Crystallization and Alkaline Degradation Behaviors of Poly(l-Lactide)/4-Armed Poly(ε-Caprolactone)-Block-Poly(d-Lactide) Blends with Different Poly(d-Lactide) Block Lengths
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
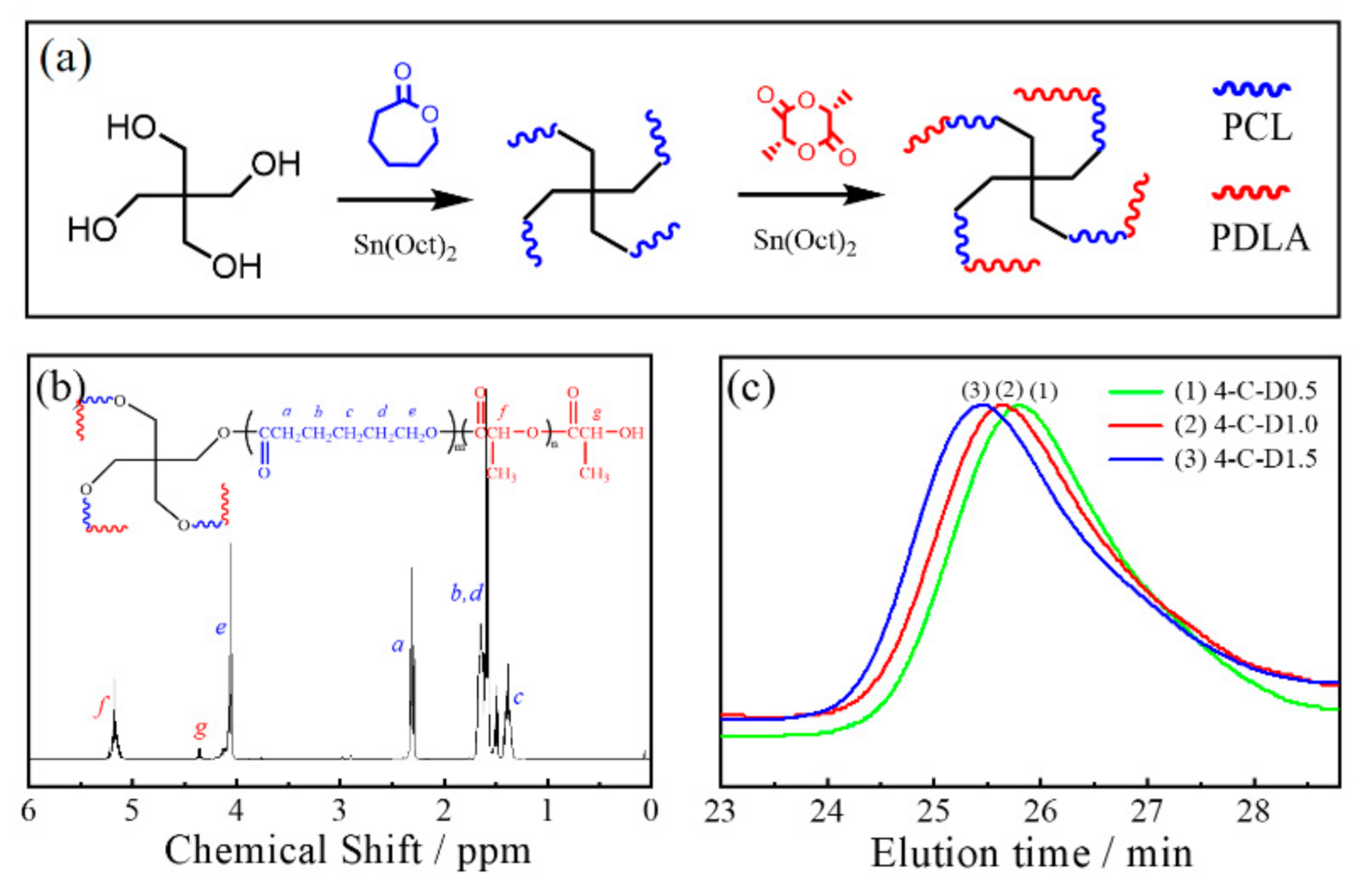
2.2.1. Synthesis of 4-C-D Copolymers
2.2.2. Sample Preparation
2.2.3. Analysis and Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of 4-C-D
3.2. Miscibility of the PLLA/4-C-D Blends
3.3. Crystal Structure of the PLLA/4-C-D Blends
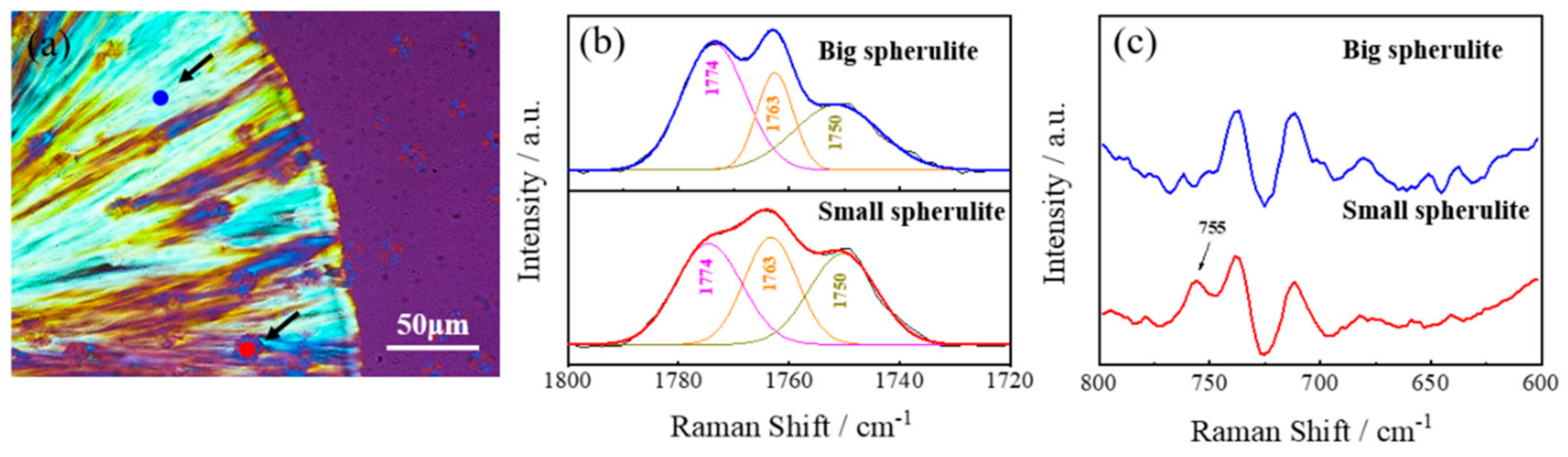
3.4. Spherulite Morphologies and Growth Rates
3.5. Isothermal Crystallization
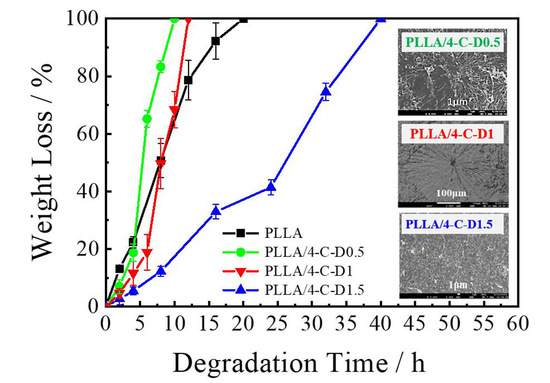
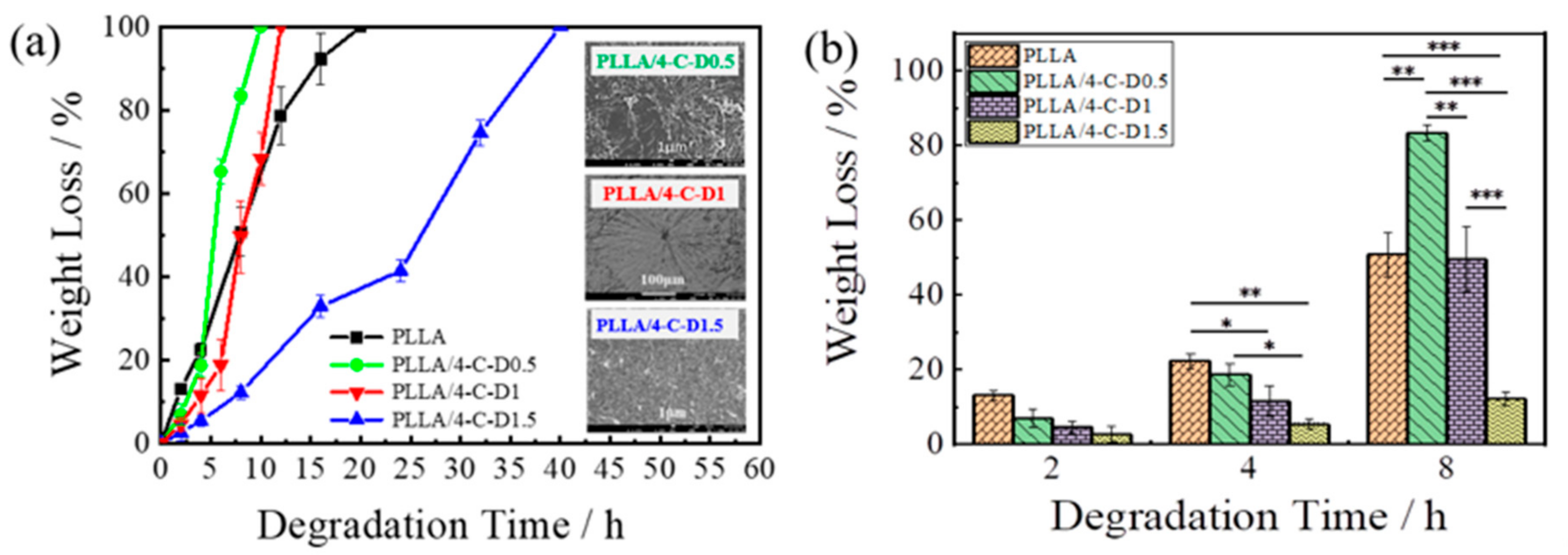
3.6. Degradation Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug. Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Amass, W.; Amass, A.; Tighe, B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Shi, X.D.; Sun, P.J.; Gan, Z.H. Preparation of porous polylactide microspheres and their application in tissue engineering. Chin. J. Polym. Sci. 2018, 36, 712–719. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.B.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Xu, H.; Teng, C.Q.; Yu, M.H. Improvements of thermal property and crystallization behavior of PLLA based multiblock copolymer by forming stereocomplex with PDLA oligomer. Polymer 2006, 47, 3922–3928. [Google Scholar] [CrossRef]
