3.1. Effect of αMSD on the Physichemical Properties of Polymers
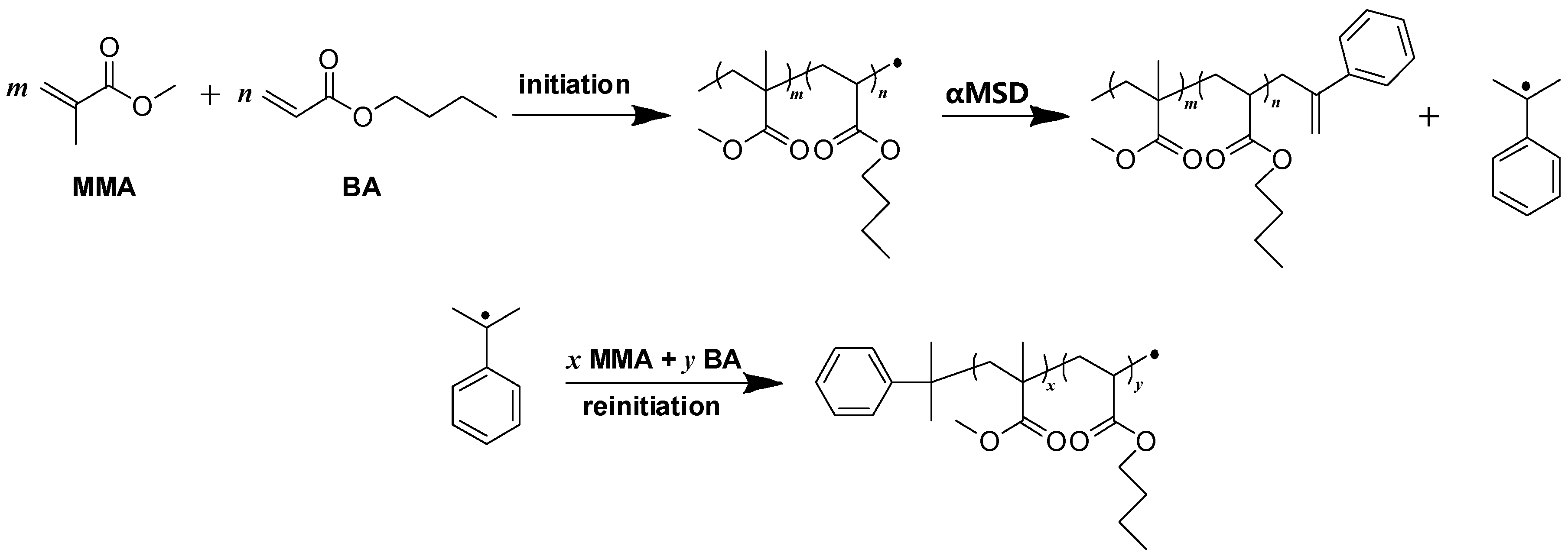
Different levels of αMSD were introduced into the emulsion polymerization of (meth) acrylate in order to effectively regulate the physical properties of the resulting polymers. Its effect on average molecular mass, polymerization degree, and monomer conversion is shown in
Table 1.
As the addition of αMSD increased from 0% to 0.1%, the number-average molecular weights were evidently decreased from 40.56 × 10
4 to 28.58 × 10
4 g/mol, whereas monomer conversion underwent a slight decrease to 97.8%. When αMSD concentration increased from 0.1% to 1%, both molecular weights and monomer conversion were significantly decreased, as the presence of αMSD was able to effectively interfere with the polymerization process. In terms of the dispersity (
Ð) of the molecular weight, the addition of αMSD contributed to the gradual decrease of the dispersity to nearly 1.60 due to a mathematical artefact in the definition of dispersity [
36]. However, despite its efficiency in adjusting molecular weights and
Ð, only 44.6% monomer conversion was accomplished when the concentration of αMSD was 3%. These results indicate that the utilization of αMSD as a chain transfer agent is effective in the adjustment of the molecular weights and the dispersity
Ð of synthesized polymers, but a further addition of αMSD might compromise the monomer conversion.
Figure 1 was obtained by plotting the change in logarithm of the concentration of αMSD (ln [αMSD]) as a function of the logarithm of
Mn (ln [
Mn]). It showed that the influence of αMSD concentration on M
n was estimated to be
Mn∝[αMSD]
−0.797, and the fitting linear expression was
y = 7.458 − 0.797
x (
R-Square = 0.983,
y-ln [
Mn],
x-ln [αMSD]). The fitting result quantitatively described the effect of αMD on molecular weight of synthesized polymers, which could be used to estimate the appropriate CTA dosage according to different mass requirements.
