Dielectric Properties of P(VDF-TrFE-CTFE) Composites Filled with Surface-Coated TiO2 Nanowires by SnO2 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2@SnO2 Hybrid Nanopaticles
2.2.1. Synthesis of TiO2 NWs
2.2.2. Synthesis of TiO2@SnO2 Hybrid Nanoparticles
2.3. Fabrication of P(VDF-TrFE-CTFE)-Based Composites
2.4. Characterization
3. Results and Discussion
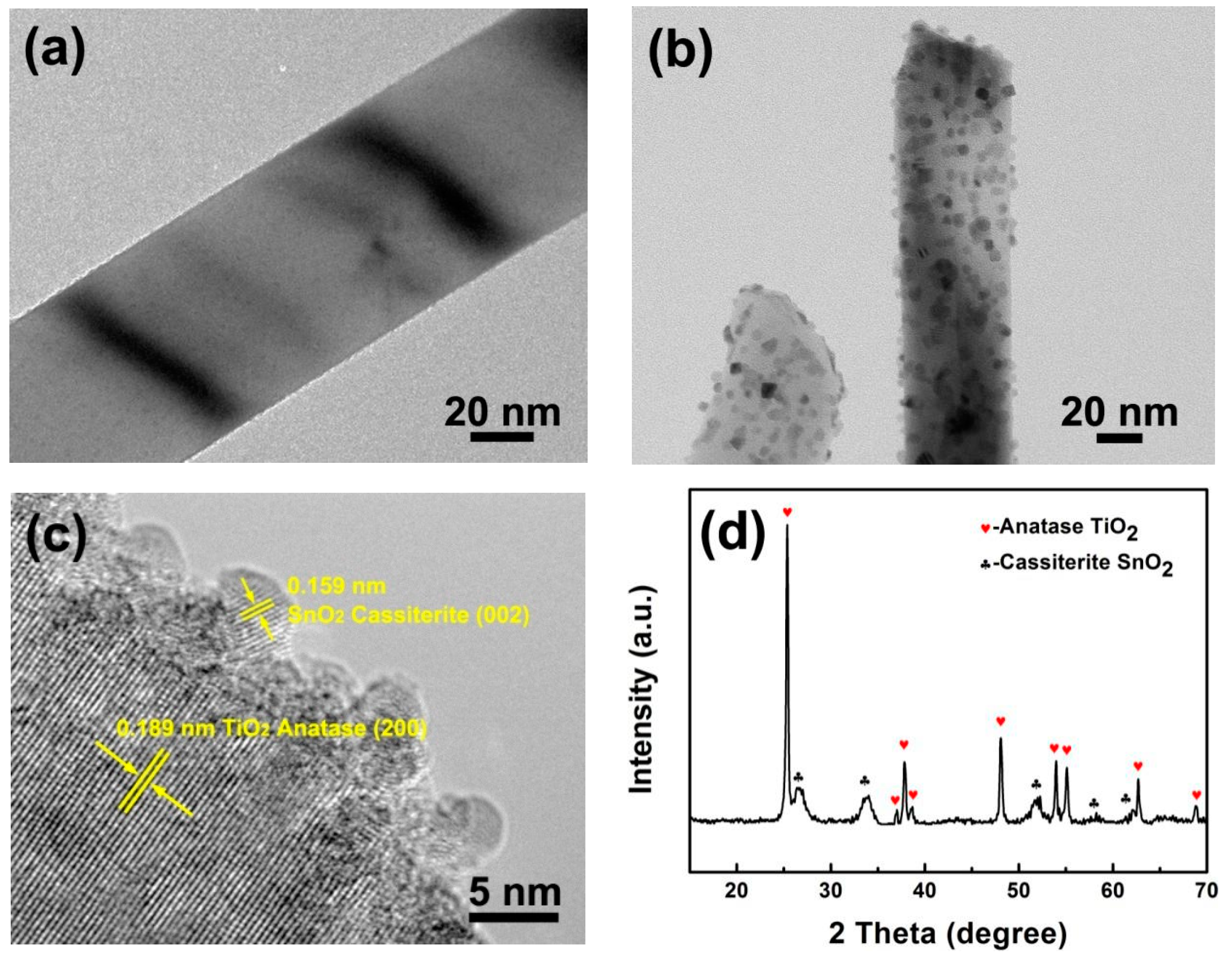
3.1. Structure and Morphology of TiO2@SnO2 Nanoparticles
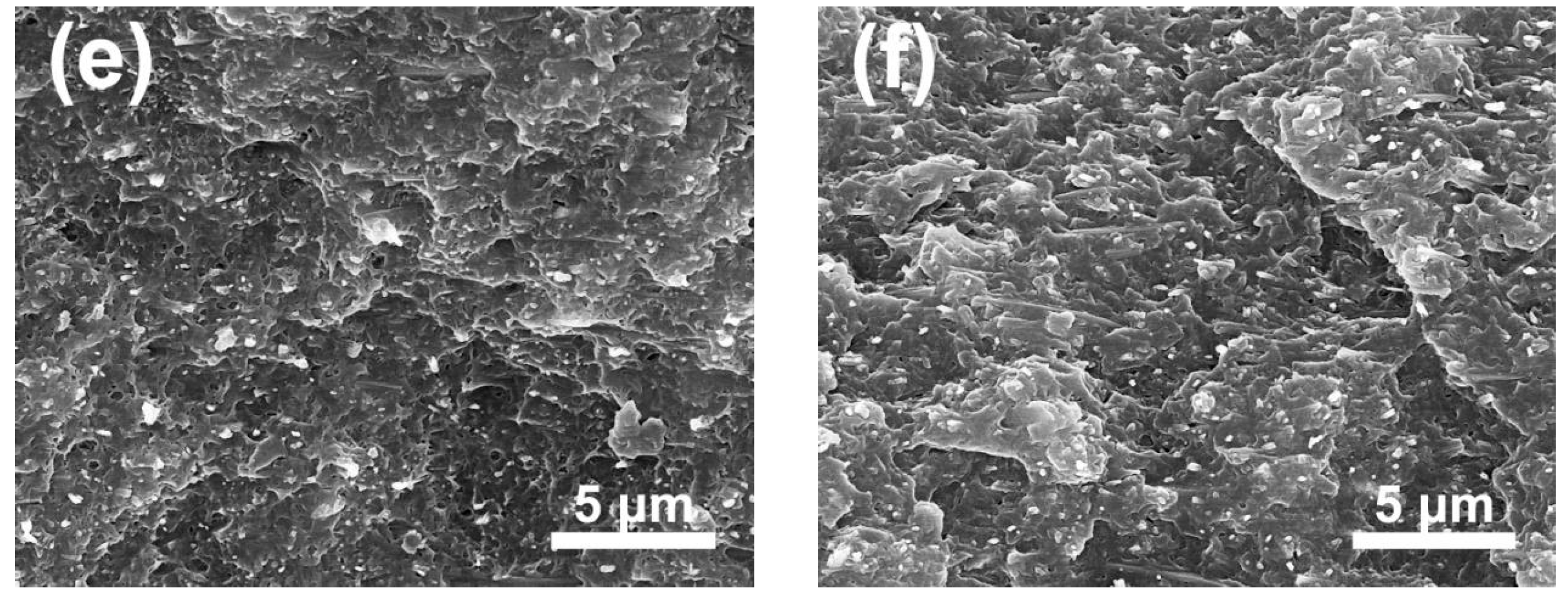
3.2. Morphology and Structure of TiO2 @SnO2/P(VDF-TrFE-CTFE) Composites
3.3. Crystallization and Melting Behavior of TiO2 @SnO2/P(VDF-TrFE-CTFE) Composites
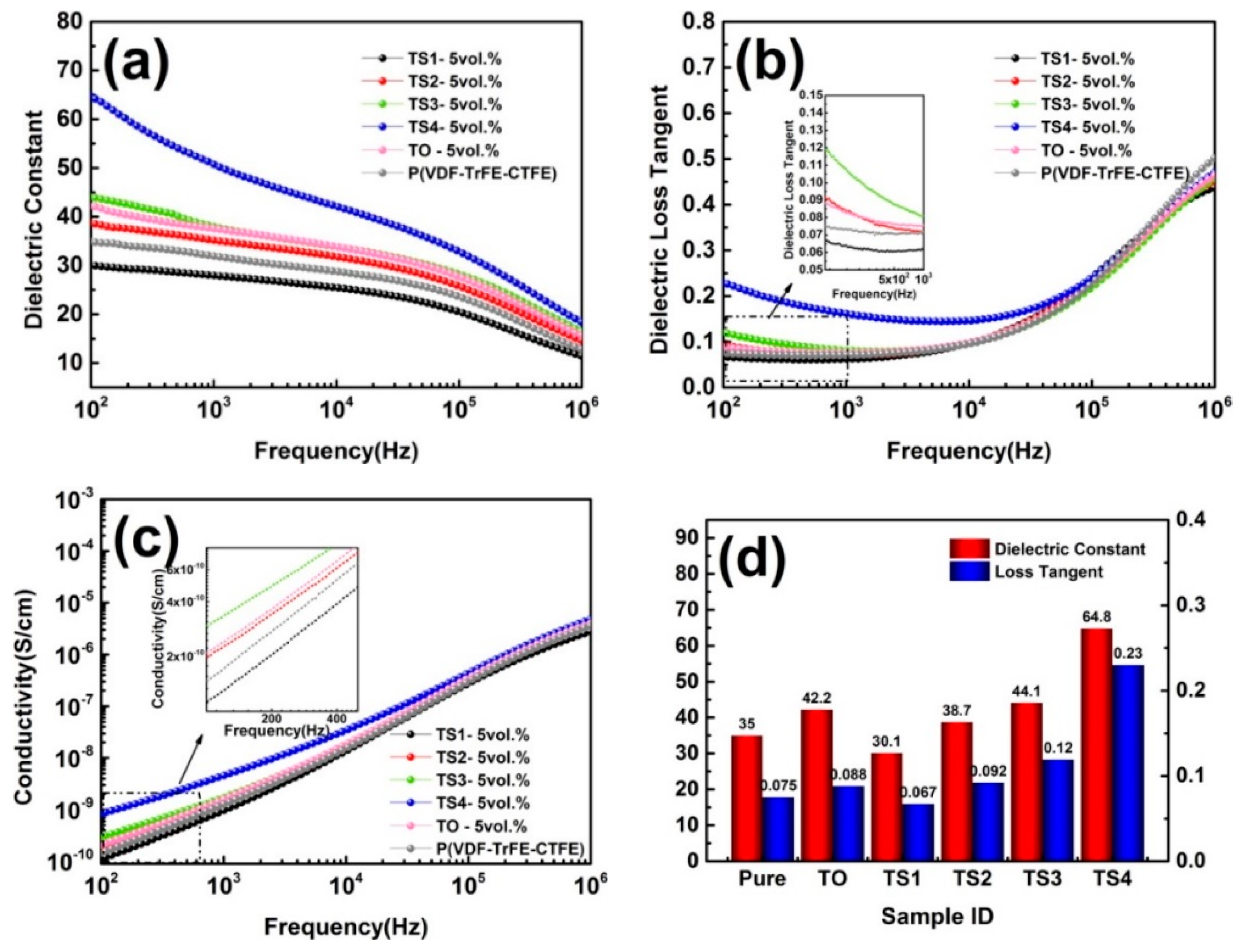
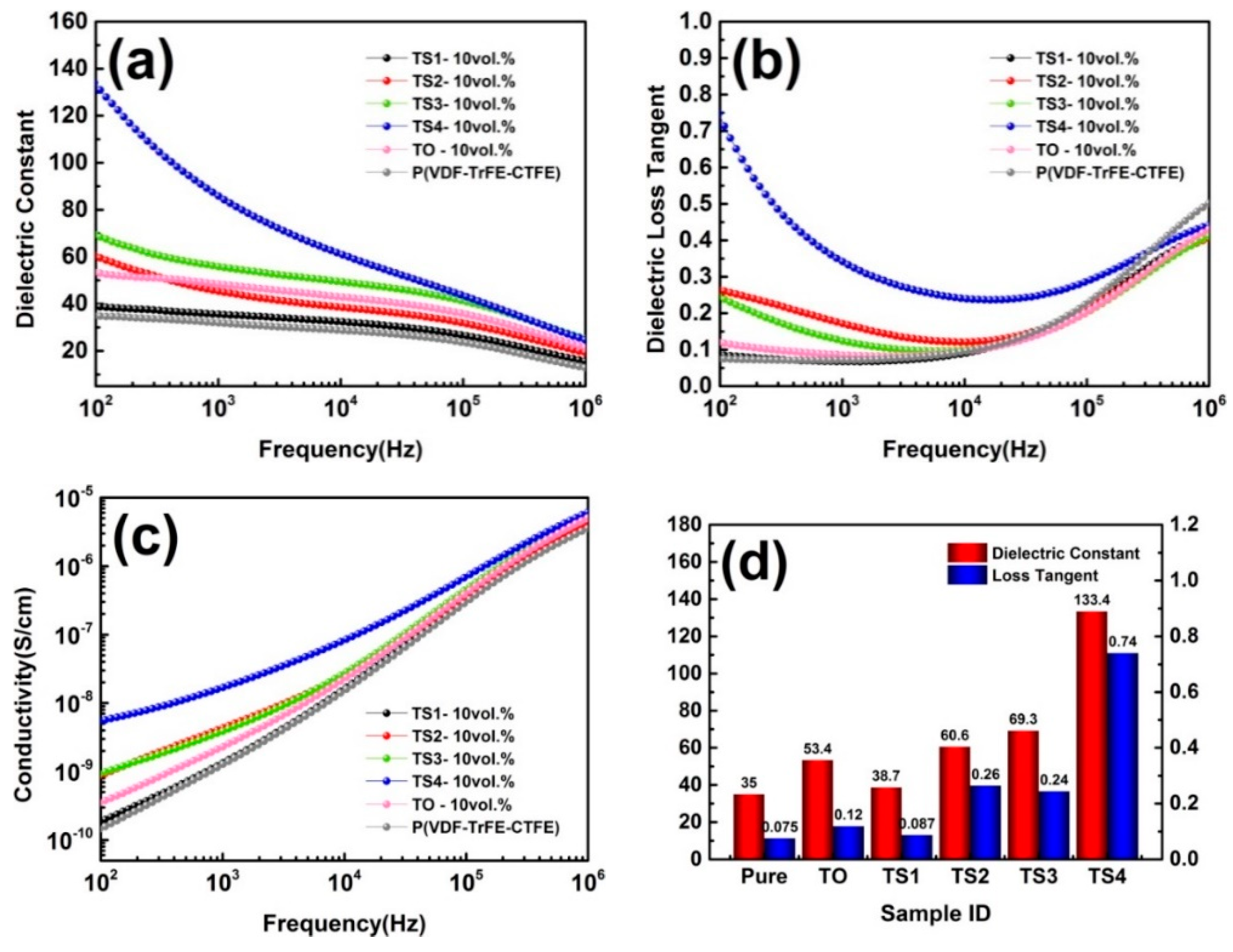
3.4. Dielectric Performances of TiO2 @SnO2/P(VDF-TrFE-CTFE) Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guo, M.F.; Jiang, J.Y.; Shen, Z.H.; Lin, Y.H.; Nan, C.W.; Shen, Y. High-energy-density ferroelectric polymer nanocomposites for capacitive energy storage: Enhanced breakdown strength and improved discharge efficiency. Mater Today 2019, 29, 49–67. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.H.; Fan, B.Y.; Ai, D.; Peng, Z.R.; Wang, Q. Nanostructured ferroelectric-polymer composites for capacitive energy storage. Small Methods 2018, 2, 1700399. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, Y.F. Recent progress in dielectric nanocomposites. Mater. Sci. Technol. 2019, 1–16. [Google Scholar] [CrossRef]