- Li, Z.B.; Muiruri, J.K.; Thitsartarn, W.; Zhang, X.; Tan, B.H.; He, C.B. Biodegradable silica rubber core-shell nanoparticles and their stereocomplex for efficient PLA toughening. Compos. Sci. Technol. 2018, 159, 11–17. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.S.; Xiong, Z.; Tang, Z.B.; Zhang, R.Y.; Zhu, J. Research progress in the heat resistance, toughening and filling modification of PLA. Sci. China Chem. 2016, 59, 1355–1368. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer-polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Castillo, R.V.; Müller, A.J.; Raquez, J.-M.; Dubois, P. Crystallization kinetics and morphology of biodegradable double crystalline PLLA-b-PCL diblock copolymers. Macromolecules 2010, 43, 4149–4160. [Google Scholar] [CrossRef]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase structure, compatibility, and toughness of PLA/PCL blends: A review. Front. Mater. 2019, 6, 13. [Google Scholar] [CrossRef]
- Song, Z.J.; Huang, X.L.; Lu, X.L.; Lv, Q.Q.; Xu, N.; Pang, S.J.; Pan, L.S.; Li, T. Improvement of microstructures and properties of poly(lactic acid)/poly(epsilon-caprolactone) blends compatibilized with polyoxymethylene. J. Appl. Polym. Sci. 2018, 135, 13. [Google Scholar] [CrossRef]
- Ferri, J.M.; Fenollar, O.; Jorda-Vilaplana, A.; Garcia-Sanoguera, D.; Balart, R. Effect of miscibility on mechanical and thermal properties of poly(lactic acid)/polycaprolactone blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Tsuji, H.; Yamada, T.; Suzuki, M.; Itsuno, S. Blends of aliphatic polyesters. Part 7. Effects of poly(L-lactide-co-epsilon-caprolactone) on morphology, structure, crystallization, and physical properties of blends of poly(L-lactide) and poly(epsilon-caprolactone). Polym. Int. 2003, 52, 269–275. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Liu, S.; Teo, W.S.; Kong, J.; He, C. Highly biodegradable and tough polylactic acid–cellulose nanocrystal composite. ACS Sustain. Chem. Eng. 2017, 5, 3929–3937. [Google Scholar] [CrossRef]