In addition,
in
Table 1 was obtained from Equation (1) [
37]. As MMA was expected to polymerize first, followed by the polymerization of BA [
38], Equation (1) is shown as follows.
where
x is the mass fraction of MMA in the synthesized copolymers;
F1 as the mole fraction of MMA in the copolymer is calculated by Meyer’s [
37] copolymerization equations (Equations (2) and (3));
m1 and
m2 are the relative molecular masses of MMA and BA, respectively;
f10 and
f1 are the mole fractions of MMA in the monomer phase at the beginning and after a certain conversion of the polymerization, respectively;
f20 and
f2 are the mole fractions of BA at different stages;
C is a monomer conversion;
α,
β,
γ, and
δ are coefficients relating to the properties of monomers, which are shown in
Table 2.
The relative variation tendencies of
x and 1-
x could be seen in
Figure 2. As the amounts of initiator and αMSD in the polymerization process were very small, their proportions in the polymer were ignored. It is evident that x increased gradually with increasing amounts of αMSD. This indicated that αMSD affected not only the molecular weights of polymers, but also its composition.
To analyze the potential reason, the reactivity ratios were calculated according to a semi-empirical formula, equation
Q-
e (Equations (4) and (5)).
where
Q and
e represent the conjugation degree and polarity of monomer substituents, respectively.
ra and
rb are the reactivity ratios of monomers
a and
b, respectively. In the case of
ra, a higher value means a larger self-polymerization trend. Alternatively, a lower value indicates a larger copolymerization trend.
During the calculation, the
Q and
e values for αMSD were estimated using
Q and
e of alpha methyl styrene (AMS), as αMSD was a dimer of AMS. The specific
Q and
e values of AMS, MMA, and BA were listed in
Table 3, and the calculated reactivity ratios of the three monomers were given in
Table 4.
Where r1 and r3 are the reactivity ratios for MMA to BA and AMS; r2 and r6 are the reactivity ratios for BA to MMA and AMS; and r4 and r5 are the reactivity ratios for AMS to MMA and BA, respectively.
As given in
Table 4,
r1 >
r2 revealed that MMA had a greater polymerization activity than BA, which contributed to a higher
x (>0.5) in the early polymerization period. For the sample without αMSD addition (Run 1), BA’s mass fraction in the polymers gradually increased up to 50% in the later polymerization period. However, this process would be terminated earlier in the presence of αMSD. Because of the chain transfer reaction of αMSD, the propagation process was terminated significantly earlier with an increase in the amount of αMSD. As a result, the growth of BA’s mass fraction in the polymer was terminated earlier and earlier. Finally, MMA’s mass fraction in the polymer increased, whereas the mass fraction of BA was lowered with an increase in the amount of αMSD.
In addition, r1 > r3 indicated that MMA had a greater tendency to copolymerize with AMS than BA. Similarly, r2 > r6 indicated that BA tended to copolymerize with AMS rather than MMA. In other words, both MMA and BA were more likely to copolymerize with AMS (αMSD). This could indirectly explain why αMSD effectively controlled the molecular weights of the polymer. For AMS, r4 ≈ r5, there was an almost equal possibility of AMS to copolymerize with MMA or BA.
To compare the regulation reactivity differences of αMSD as a chain transfer agent to MMA and BA more accurately, its transfer coefficients (
Ctr) to each single monomer were calculated with the following expression (Equation (6)). It was derived by Alfrey [
39] for the case of binary copolymerization in the presence of small amounts of CTA, based on the assumption that the only effect of the chain transfer reaction was to terminate one growing molecular chain and start another, i.e., ignoring cases in which a chain transfer also leaded to the termination of the kinetic chain.
where
A and
B are mole fractions of monomer A and monomer B, respectively, in the polymer; [
A] and [
B] are the mole fractions of monomer A and monomer B in the whole monomers;
ra and
rb are the reactivity of the two monomers; and
K is the slope of a Mayo-like plot (i.e., 1/
vs. total monomer concentration, Equation (7)) [
40,
41].
where [
C] and [
M] are concentrations of the CTA and whole monomers, respectively;
is the average degree of polymerization for the polymer at a given [
C]; and
is for the polymer in the absence of CTA.