- Zou, K.L.; Dan, Y.; Xu, H.J.; Zhang, Q.F.; Lu, Y.M.; Huang, H.T.; He, Y.B. Recent advances in lead-free dielectric materials for energy storage. Mater. Res. Bull. 2019, 113, 190–201. [Google Scholar] [CrossRef]
- Yang, L.T.; Kong, X.; Li, F.; Hao, H.; Cheng, Z.X.; Liu, H.X.; Li, J.F.; Zhang, S.J. Perovskite lead-free dielectrics for energy storage applications. Prog. Mater. Sci. 2019, 102, 72–108. [Google Scholar] [CrossRef]
- Yao, Z.H.; Song, Z.; Hao, H.; Yu, Z.Y.; Cao, M.H.; Zhang, S.J.; Lanagan, M.T.; Liu, H.X. Homogeneous/Inhomogeneous-Structured dielectrics and their energy-storage performances. Adv. Mater. 2017, 29, 1601727. [Google Scholar] [CrossRef]
- Li, H.; Ai, D.; Ren, L.L.; Yao, B.; Han, Z.B.; Shen, Z.H.; Wang, J.J.; Chen, L.Q.; Wang, Q. Scalable polymer nanocomposites with record high-temperature capacitive performance enabled by rationally designed nanostructured inorganic fillers. Adv. Mater. 2019, 31, 1900875. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Jiang, P.K.; Zhang, Z.C.; Huang, X.Y. Dielectric phenomena and electrical energy storage of poly (vinylidene fluoride) based high-k polymers. Chinese. Chem. Lett. 2017, 28, 2027–2035. [Google Scholar] [CrossRef]
- Chen, Q.; Shen, Y.; Zhang, S.H.; Zhang, Q.M. Polymer-based dielectrics with high energy storage density. Annu. Rev. Mater. Res. 2015, 45, 433–458. [Google Scholar] [CrossRef]