- Ning, Z.B.; Liu, J.J.; Jiang, N.; Gan, Z.H. Enhanced crystallization rate and mechanical properties of poly(l-lactic acid) by stereocomplexation with four-armed poly(ε-caprolactone)-block-poly(d-lactic acid) diblock copolymer. Polym. Int. 2017, 66, 968–976. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, L.P.; Lu, X.H.; He, C.B. Biodegradable and renewable poly(lactide)-lignin composites: Synthesis, interface and toughening mechanism. J. Mater. Chem. A 2015, 3, 3699–3709. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(Lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Schmidt, S.C.; Hillmyer, M.A. Polylactide stereocomplex crystallites as nucleating agents for isotactic polylactide. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 300–313. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactic acid) stereocomplexes: A decade of progress. Adv. Drug. Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef]
- Pan, P.J.; Liang, Z.C.; Zhu, B.; Dong, T.; Inoue, Y. Blending effects on polymorphic crystallization of poly(L-lactide). Macromolecules 2009, 42, 3374–3380. [Google Scholar] [CrossRef]
- Shao, J.; Xiang, S.; Bian, X.C.; Sun, J.R.; Li, G.; Chen, X.S. Remarkable melting behavior of PLA stereocomplex in linear PLLA/PDLA blends. Ind. Eng. Chem. Res. 2015, 54, 2246–2253. [Google Scholar] [CrossRef]
- Song, Y.; Wang, D.J.; Jiang, N.; Gan, Z.H. Role of PEG segment in stereocomplex crystallization for PLLA/PDLA-b-PEG-b-PDLA blends. ACS Sustain. Chem. Eng. 2015, 3, 1492–1500. [Google Scholar] [CrossRef]
