A.Y.; Jung, G.Y.; Chung, Y.-S.; Lee, S. Accelerating the stabilization of polyacrylonitrile fibers by UV irradiation. J. Ind. Eng. Chem. 2019, 73, 47–51. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, Y.; Wang, J.; Chen, Q.; Zhou, L.; Jiang, J.; Chen, L. Improving crosslinking of stabilized polyacrylonitrile fibers and mechanical properties of carbon fibers by irradiating with γ-ray. Polym. Degrad. Stab. 2016, 133, 16–26. [Google Scholar] [CrossRef]
- Park, S.; Kil, H.-S.; Choi, D.; Song, S.-K.; Lee, S. Rapid stabilization of polyacrylonitrile fibers achieved by plasma-assisted thermal treatment on electron-beam irradiated fibers. J. Ind. Eng. Chem. 2019, 69, 449–454. [Google Scholar] [CrossRef]
- Qin, X.; Lu, Y.; Xiao, H.; Song, Y. Improving stabilization degree of stabilized fibers by pretreating polyacrylonitrile precursor fibers in nitrogen. Mater. Lett. 2012, 76, 162–164. [Google Scholar] [CrossRef]
- Qin, X.; Lu, Y.; Xiao, H.; Hao, Y.; Pan, D. Improving preferred orientation and mechanical properties of PAN-based carbon fibers by pretreating precursor fibers in nitrogen. Carbon 2011, 49, 4598–4600. [Google Scholar] [CrossRef]
- Chai, X.; Mi, H.; Zhu, C.; He, C.; Xu, J.; Zhou, X.; Liu, J. Low-temperature thermal stabilization of polyacrylontrile-based precursor fibers towards efficient preparation of carbon fibers with improved mechanical properties. Polymer 2015, 76, 131–139. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, X.; Wang, X.; Huang, J.; Huang, X.; Chen, Y. Simultaneous DSC/TG analysis on the thermal behavior of PAN polymers prepared by aqueous free-radical polymerization. Polym. Degrad. Stab. 2016, 130, 320–327. [Google Scholar] [CrossRef]
- Szepcsik, B.; Pukánszky, B. The mechanism of thermal stabilization of polyacrylonitrile. Thermochim. Acta 2019, 671, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, J.; Wu, G. Evolution of structure and properties of PAN precursors during their conversion to carbon fibers. Carbon 2003, 41, 2805–2812. [Google Scholar] [CrossRef]
- Yu, M.; Wang, C.; Bai, Y.; Wang, Y.; Xu, Y. Influence of precursor properties on the thermal stabilization of polyacrylonitrile fibers. Polym. Bull. 2006, 57, 757–763. [Google Scholar] [CrossRef]
- Meinl, J.; Schönfeld, K.; Kirsten, M.; Kittler, K.; Michaelis, A.; Cherif, C. Optimization of the temperature program to scale up the stabilization of polyacrylonitrile fibers. Compos. Part A Appl. Sci. Manuf. 2017, 96, 37–45. [Google Scholar] [CrossRef]
- Wang, J.; Hu, L.; Yang, C.; Zhao, W.; Lu, Y. Effects of oxygen content in the atmosphere on thermal oxidative stabilization of polyacrylonitrile fibers. RSC Adv. 2016, 6, 73404–73411. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Lian, F.; Liang, J. Effect of the oxygen-induced modification of polyacrylonitrile fibers during thermal-oxidative stabilization on the radial microcrystalline structure of the resulting carbon fibers. Polym. Degrad. Stab. 2013, 98, 2259–2267. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Zhou, T.; Liu, X.; Yuan, Q.; Zhang, A. New understanding on the reaction pathways of the polyacrylonitrile copolymer fiber pre-oxidation: Online tracking by two-dimensional correlation FTIR spectroscopy. RSC Adv. 2016, 6, 4397–4409. [Google Scholar] [CrossRef]
- Nguyen-Thai, N.U.; Hong, S.C. Structural evolution of poly(acrylonitrile-co-itaconic acid) during thermal oxidative stabilization for carbon materials. Macromolecules 2013, 46, 5882–5889. [Google Scholar] [CrossRef]
- Nunna, S.; Creighton, C.; Fox, B.L.; Naebe, M.; Maghe, M.; Tobin, M.J.; Bambery, K.; Vongsvivut, J.; Hameed, N. The effect of thermally induced chemical transformations on the structure and properties of carbon fibre precursors. J. Mater. Chem. A 2017, 5, 7372–7382. [Google Scholar] [CrossRef]
- Ge, Y.; Fu, Z.; Deng, Y.; Zhang, M.; Zhang, H. The effects of chemical reaction on the microstructure and mechanical properties of polyacrylonitrile (PAN) precursor fibers. J. Mater. Sci. 2019, 54, 12592–12604. [Google Scholar] [CrossRef]
- Saha, B.; Schatz, G.C. Carbonization in polyacrylonitrile (PAN) based carbon fibers studied by ReaxFF molecular dynamics simulations. J. Phys. Chem. B 2012, 116, 4684–4692. [Google Scholar] [CrossRef]