- Huan, T.D.; Boggs, S.; Teyssedre, G.; Laurent, C.; Cakmak, M.; Kumar, S.; Ramprasad, R. Advanced polymeric dielectrics for high energy density applications. Prog. Mater. Sci. 2016, 83, 236–269. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Chen, Q.; Chu, B.J.; Zhang, Q.M. Recent development of high energy density polymers for dielectric capacitors. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1036–1042. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.H.; Tian, H.D.; Wang, C.; Guo, Z.H.; Liu, P.; Peng, Z.R.; Wang, Q. Synergetic enhancement of mechanical and electrical strength in epoxy/silica nanocomposites via chemically-bonded interface. Compos. Sci. Technol. 2018, 167, 539–546. [Google Scholar] [CrossRef]
- Hao, Y.N.; Wang, X.H.; Bi, K.; Zhang, J.M.; Huang, Y.H.; Wu, L.W.; Zhao, P.Y.; Xu, K.; Lei, M.; Li, L.T. Significantly enhanced energy storage performance promoted by ultimate sized ferroelectric BaTiO3 fillers in nanocomposite films. Nano Energy 2017, 31, 49–56. [Google Scholar] [CrossRef]
- Tang, H.X.; Lin, Y.R.; Sodano, H.A. Enhanced energy storage in nanocomposite capacitors through aligned PZT nanowires by uniaxial strain assembly. Adv. Energy. Mater. 2012, 2, 469–476. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, H.; Wang, H.; Ding, X.G.; Zhang, Q.L.; Zhu, Z.C. Enhanced dielectric properties and energy density of flexible KTa0.2Nb0.8O3-BaTiO3/P(VDF-TrFE-CTFE) nanocomposite. J. Mater. Sci. Mater. Electron. 2019, 30, 2501–2511. [Google Scholar] [CrossRef]