- de Jong, S.J.; van Dijk-Wolthuis, W.N.E.; Kettenes-van den Bosch, J.J.; Schuyl, P.J.W.; Hennink, W.E. Monodisperse enantiomeric lactic acid oligomers: Preparation, characterization, and stereocomplex formation. Macromolecules 1998, 31, 6397–6402. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.P.; Wang, M.F.; Ozaki, Y.; Sato, H.; Zhang, J.M. Investigation of crystallization behavior of asymmetric PLLA/PDLA blend using raman imaging measurement. Polymer 2019, 172, 1–6. [Google Scholar] [CrossRef]
- Iniguez-Franco, F.; Auras, R.; Burgess, G.; Holmes, D.; Fang, X.; Rubino, M.; Soto-Valdez, H. Concurrent solvent induced crystallization and hydrolytic degradation of PLA by water-ethanol solutions. Polymer 2016, 99, 315–323. [Google Scholar] [CrossRef]
- Ning, Z.B.; Jiang, N.; Gan, Z.H. Four-armed PCL-b-PDLA diblock copolymer: 1. Synthesis, crystallization and degradation. Polym. Degrad. Stab. 2014, 107, 120–128. [Google Scholar] [CrossRef]
- Jing, Y.H.; Quan, C.Y.; Liu, B.; Jiang, Q.; Zhang, C. A mini review on the functional biomaterials based on poly (lactic acid) stereocomplex. Polym. Rev. 2016, 56, 262–286. [Google Scholar] [CrossRef]



| Sample | Mn,PCL(a) (g/mol) | Mn,PDLA(a) (g/mol) | Mn,NMR(b) (g/mol) | Mn,GPC(c) (kg/mol) | Mw,GPC(c) (kg/mol) | PDI(c) | Conversion (%) |
|---|---|---|---|---|---|---|---|
| PLLA | - | - | - | 49.1 | 71.2 | 1.45 | 95.3 |
| 4-C-D0.5 | 1381 | 513 | 7715 | 8.3 | 10.3 | 1.16 | 78.4 |
| 4-C-D1 | 911 | 876 | 7283 | 10.9 | 13.0 | 1.19 | 81.8 |
| 4-C-D1.5 | 897 | 1360 | 9163 | 14.0 | 16.1 | 1.15 | 84.1 |