- Lian, F.; Liu, J.; Ma, Z.; Liang, J. Stretching-induced deformation of polyacrylonitrile chains both in quasicrystals and in amorphous regions during the in situ thermal modification of fibers prior to oxidative stabilization. Carbon 2012, 50, 488–499. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Liu, F.; Wang, H.; Xue, L.; Fan, L.; Zhu, Z. Effect of microstructure on the mechanical properties of PAN-based carbon fibers during high-temperature graphitization. J. Mater. Sci. 2008, 43, 4316–4322. [Google Scholar] [CrossRef]
- Gutmann, P.; Moosburger-Will, J.; Kurt, S.; Xu, Y.; Horn, S. Carbonization of polyacrylonitrile-based fibers under defined tensile load: Influence on shrinkage behavior, microstructure, and mechanical properties. Polym. Degrad. Stab. 2019, 163, 174–184. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.H.; Li, R.Y. Continuous carbonization of polyacrylonitrile-based oxidized fibers: Aspects on mechanical properties and morphological structure. J. Appl. Polym. Sci. 1994, 52, 945–950. [Google Scholar] [CrossRef]
- Hameed, N.; Sharp, J.; Nunna, S.; Creighton, C.; Magniez, K.; Jyotishkumar, P.; Salim, N.V.; Fox, B. Structural transformation of polyacrylonitrile fibers during stabilization and low temperature carbonization. Polym. Degrad. Stab. 2016, 128, 39–45. [Google Scholar] [CrossRef]