- Bobić, J.D.; Teixeira, G.F.; Grigalaitis, R.; Gyergyek, S.; Petrović, M.M.V.; Zaghete, M.A.; Stojanovic, B.D. PZT–NZF/CF ferrite flexible thick films: Structural, dielectric, ferroelectric, and magnetic characterization. J. Adv. Ceram. 2019, 8, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.H.; Zhai, J.W.; Wang, J.W.; Xue, S.X.; Zhang, W.Q. Enhanced energy storage density in poly(Vinylidene Fluoride) nanocomposites by a small loading of surface-hydroxylated Ba0.6Sr0.4TiO3 nanofibers. ACS Appl. Mater. Interfaces 2014, 6, 1533–1540. [Google Scholar] [CrossRef]
- Huang, X.Y.; Sun, B.; Zhu, Y.K.; Li, S.T.; Jiang, P.K. High-k polymer nanocomposites with 1D filler for dielectric and energy storage applications. Prog. Mater. Sci. 2019, 100, 187–225. [Google Scholar] [CrossRef]
- Liang, L.Y.; Kang, X.L.; Sang, Y.H.; Liu, H. One-Dimensional ferroelectric nanostructures: Synthesis, properties, and applications. Adv. Sci. 2016, 3, 1500358. [Google Scholar] [CrossRef] [Green Version]
- Tomer, V.; Randall, C.A. High field dielectric properties of anisotropic polymer-ceramic composites. J. Appl. Phys. 2008, 104, 074106. [Google Scholar] [CrossRef]
- Hu, P.H.; Wang, J.J.; Shen, Y.; Guan, Y.H.; Lin, Y.H.; Nan, C.W. Highly enhanced energy density induced by hetero-interface in sandwich-structured polymer nanocomposites. J. Mater. Chem. A 2013, 1, 12321–12326. [Google Scholar] [CrossRef]
- Yao, L.M.; Pan, Z.B.; Liu, S.H.; Zhai, J.W.; Chen, H.H.D. Significantly enhanced energy density in nanocomposite capacitors combining the TiO2 nanorod array with poly (vinylidene fluoride). ACS Appl. Mater. Interfaces 2016, 8, 26343–26351. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.W.; Wang, J.J.; Shen, Y.; Gu, L.; Lin, Y.H.; Nan, C.W. Hierarchical interfaces induce high dielectric permittivity in nanocomposites containing TiO2@BaTiO3 nanofibers. Nanoscale 2014, 6, 6701–6709. [Google Scholar] [CrossRef]
- Tang, H.X.; Sodano, H.A. High energy density nanocomposite capacitors using non-ferroelectric nanowires. Appl. Phys. Lett. 2013, 102, 063901. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.H.; Zhu, H. Enhanced dielectric properties of polyvinylidene fluoride with addition of SnO2 nanoparticles. Phys. Status Solidi R 2016, 10, 753–756. [Google Scholar] [CrossRef]
- Hoque, N.A.; Thakur, P.; Bala, N.; Kool, A.; Das, S.; Ray, P.P. Tunable photoluminescence emissions and large dielectric constant of the electroactive poly(vinylidene fluoride-hexafluoropropylene) thin films modified with SnO2 nanoparticles. RSC Adv. 2016, 6, 29931–29943. [Google Scholar] [CrossRef]
- Zha, J.W.; Meng, X.; Wang, D.R.; Dang, Z.M.; Li, R.K.Y. Dielectric properties of poly(vinylidene fluoride) nanocomposites filled with surface coated BaTiO3 by SnO2 nanodots. Appl. Phys. Lett. 2014, 104, 072906. [Google Scholar] [CrossRef]
- Tian, Q.H.; Zhang, Z.X.; Yang, L.; Hirano, S. Encapsulation of SnO2 nanoparticles into hollow TiO2 nanowires as high performance anode materials for lithium ion batteries. J. Power Sources 2014, 253, 9–16. [Google Scholar] [CrossRef]
- Bertoti, I.; Mohai, M.; Sullivan, J.L.; Saied, S.O. Surface characterization of plasma-nitrided titanium—An xps study. Appl. Surf. Sci. 1995, 84, 357–371. [Google Scholar] [CrossRef]
