Development of Biodegradable Flame-Retardant Bamboo Charcoal Composites, Part I: Thermal and Elemental Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA Composites
2.3. Materials Characterization
2.4. Cone Calorimetry and Limiting Oxygen Index (LOI) Tests
3. Results and Discussion
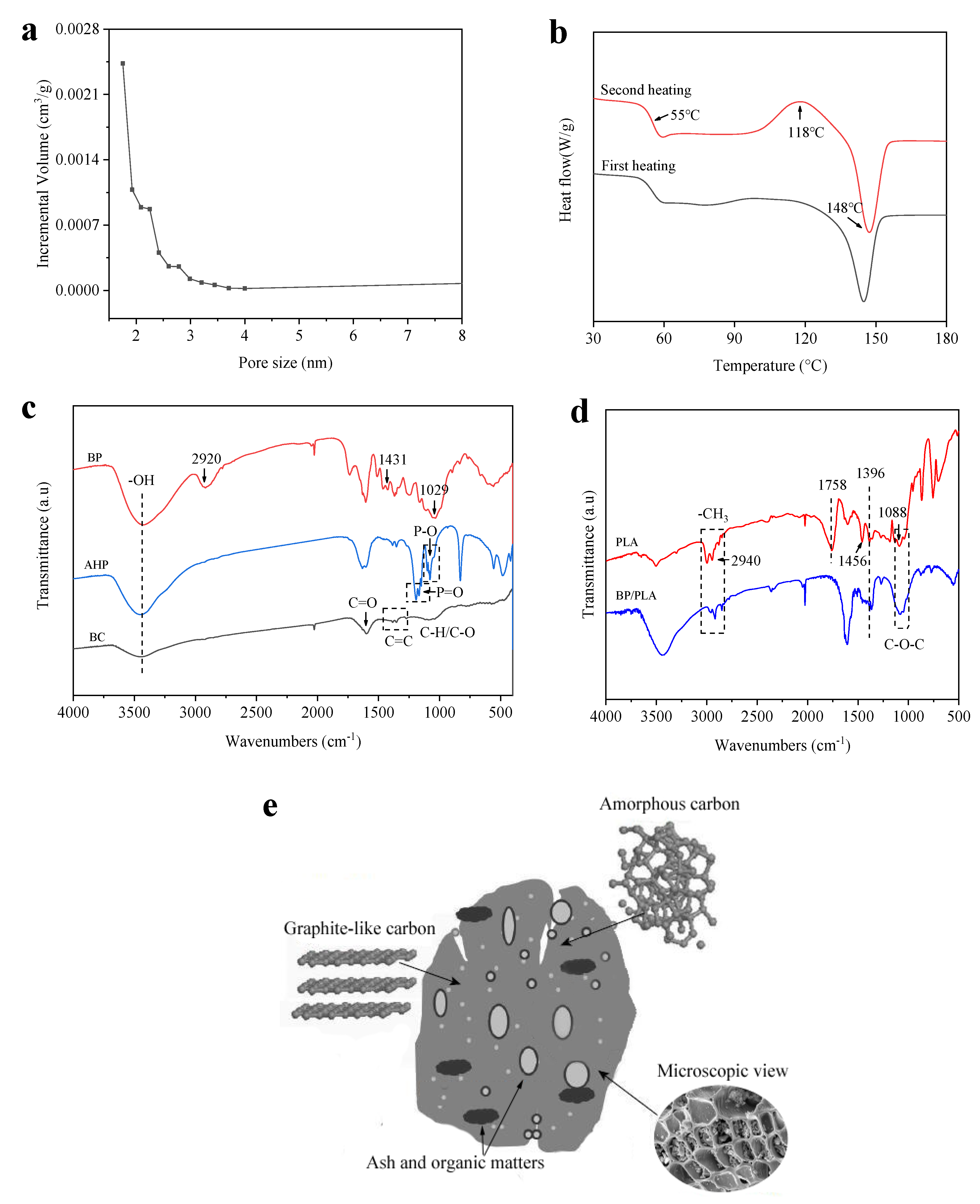
3.1. Characterization of BC, BP, AHP and PLA Materials
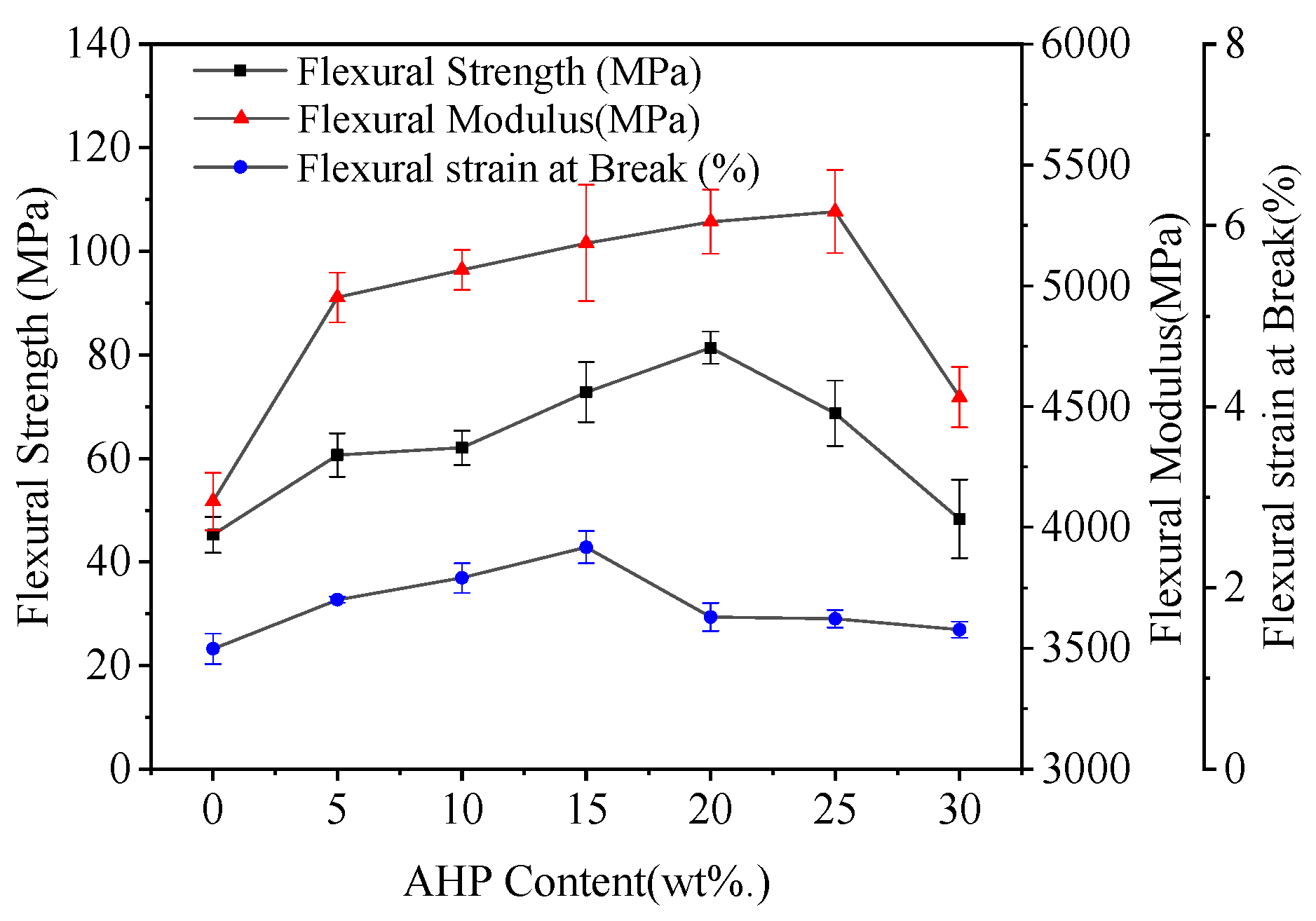
3.2. Mechanical Properties
3.3. Early Burning Behavior and Flame Retardancy (LOI and UL94 Vertical Flame Tests)
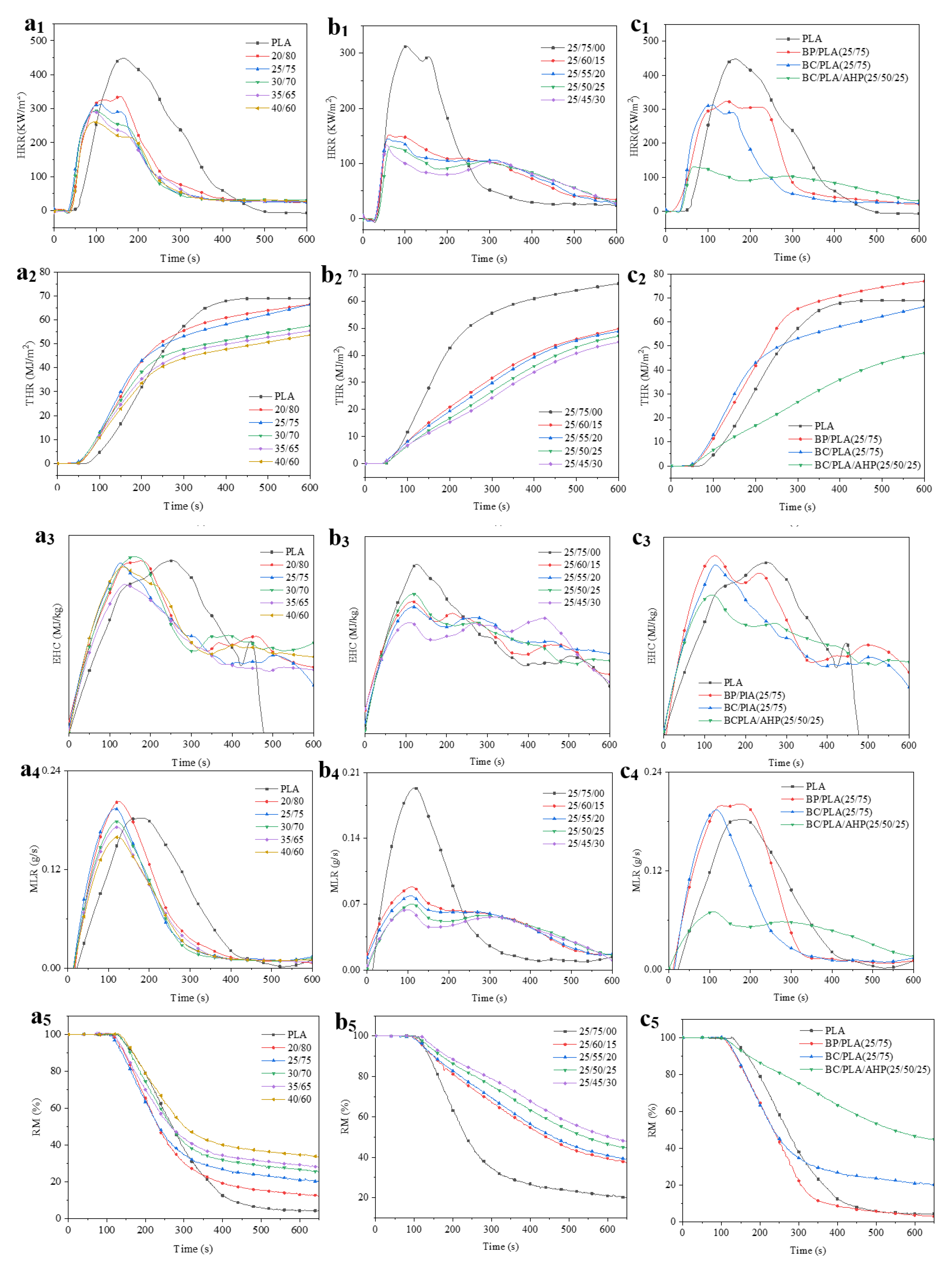
3.4. Cone Calorimetery Testing
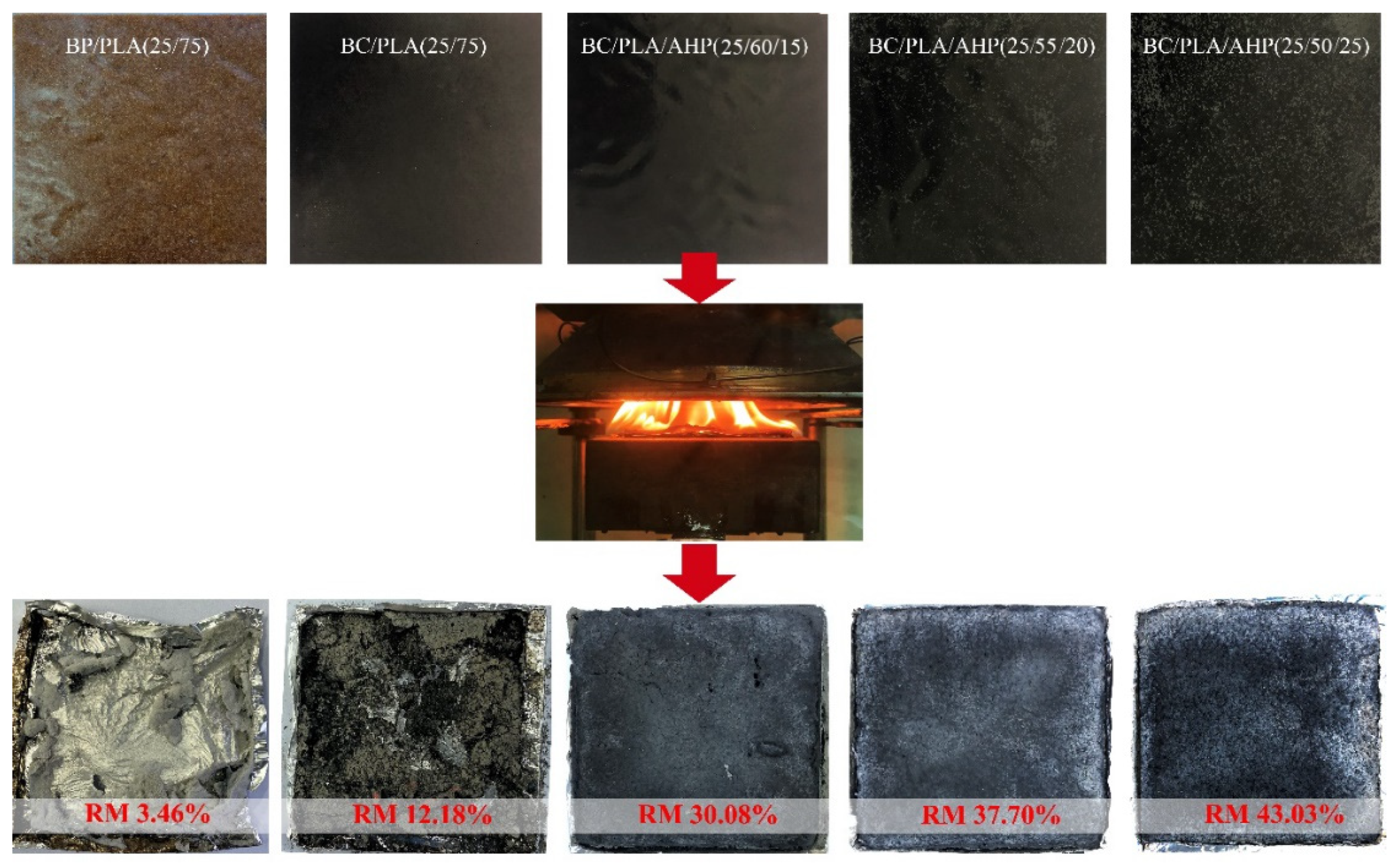
3.5. SEM Images of Residues
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Momani, B.L. Assessment of the Impacts of Bioplastics: Energy Usage, Fossil Fuel Usage, Pollution, Health Effects, Effects on the Food Supply, and Economic Effects Compared to Petroleum Based Plastics; Worcester Polytechnic Institute: Worcester, UK, 2009; p. 59. Available online: https://digitalcommons.wpi.edu/iqp-all (accessed on 6 March 2020).
