Copolymerization of Styrene and Pentadecylphenylmethacrylate (PDPMA): Synthesis, Characterization, Thermomechanical and Adhesion Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Monomer Synthesis
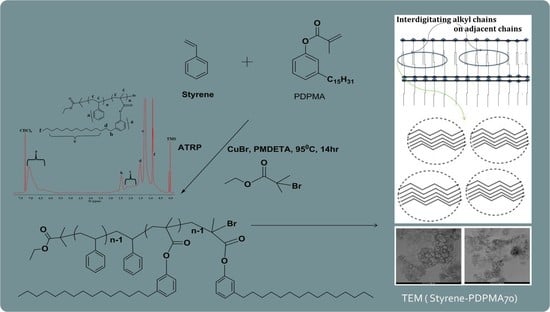
2.3. Atom Transfer Radical Polymerization (ATRP)
2.4. Characterization
3. Results and Discussion
3.1. Polymer Synthesis and Characterization
3.2. Molecular Characterization of Styrene-PDPMA Copolymers
3.3. Glass Transition Behaviour of Styrene-PDPMA Copolymers
3.4. Thermal Stability of Copolymers
3.5. Rheological Properties
3.6. Adhesion Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coltelli, M.-B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the Art in the Development and Properties of Protein-Based Films and Coatings and Their Applicability to Cellulose Based Products: An Extensive Review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Teli, M.D.; Pandit, P. Novel method of ecofriendly single bath dyeing and functional finishing of wool protein with coconut shell extract biomolecules. ACS Sustain. Chem. Eng. 2017, 5, 8323–8333. [Google Scholar] [CrossRef]
- Teli, M.D.; Pandit, P. Development of thermally stable and hygienic colored cotton fabric made by treatment with natural coconut shell extract. J. Ind. Text. 2018, 48, 87–118. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.; Patel, S.; Pandit., P.; Nachimuthu, S.; Jose, S. Colouration of textiles using roasted peanut skin-an agro processing residue. J. Clean. Prod. 2018, 172, 1319–1326. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M. Recent developments in sugar palm (Arenga pinnata) based biocomposites and their potential industrial applications: A review. Renew. Sustain. Energy Rev. 2016, 54, 533–549. [Google Scholar] [CrossRef]
- Mekonnen, T.; Misra, M.; Mohanty, A.K. Fermented soymeals and their reactive blends with poly (butylene adipate-co-terephthalate) in engineering biodegradable cast films for sustainable packaging. ACS Sustain. Chem. Eng. 2016, 4, 782–793. [Google Scholar] [CrossRef] [Green Version]
- Coma, M.; Martinez-Hernandez, E.; Abeln, F.; Raikova, S.; Donnelly, J.; Arnot, T.C.; Allen, M.J.; Honge, D.D.; Chuck, C. Organic waste as a sustainable feedstock for platform chemicals. Faraday Discuss. 2017, 202, 175–195. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Fevre, M.; Jones, G.O.; Waymouth, R.M. Catalysis as an enabling science for sustainable polymers. Chem. Rev. 2018, 118, 839–885. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Hillmyer, M.A. 50th anniversary perspective: There is a great future in sustainable polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Wang, S.; Kesava, S.V.; Gomez, E.D.; Robertson, M.L. Sustainable thermoplastic elastomers derived from fatty acids. Macromolecules 2013, 46, 7202–7212. [Google Scholar] [CrossRef]
- Bocquè, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Uyama, H.; Kuwabara, M.; Sujimoto, T.T.; Nakano, M.; Usuki, A.; Kobayashi, S. Green nanocomposites from renewable resources: Plant oil-Clay hybrid materials. Chem. Mater. 2003, 15, 2492–2494. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Green nanocomposites from renewable resources: Biodegradable plant oil-silica hybrid coatings. Macromol. Rapid Commun. 2003, 24, 711–714. [Google Scholar] [CrossRef]
- Maffezzoli, A.; Calò, E.; Zurlo, S.; Mele, G.; Tarzia, A.; Stifani, C. Cardanol based matrix biocomposites reinforced with natural fibres. Compos. Sci. Technol. 2004, 64, 839–845. [Google Scholar] [CrossRef]