In this work, MMA and BA were regarded as monomers A and B, respectively, as αMSD was used as the CTA. On the basis of the above analysis, the values of the individual transfer coefficients (Ctr,a and Ctr,b) for a particular monomer ratio were successfully obtained by the two simultaneous equations (Equations (6) and (7)).
Figure 3 displayed the fitting result of the Mayo plot, and
K was derived from the slope of the plot: 0.56 (R-Square = 0.986).
K remained unchanged as long as the monomer ratio of MMA and BA was a constant. As a consequence,
Ctr,a and
Ctr,b could be calculated through Equation (6) by choosing any two runs (including αMSD) in
Table 1, such as Run 2 and Run 4, and the result was shown in
Table 5.
If this polymerization system was treated as a terpolymerization of MMA, BA, and αMSD, the two
Ctrs of αMSD could be calculated by the reactivity ratios of the three monomers on the basis of
Q-
e values. According to the definition of
Ctr, the
Ctrs values of αMSD should be kinetically equivalent to the reciprocals of the reactivity ratios of any two monomers. The calculation results were shown in
Table 5.
As shown above, the Ctrs values of αMSD determined by different methods were obviously different. Ctr,MMA and Ctr,BA were 0.62 and 0.47 according to Alfrey’s theory, respectively. However, when using Q-e method, the former was 2.12 and the latter was increased to 14.28. The difference could be explained from two aspects: First, the Ctr calculations based on the Q-e method utilized AMS’s reactivity ratios to represent αMSD’s. Nevertheless, as a dimer of AMS, αMSD’s structure was more complex than AMS, and the former had more steric effects when it reacted with other monomers. Thus, their reactivity ratios as well as Ctrs should be different. Second, there were some inherent errors in calculating the reactivity ratios using the Q-e method.
In contrast to the
Q-e method, the substitution of AMS for αMSD was avoided in Alfrey’s theory, so the
Ctrs values of αMSD should be more accurate. In addition, the almost same values of
Ctr,MMA and
Ctr,BA indicated that αMSD has almost same chain transfer possibilities with both MMA and BA, which was consistent with the above analysis of reactivity ratios in
Table 4.
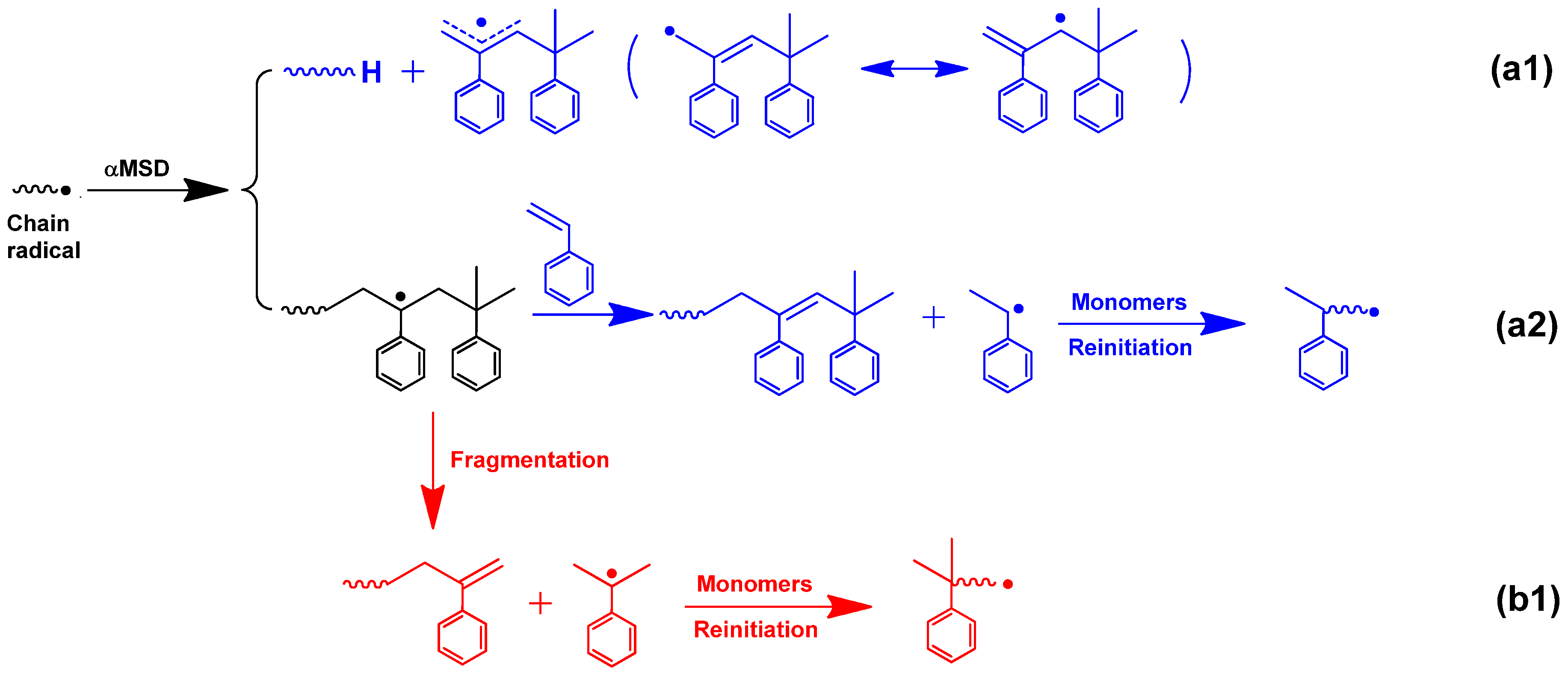
As described above, few studies have been conducted to use αMSD as a chain transfer agent in the emulsion polymerization. Therefore, we have used NMR analysis to reveal its potential mechanism. In the polymerization process, chain propagation would be terminated by the irreversible AFCT agent. Therefore, a part of the polymer’s terminal structures consists of the AFCT agent. On this basis, the mechanism for αMSD could be revealed by a polymer terminal structure analysis. The
1H NMR analysis of the resulting polymer using 1% αMSD showed that two signals of similar intensities at approximately 5.0, 5.1, and 5.6 ppm were observed as the characteristic peaks of the two syntonic hydrogen atoms of the terminal double bonds (