- Serra, T.; Planell, J.A.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomaterialia 2013, 9, 5521–5530. [Google Scholar] [CrossRef]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly (lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Fazita, M.R.; Jayaraman, K.; Bhattacharyya, D.; Mohamad Haafiz, M.K.; Saurabh, C.K.; Hussin, M.H.; Abdul-Kalil, H.P.S. Green composites made of bamboo fabric and poly (lactic) acid for packaging applications—A review. Materials 2016, 9, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Rapp, F.; Schneider, A.; Elsner, P. Biopolymer foams-relationship between material characteristics and foaming behavior of cellulose based foams. In AIP Conference Proceedings; American Institute of Physics: College Park, Maryland, 2014; pp. 362–366. [Google Scholar] [CrossRef]
- Wang, L.; Lee, R.E.; Wang, G.; Chu, R.K.; Zhao, J.; Park, C.B. Use of stereocomplex crystallites for fully-biobased microcellular low-density poly (lactic acid) foams for green packaging. Chem. Eng. J. 2017, 327, 1151–1162. [Google Scholar] [CrossRef]
- Lee, J.T.; Kim, M.W.; Song, Y.S.; Kang, T.J.; Youn, J.R. Mechanical properties of denim fabric reinforced poly (lactic acid). Fibers Polym. 2010, 11, 60–66. [Google Scholar] [CrossRef]