- Li, J.J.; Seok, S.I.; Chu, B.J.; Dogan, F.; Zhang, Q.M.; Wang, Q. Nanocomposites of ferroelectric polymers with TiO2 nanoparticles exhibiting significantly enhanced electrical energy density. Adv. Mater. 2009, 21, 217. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Claude, J.; Norena-Franco, L.E.; Wang, Q. Structural dependence of phase transition and dielectric relaxation in ferroelectric poly(vinylidene fluoride-chlorotrifluoroethylene-trifluoroethylene)s. J. Phys. Chem. B 2008, 112, 10411–10416. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Claude, J.; Neese, B.; Zhang, Q.M.; Wang, Q. A modular approach to ferroelectric polymers with chemically tunable Curie temperatures and dielectric constants. J. Am. Chem. Soc. 2006, 128, 8120–8121. [Google Scholar] [CrossRef]
- Brus, L. Electronic wave-functions in semiconductor clusters-experiment and theory. J. Phys. Chem. 1986, 90, 2555–2560. [Google Scholar] [CrossRef]
- Lee, E.J.H.; Ribeiro, C.; Giraldi, T.R.; Longo, E.; Leite, E.R.; Varela, J.A. Photoluminescence in quantum-confined SnO2 nanocrystals: Evidence of free exciton decay. Appl. Phys. Lett. 2004, 84, 1745–1747. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.Y.; Huang, X.Y.; Li, B.W.; Zhi, C.Y.; Tanaka, T.; Jiang, P.K. Core-satellite Ag@BaTiO3 nanoassemblies for fabrication of polymer nanocomposites with high discharged energy density, high breakdown strength and low dielectric loss. Phys. Chem. Chem. Phys. 2013, 15, 17560–17569. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.Y.; Hu, Z.W.; Jiang, P.K.; Li, S.T.; Tanaka, T. Large dielectric constant and high thermal conductivity in poly(vinylidene fluoride)/barium titanate/silicon carbide three-phase nanocomposites. ACS Appl. Mater. Interfaces 2011, 3, 4396–4403. [Google Scholar] [CrossRef]






| Sample | Tm (°C) | Tc (°C) | ||
|---|---|---|---|---|
| P(VDF-TrFE-CTFE) | 122.85 | 100 | 92.15 | |
| 5 vol.% | TS2 | 122.35 | 104.56 | 93.15 |
| TS3 | 122.35 | 103.74 | 92.98 | |
| TS4 | 122.35 | 104.14 | 93.15 | |
| 10 vol.% | TO | 121.52 | 100.08 | 94.15 |
| TS1 | 122.02 | 103.85 | 94.15 | |
| TS2 | 121.85 | 99.29 | 93.82 | |
| TS3 | 121.35 | 99.92 | 93.82 | |
| TS4 | 121.18 | 96.07 | 93.65 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhang, Z.; Xu, N.; Yang, H. Dielectric Properties of P(VDF-TrFE-CTFE) Composites Filled with Surface-Coated TiO2 Nanowires by SnO2 Nanoparticles. Polymers 2020, 12, 85. https://doi.org/10.3390/polym12010085
Zhang Q, Zhang Z, Xu N, Yang H. Dielectric Properties of P(VDF-TrFE-CTFE) Composites Filled with Surface-Coated TiO2 Nanowires by SnO2 Nanoparticles. Polymers. 2020; 12(1):85. https://doi.org/10.3390/polym12010085
Chicago/Turabian StyleZhang, Qilong, Zhao Zhang, Nuoxin Xu, and Hui Yang. 2020. "Dielectric Properties of P(VDF-TrFE-CTFE) Composites Filled with Surface-Coated TiO2 Nanowires by SnO2 Nanoparticles" Polymers 12, no. 1: 85. https://doi.org/10.3390/polym12010085