Figure 4). Moreover, multiple signals at ~7.0 ppm were present, which were ascribed to the substituted benzene ring from the αMSD. Accordingly, these results confirmed that emulsion polymerization of acrylates using αMSD as a chain transfer agent followed Yasummasa’s mechanism. The detailed regulation mechanism is illustrated in
Scheme 4.
3.2. Effect of αMSD on the Polymerization Rate
As αMSD has an obvious influence on polymer molecular weights, it was necessary to study if its presence would also affect the polymerization rate (
Rp). In the emulsion polymerization, the constant speed stage was typically used as the indicator of total polymerization rate, and it could be calculated from the conversion–time curve.
Figure 5 showed the monomer conversion versus reaction time in the presence of αMSD at various concentrations. The slopes of the curves directly reflected
Rp. It was clear that the presence of αMSD could decrease the
Rp as compared to samples without αMSD. Moreover, as the increase in the quantities of αMSD,
Rp was decreased more significantly. For example, the monomer conversion only reached 0.4 after 250 min when 3% of αMSD was added, whereas its conversion approached 100% without αMSD addition. Furthermore, the effect of αMSD concentration ([αMSD]) on
Rp was further correlated via constructing a log–log plot of
Rp and [αMSD], as shown in
Figure 6. As expected, the result was quantitatively estimated to be
Rp∝[αMSD]
−0.945 (R-Square = 0.905).
The potential reasons for the decrease in
Rp in the presence of αMSD may be explained from two aspects. First of all, the overall rate constant of the scission process of the leaving group may be lower than propagation [
20]. The efficient transfer reaction of an irreversible AFCT agent required that transitional radicals, created by the addition reaction of the irreversible AFCT agent and chains radicals, could easily fragment to generate new radicals to reinitiate the polymerization process. As a result, retardation would happen. In fact, the novel radical (isopropyl benzene radical, seen in
Scheme 4) in this paper, had less access to reinitiate polymerization than the primary radical (sulfate radical anion) due to its steric hindrance. Thus, the macroscopic reaction rate decreased. Second, radicals derived from both the initiator and leaving groups might escape from micelles or latex particles (desorption) and then be terminated with radicals in either the water phase or polymer particle (reabsorption). This desorption phenomenon had been successfully verified in the emulsion polymerization, and the related theoretical model had been previously established [
42,
43,
44]. The sulfate radical anion is more hydrophilic than the isopropyl benzene radical. As a result, the desorption number of the former was much greater than latter, and the latter tended to stay in the original particles. This led to serious loss of the high-active free radicals, and in addition, the remaining free radicals in the latex particles were not active enough, which further exacerbated the decline in
Rp.