- Hofman, A.H.; Chen, Y.; Ten Brinke, G.; Loos, K. Interaction strength in poly (4-vinylpyridine)-n-alkyl phenol supramolecular comb-shaped copolymers. Macromolecules 2015, 48, 1554–1562. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, Y.; Dong, X.; Zhoua, Y.; Wang, D. Frustrated crystallisation and hierarchical self-assembly behaviour of comb-like polymers. Chem. Soc. Rev. 2013, 42, 2075–2099. [Google Scholar] [CrossRef]
- O’Leary, K.A.; Paul, D.R. Physical properties of poly (n-alkyl acrylate) copolymers. Part 1. Crystalline/crystalline combinations. Polymer 2006, 47, 1226–1244. [Google Scholar]
- Kazantsev, O.A.; Kamorina, S.I.; Rumyantsev, M.; Kamorin, D.M.; Sivokhin, A.P. Radical copolymerization of higher alkyl methacrylates with acrylic esters and amides in toluene: Influence of monomer association on copolymer composition. J. Polym. Res. 2016, 23, 89. [Google Scholar] [CrossRef]
- Ternorutsky, L.; Kilian, L.; Dutta, S.; Lee, K.I.; Ramsey, N.K. Ultra-High Solids Emulsion and Application. U.S. Patent 20180134888 A1, 17 May 2018. [Google Scholar]
- Kim, T.H.; Kim, J.C. Redox-responsive solid lipid microparticles composed of octadecyl acrylate and allyl disulfide. J. Biomater. Sci. Polym. Ed. 2018, 29, 476–490. [Google Scholar] [CrossRef]
- Gu, Z.X.; Cheng, J.; Zhang, M.Z.; He, J.I.; Ni, P.H. Effect of sequence structure on wetting behaviors of fluorinated methacrylate polymers based on perfluorohexylethyl methacrylate and stearyl acrylate. Chin. J. Polym. Sci. 2017, 35, 1061–1072. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, S.; Huang, C.; Tian, Y.; Yang, X.; Jing, X.; Li, A. Screening of synthesis conditions of polymers and the effects on viscosity reduction performance. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 022032. [Google Scholar] [CrossRef] [Green Version]
- Navarro, J.R.G.; Edlund, U. Surface-initiated controlled radical polymerization approach to enhance nanocomposite integration of cellulose nanofibrils. Biomacromolecules 2017, 18, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Arua, U.N.; Blum, F.D. Disruptions in the crystallinity of poly (lauryl methacrylate) due to adsorption on silica. J. Polym. Sci. Part. B Polym. Phys. 2018, 56, 89–96. [Google Scholar] [CrossRef]
- Gupta, J.; Wan, C.; Haddleton, D.M.; Tony, M.N. Plasticisation and compatibilisation of poly (propylene) with poly (lauryl acrylate) surface modified MWCNTs. Polymer 2017, 133, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.F.J.; Chen., J.B. Synthesis of high-solid-content, acrylic pressure-sensitive adhesives by solvent polymerization. J. Appl. Polym. Sci. 2018, 135, 46257. [Google Scholar] [CrossRef]
- Gupta, J.; Keddie, D.J.; Wan, C.G.; Haddleton, D.M.; McNally, T. Functionalisation of MWCNTs with poly (lauryl acrylate) polymerised by Cu (0)-mediated and RAFT methods. Polym. Chem. 2016, 7, 3884–3896. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Wang, Q.; Jiang, J.; Zhan, X.; Chen, F. Microphase structure, crystallization behavior, and wettability properties of novel fluorinated copolymers poly (perfluoroalkyl acrylate-co-stearyl acrylate) containing short perfluorohexyl chains. Langmuir 2015, 31, 4752–4760. [Google Scholar] [CrossRef]
- Suresh, K.I.; Prasad, V.S.K. Synthesis, structure, and properties of novel polyols from cardanol and developed polyurethanes. Ind. Eng. Chem. Res. 2005, 44, 4504–4512. [Google Scholar] [CrossRef]
- Gedam, P.H.; Sampathkumaran, P.S.; Sivasamban, M.A. Examination of the components of cashew nut shell liquid by NMR. Indian J. Chem. 1972, 10, 388–391. [Google Scholar]
- Li, W.S.J.; Negrell, C.; Ladmiral, V.; laikeehim, J.; Bron, P.; Lacroix-Desmazes, P.; Duhamel, C.J.; Caillol, S. Cardanol based polymer Latex by radical aqueous miniemulsionpolymerization. Polym. Chem. 2018, 9, 2468–2477. [Google Scholar] [CrossRef]