- Huang, J.; Ouyang, Q.; Li, M.; Heng, F.; Ma, H.; Chen, Y. Thermal behavior and thermal stabilization of guanidine hydrochloride-modified acrylic fiber for preparation of low-cost carbon fiber. J. Therm. Anal. Calorim. 2018, 136, 2195–2203. [Google Scholar] [CrossRef]


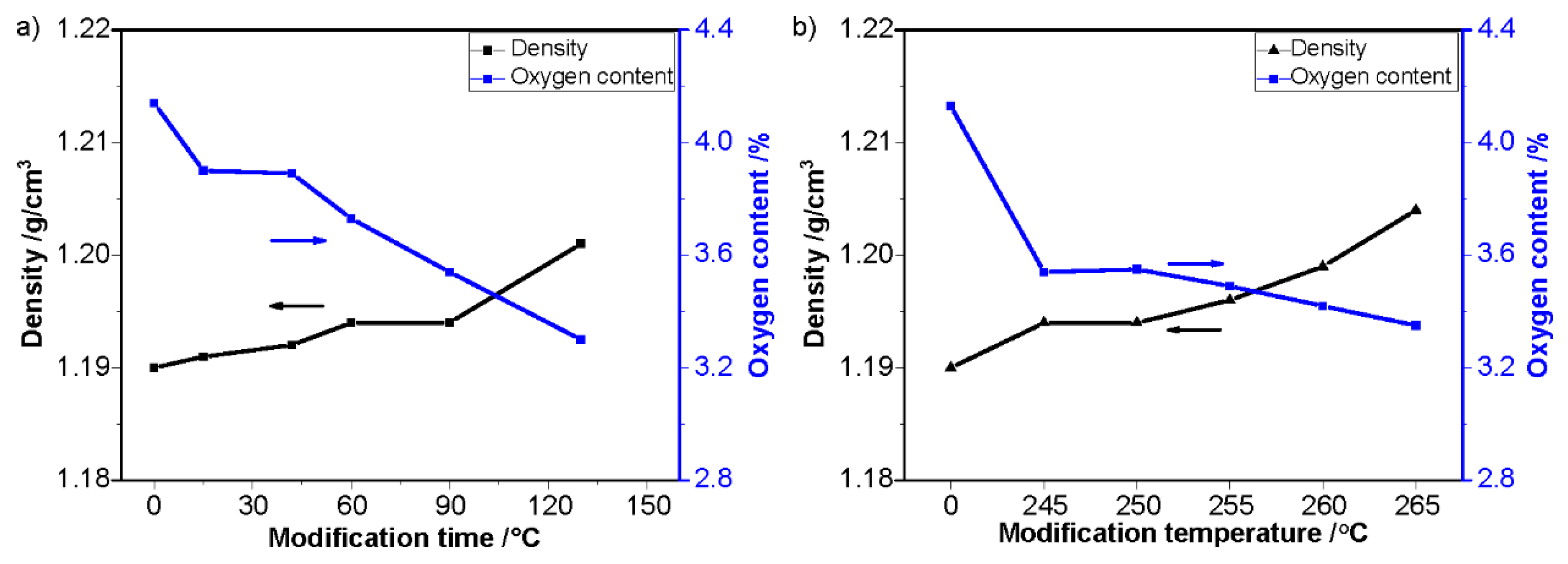
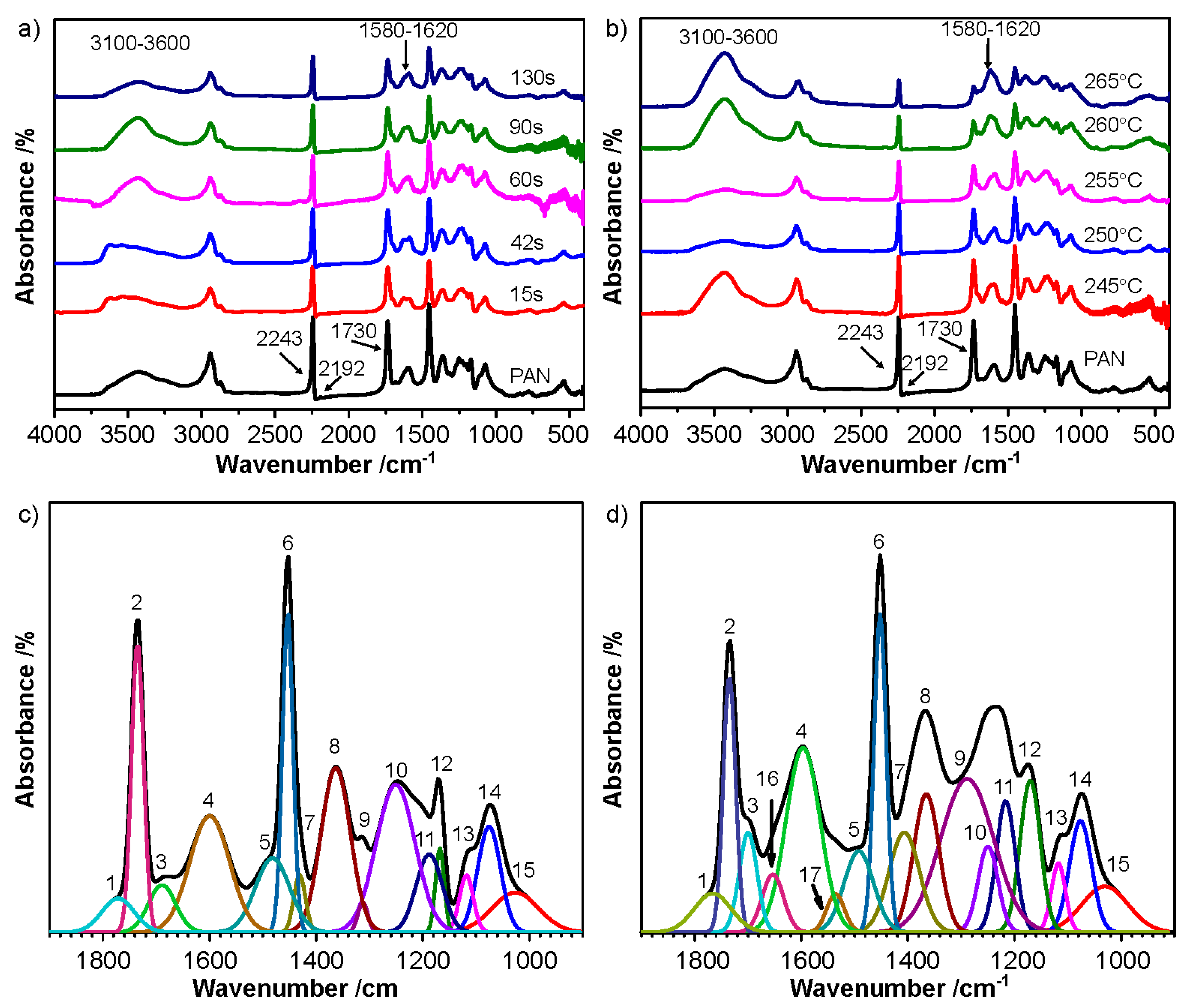
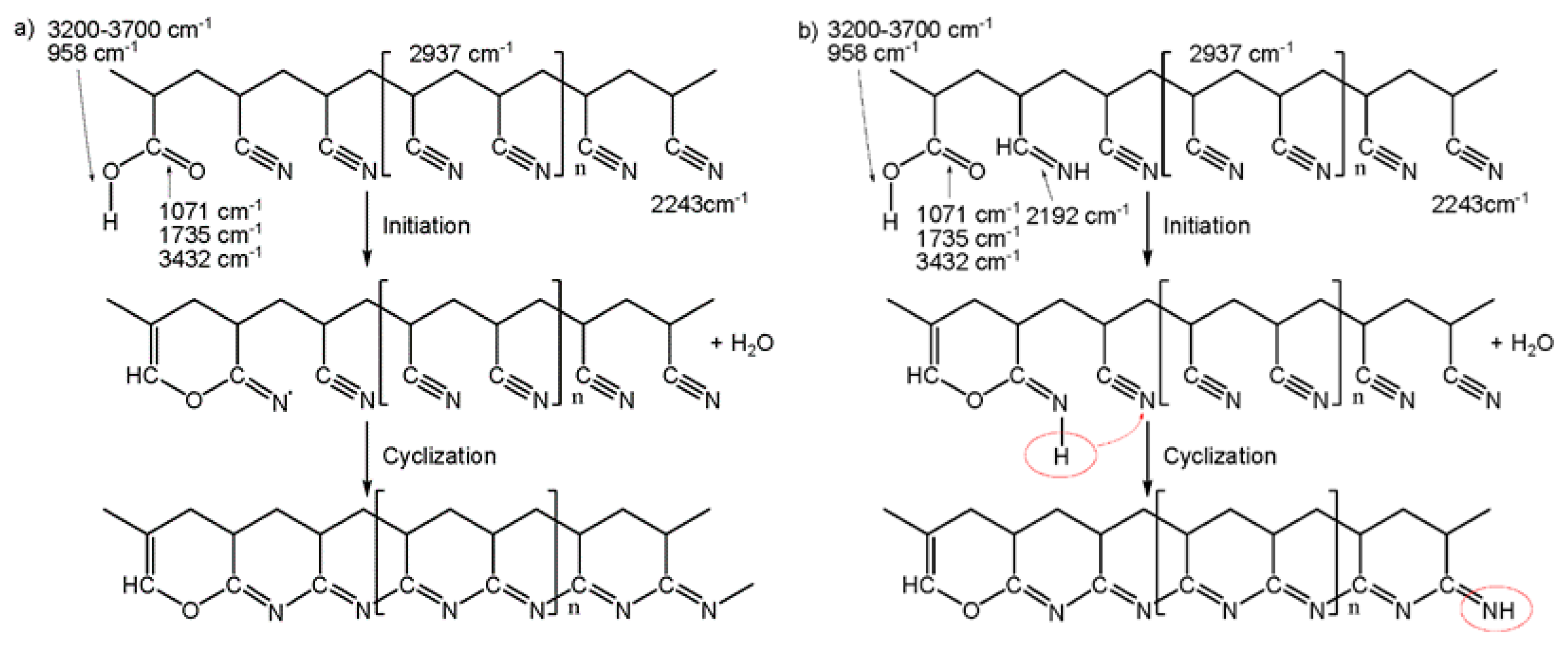
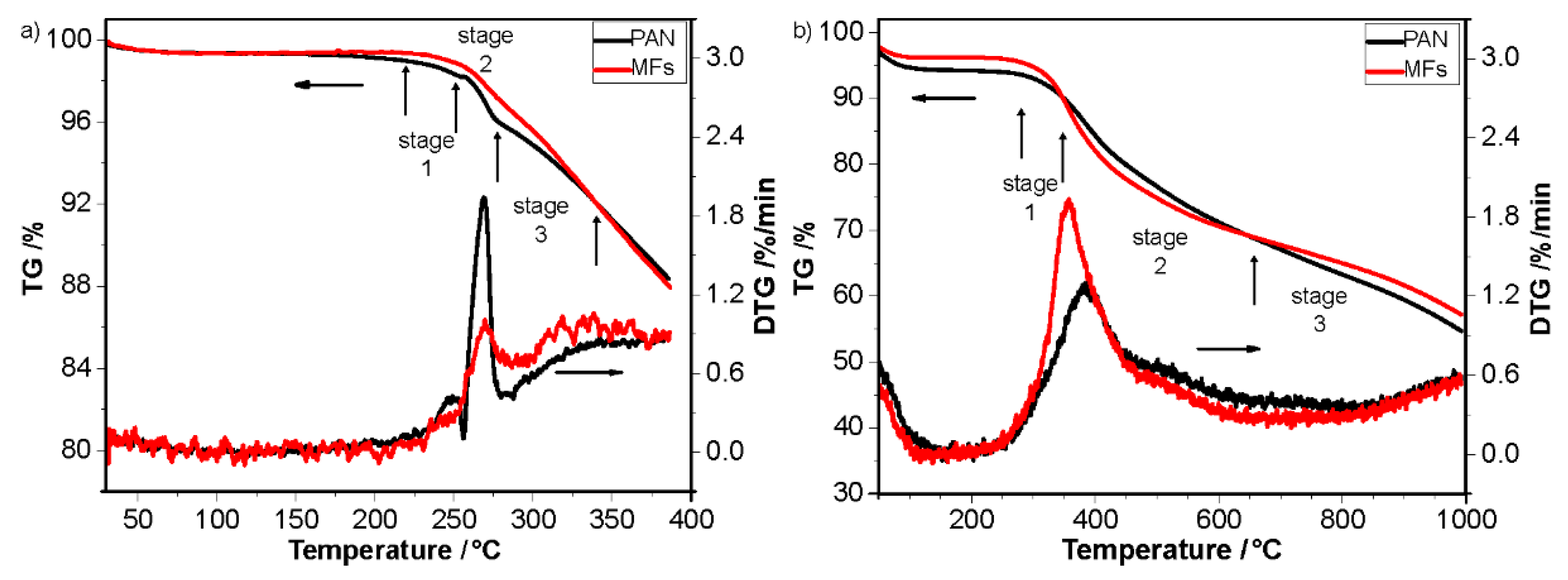
| Sample | Temperature/°C | Time/s | Sample | Temperature/°C | Time/s |
|---|---|---|---|---|---|
| MFs-1 | 245 | 15 | MFs-6 | 245 | 90 |
| MFs-2 | 245 | 42 | MFs-7 | 250 | 90 |
| MFs-3 | 245 | 60 | MFs-8 | 255 | 90 |
| MFs-4 | 245 | 90 | MFs-9 | 260 | 90 |
| MFs-5 | 245 | 130 | MFs-10 | 265 | 90 |
| Designation of Peaks | Position of the Peaks (cm−1) | Corresponding Vibration Modes | References |
|---|---|---|---|
| 1 | 1773 | νC = O | [35] |
| 2 | 1735 | ||
| 3 | 1690 | νC = O in dimer COOH | [35] |
| 4 | 1600 | νC = C + νC = N + δN-H | [35,38] |
| 5 | 1481 | νC = C of ring | [36] |
| 6 | 1452 | δC-H in CH2 | [35,36] |
| 7 | 1430 | δO-H and ωC-H | [35] |
| 8 | 1362 | ||
| 9 | 1312 | ||
| 10 | 1250 | νasC-O-C in ether | [36] |
| 11 | 1189 | νC-O | [38] |
| 12 | 1168 | νC-C + νC-N + νC-O + δO-H of COOH | [22,35] |
| 13 | 1117 | ||
| 14 | 1074 | νsC-O-C in ether | [22,35] |
| 15 | 1027 | νC-N in aliphatic amine | [35,36] |
| 16 | 1654 | νC = O in acridoneνC = C and νC = N | [24,35] |