- Wang, D.Y.; Song, Y.P.; Lin, L.; Wang, X.L.; Wang, Y.Z. A novel phosphorus-containing poly(lactic acid) toward its flame retardation. Polymer 2011, 52, 233–238. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Properties of lactic acid-based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Li, L.; Mao, X.; Ju, R.; Chen, Y.; Qian, L. Synergistic effect of organo-montmorillonite on intumescent flame-retardant PLA. Ferroelectrics 2018, 527, 25–36. [Google Scholar] [CrossRef]
- Lou, C.W.; Lin, C.W.; Lei, C.H.; Su, K.H.; Hsu, C.H.; Liu, Z.H.; Lin, J.H. PET/PP blend with bamboo charcoal to produce functional composites. J. Mater. Process. Technol. 2007, 192, 428–433. [Google Scholar] [CrossRef]
- Srisuk, R.; Techawinyutham, L.; Koetniyom, W.; Dangtungee, R. Mechanical Properties of Bamboo Charcoal (BC)/Poly (Lactic) Acid (PLA) Composites. In Key Engineering Materials; Trans Tech Publications: Kapellweg, Switzerland, 2019; Volume 801, pp. 121–126. [Google Scholar] [CrossRef]
- Zawawi, E.E.; Amirrudin, M.; Kamarun, D.; Bonnia, N.N.; Rozana, M.D. Characterizations of poly-lactic acid/polypropylene filled with bamboo charcoal powder. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1349, p. 012122. [Google Scholar] [CrossRef] [Green Version]