| Sample | tp (a) (min) | Tm,hc (b) (°C) | Tm,sc (b) (°C) | ΔHm,hc (c) (J/g) | ΔHm,sc (c) (J/g) | Xc,hc (d) (%) | Xc,sc (e) (%) |
|---|---|---|---|---|---|---|---|
| PLLA | 15.0 | 170.0 | - | 42.7 | - | 45.9 | - |
| PLLA/4-C-D0.5 | - | 164.8, 169.7 | - | 1.73 | - | 1.9 | - |
| PLLA/4-C-D1 | 47.6 | 162.4, 168.4 | 182.6 | 46.3 | 0.44 | 49.8 | 0.78 |
| PLLA/4-C-D1.5 | 72.3 | 163.0 | 184.3 | 41.4 | 4.42 | 44.5 | 7.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, S.; Wang, M.; Zhuang, Z.; Ning, Z. Crystallization and Alkaline Degradation Behaviors of Poly(l-Lactide)/4-Armed Poly(ε-Caprolactone)-Block-Poly(d-Lactide) Blends with Different Poly(d-Lactide) Block Lengths. Polymers 2020, 12, 2195. https://doi.org/10.3390/polym12102195
Dai S, Wang M, Zhuang Z, Ning Z. Crystallization and Alkaline Degradation Behaviors of Poly(l-Lactide)/4-Armed Poly(ε-Caprolactone)-Block-Poly(d-Lactide) Blends with Different Poly(d-Lactide) Block Lengths. Polymers. 2020; 12(10):2195. https://doi.org/10.3390/polym12102195
Chicago/Turabian StyleDai, Suyang, Min Wang, Zhuoxin Zhuang, and Zhenbo Ning. 2020. "Crystallization and Alkaline Degradation Behaviors of Poly(l-Lactide)/4-Armed Poly(ε-Caprolactone)-Block-Poly(d-Lactide) Blends with Different Poly(d-Lactide) Block Lengths" Polymers 12, no. 10: 2195. https://doi.org/10.3390/polym12102195
APA StyleDai, S., Wang, M., Zhuang, Z., & Ning, Z. (2020). Crystallization and Alkaline Degradation Behaviors of Poly(l-Lactide)/4-Armed Poly(ε-Caprolactone)-Block-Poly(d-Lactide) Blends with Different Poly(d-Lactide) Block Lengths. Polymers, 12(10), 2195. https://doi.org/10.3390/polym12102195