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162. [Google Scholar] [CrossRef]
- Jhon, G.; Pillai, C.K.S. Synthesis and characterization of a self crosslinkable polymer from cardanol: Autooxidation of poly (cardanyl acrylate) to crosslinked film. J. Polym. Sci. Part. A Polym. Chem. 1993, 31, 1069–1073. [Google Scholar] [CrossRef]
- Sitaramam, B.S.; Chatterjee, P.C. Synthesis, polymerization, and end-use evaluation of 3-pentadecylphenyl acrylate and methacrylate. J. Appl. Polym. Sci. 1989, 37, 33–37. [Google Scholar] [CrossRef]
- Suresh, K.I.; Jaikrishna, M. Synthesis of novel crosslinkable polymers by atom transfer radical polymerization of cardanyl acrylate. J. Polym. Sci. Part. A Polym. Chem. 2005, 43, 5953–5961. [Google Scholar] [CrossRef]
- Rajender, N.; Suresh, K.I.; Sreedhar, B. Comb-like polymer-graphene nanocomposites with improved adhesion properties via surface-initiated atom transfer radical polymerization (SI-ATRP). J. Appl. Polym. Sci. 2018, 135, 45885. [Google Scholar] [CrossRef]
- Nutenki, R.; Darapureddi, P.R.; Nayak, R.R.; Suresh, K.I. Amphiphilic comb-like polymer-modified graphene oxide and its nanocomposite with polystyrene via emulsion polymerization. Colloid Polym. Sci. 2018, 296, 133–144. [Google Scholar] [CrossRef]
- Ruokolainen, J.; ten Brinke, G.; Ikkala, O.; Torkkeli, M.; Serimaa, R. Mesomorphic structures in flexible polymer-Surfactant systems due to hydrogen bonding: Poly(4-vinylpyridine)-Pentadecylphenol. Macromolecules 1996, 29, 3409–3415. [Google Scholar] [CrossRef]
- Ruokolainen, J.; Saariaho, M.; Ikkala, O.; ten Brinke, G.; Thomas, E.L.; Torkkeli, M.; Serimaa, R. Supramolecular routes to hierarchical structures: comb-coil diblock copolymers organized with two length scales. Macromolecules 1999, 32, 1152–1158. [Google Scholar] [CrossRef]
- Luyten, M.C.; Alberda van Ekenstein, G.O.R.; ten Brinke, G.; Ruokolainen, J.; Ikkala, O.; Torkkeli, M.; Serimaa, R. Crystallization and cocrystallization in supramolecular comb copolymer-like systems: blends of poly(4-vinylpyridine) and pentadecylphenol. Macromolecules 1999, 32, 4404–4410. [Google Scholar] [CrossRef] [Green Version]
- Faber, M.; Hofman, A.H.; Polushkin, E.; vanEkenstein, G.A.; Seitsonen, J.; Ruokolainen, J.; Loos, K.; ten Brinke, G. Hierarchical self-assembly in supramolecular double-comb diblock copolymer complexes. Macromolecules 2013, 46, 500–517. [Google Scholar] [CrossRef]
- Suresh, K.I.; Foerst, G.; Schubert, R.; Bartsch, E. Synthesis and micellization properties of new anionic reactive surfactants based on hydrogenated cardanol. J. Surfactants Deterg. 2012, 15, 207–215. [Google Scholar]
- Suresh, K.I.; Bartsch, E. Effect of sulfonated 3-pentadecyl phenyl acrylate as surfmer in the emulsion polymerization of styrene: Synthesis and polymer properties. Colloid Polym. Sci. 2013, 291, 1843–1853. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Koerner, H.; Lederer, K. Free radical co- and terpolymerization of styrene, hydrogenated cardanyl acrylate, and cardanyl acetate. J. Appl. Polym. Sci. 2003, 88, 1399–1409. [Google Scholar] [CrossRef]
- Chanda, M. Introduction to Polymer Science; CRC Press: Florida, FL, USA, 2006; pp. 429–436. ISBN 0849373840. [Google Scholar]
- Koiry, B.P.; Singha, N.K. Copper mediated controlled radical copolymerization of styrene and 2-ethylhexyl acrylate and determination of their reactivity ratios. Front. Chem. 2014, 2, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mieras, H.J. Solution and bulk flow properties of linear and branched styrene–butadiene rubbers. J. Polym. Sci. Polym. Symp. 1973, 42, 987–1000. [Google Scholar] [CrossRef]