- Ho, M.P.; Lau, K.T. Enhancement of impact resistance of biodegradable polymer using bamboo charcoal particles. Mater. Lett. 2014, 136, 122–125. [Google Scholar] [CrossRef]
- Ho, M.P.; Lau, K.T.; Wang, H.; Hui, D. Improvement on the properties of polylactic acid (PLA) using bamboo charcoal particles. Compos. Part B Eng. 2015, 81, 14–25. [Google Scholar] [CrossRef]
- Qian, S.; Yan, W.; Zhu, S.; Fontanillo Lopez, C.A.; Sheng, K. Surface modification of bamboo-char and its reinforcement in PLA biocomposites. Polym. Compos. 2018, 39, 633–639. [Google Scholar] [CrossRef]
- Wang, S.S.; Zhao, L.; Zhou, H.Y.; Li, W.Z.; Zhang, W.B. Flame retardancy and mechnical property studies of Aluminum hypophosphite on bamboo charcoal/polylactic acid composites. Mater. Rep. 2020, 34, 14214–14217. (In Chinese) [Google Scholar] [CrossRef]
- Irvine, D.J.; McCluskey, J.A.; Robinson, I.M. Fire hazards and some common polymers. Polym. Degrad. Stab. 2000, 67, 383–396. [Google Scholar] [CrossRef]
- Zhou, K.; Gui, Z.; Hu, Y. The influence of graphene based smoke suppression agents on reduced fire hazards of polystyrene composites. Compos. Part A Appl. Sci. Manuf. 2016, 80, 217–227. [Google Scholar] [CrossRef]