| 17 | 1535 | νC-N and νN-H of amide | [24,35] |
| Sample | Crystallinity/% | Lc/nm | d(100)/Å | Orientation/% |
|---|---|---|---|---|
| PAN | 61.9 | 6.58 | 5.29 | 85.3 |
| MFs-1 | 63.3 | 7.42 | 5.28 | 86.3 |
| MFs-2 | 66.1 | 7.69 | 5.30 | 86.5 |
| MFs-3 | 67.1 | 7.92 | 5.32 | 86.5 |
| MFs-4 | 67.1 | 7.90 | 5.31 | 85.5 |
| MFs-5 | 67.7 | 7.45 | 5.30 | 86.7 |
| MFs-6 | 67.1 | 7.90 | 5.35 | 85.5 |
| MFs-7 | 68.1 | 8.04 | 5.31 | 86.4 |
| MFs-8 | 67.7 | 7.96 | 5.28 | 86.6 |
| MFs-9 | 67.6 | 7.69 | 5.29 | 86.6 |
| MFs-10 | 67.0 | 7.49 | 5.30 | 85.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, W.; Liu, J.; Wang, X.; Yan, K.; Zhang, A.; Yang, J.; Chen, L.; Liang, J. Structural Transformation of Polyacrylonitrile (PAN) Fibers during Rapid Thermal Pretreatment in Nitrogen Atmosphere. Polymers 2020, 12, 63. https://doi.org/10.3390/polym12010063
Dang W, Liu J, Wang X, Yan K, Zhang A, Yang J, Chen L, Liang J. Structural Transformation of Polyacrylonitrile (PAN) Fibers during Rapid Thermal Pretreatment in Nitrogen Atmosphere. Polymers. 2020; 12(1):63. https://doi.org/10.3390/polym12010063
Chicago/Turabian StyleDang, Wei, Jie Liu, Xiaoxu Wang, Kaiqi Yan, Aolin Zhang, Jia Yang, Liang Chen, and Jieying Liang. 2020. "Structural Transformation of Polyacrylonitrile (PAN) Fibers during Rapid Thermal Pretreatment in Nitrogen Atmosphere" Polymers 12, no. 1: 63. https://doi.org/10.3390/polym12010063