- Donth, E.; Beiner, M.; Reissig, S.; Korus, J.; Garwe, F.; Vieweg, S.; Kahle, S.; Hempel, E.; Schröter, K. Fine structure of the main transition in amorphous polymers: entanglement spacing and characteristic length of the glass transition. discussion of examples. Macromolecules 1996, 29, 6589–6600. [Google Scholar] [CrossRef]
- Greenberg, S.A.; Alfrey, T. Side Chain Crystallization of n-Alkyl Polymethacrylates and Polyacrylates1. J. Am. Chem. Soc. 1954, 76, 6280–6285. [Google Scholar] [CrossRef]












| Sample Code | Composition | GPC | Mn Theory | PDI | ||||
|---|---|---|---|---|---|---|---|---|
| Feed | 1H NMR | Mw g/mole | Mn g/mole | |||||
| St | PDPMA | St | PDPMA | |||||
| PS | 100 | 0 | 100 | - | 3012 | 2551 | 3112 | 1.18 |
| PDPMA | 0 | 100 | - | 100 | 17,981 | 10,964 | 22,320 | 1.64 |
| SPDPMA10 | 90 | 10 | - | - | 3758 | 2828 | 3338 | 1.33 |
| SPDPMA30 | 70 | 30 | 75.35 | 24.65 | 31,923 | 23,047 | 36,521 | 1.38 |
| SPDPMA50 | 50 | 50 | 65.5 | 35.5 | 29,068 | 13,896 | 37,378 | 2.09 |
| SPDPMA70 | 30 | 70 | 57 | 43 | 21,325 | 7872 | 34,778 | 2.71 |
| SPDPMA90 | 10 | 90 | - | - | 26,690 | 17,554 | 33,711 | 1.52 |
| Styrene: PDPMAComposition | M = M1/M2 | P = m1/m2 | M/P | G | H | α + H | η | ξ | |
|---|---|---|---|---|---|---|---|---|---|
| Sample | 1H NMR | ||||||||
| SPDPMA30 | 75.35:24.65 | 2.33 | 3.05 | 0.76 | 1.57 | 1.77 | 1.95 | 0.80 | 0.90 |
| SPDPMA50 | 65.5:35.5 | 1 | 1.84 | 0.54 | 0.46 | 0.54 | 0.72 | 0.63 | 0.74 |
| SPDPMA70 | 57:43 | 0.42 | 1.32 | 0.31 | 0.11 | 0.13 | 0.32 | 0.33 | 0.42 |
| Methods Used | r1 (Styrene) | r2 (PDPMA) | r1:r2 |
|---|---|---|---|
| FR | 0.8965 | 0.0182 | 0.016 |
| KT | 0.9732 | 0.0816 | 0.073 |
| Monomer Feed Styrene: PDPMA | M1/M2 | M2/M1 | l1 | l2 | l1:l2 |
|---|---|---|---|---|---|
| 70:30 | 2.33 | 0.42 | 3.08 | 1.01 | 3.05 |
| 50:50 | 1 | 1 | 1.89 | 1.02 | 1.85 |
| 30:70 | 0.42 | 2.33 | 1.38 | 1.04 | 1.33 |
| S70 PDPMA30 | S50-PDPMA50 | |||
|---|---|---|---|---|
| Substrate Tested | Lap Shear Strength (Pa)a | Peel Strength (Pa) a | Lap Shear Strength (Pa) a | Peel Strength (Pa) a |
| Paper-Paper | 3.93 × 105 | 1.75 × 104 | 1.5 × 105 | 2.5 × 103 |
| PET-PET | 6.0 × 104 | 2.25 × 103 | 2.91 × 104 | 2.22 × 103 |
| Paper-PET | 3.67 × 105 | 2.25 × 103 | 3.23 × 105 | 1.3 × 103 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muringayil Joseph, T.; Murali Nair, S.; Kattimuttathu Ittara, S.; Haponiuk, J.T.; Thomas, S. Copolymerization of Styrene and Pentadecylphenylmethacrylate (PDPMA): Synthesis, Characterization, Thermomechanical and Adhesion Properties. Polymers 2020, 12, 97. https://doi.org/10.3390/polym12010097
Muringayil Joseph T, Murali Nair S, Kattimuttathu Ittara S, Haponiuk JT, Thomas S. Copolymerization of Styrene and Pentadecylphenylmethacrylate (PDPMA): Synthesis, Characterization, Thermomechanical and Adhesion Properties. Polymers. 2020; 12(1):97. https://doi.org/10.3390/polym12010097
Chicago/Turabian StyleMuringayil Joseph, Tomy, Sumi Murali Nair, Suresh Kattimuttathu Ittara, Józef T. Haponiuk, and Sabu Thomas. 2020. "Copolymerization of Styrene and Pentadecylphenylmethacrylate (PDPMA): Synthesis, Characterization, Thermomechanical and Adhesion Properties" Polymers 12, no. 1: 97. https://doi.org/10.3390/polym12010097
APA StyleMuringayil Joseph, T., Murali Nair, S., Kattimuttathu Ittara, S., Haponiuk, J. T., & Thomas, S. (2020). Copolymerization of Styrene and Pentadecylphenylmethacrylate (PDPMA): Synthesis, Characterization, Thermomechanical and Adhesion Properties. Polymers, 12(1), 97. https://doi.org/10.3390/polym12010097