- Basu, A.; Nazarkovsky, M.; Ghadi, R.; Khan, W.; Domb, A.J. Poly (lactic acid)-based nanocomposites. Polymers Adv. Technol. 2017, 28, 919–930. [Google Scholar] [CrossRef]
- Tipachan, C.; Gupta, R.K.; Agarwal, S.; Kajorncheappunngam, S. Flame Retardant Properties and Thermal Stability of Polylactic Acid Filled with Layered Double Hydroxide and Rice Husk Ash Silica. J. Polymers Environ. 2020, 28, 948–961. [Google Scholar] [CrossRef]
- Feng, J.; Sun, Y.; Song, P.; Lei, W.; Wu, Q.; Liu, L.; Yu, Y.; Wang, H. Fire-resistant, strong, and green polymer nanocomposites based on poly (lactic acid) and core–shell nanofibrous flame retardants. ACS Sustain. Chem. Eng. 2017, 5, 7894–7904. [Google Scholar] [CrossRef]
- Mousa, M.; Dong, Y.; Davies, I.J. Eco-friendly polyvinyl alcohol (PVA)/bamboo charcoal (BC) nanocomposites with superior mechanical and thermal properties. Adv. Compos. Mater. 2018, 27, 499–509. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Wang, X.; Xing, W.; Zhang, P.; Wang, B.; Hong, N.; Yang, W.; Hu, Y.; Song, L. Thermal degradation and flame retardance of biobased polylactide composites based on aluminum hypophosphite. Ind. Eng. Chem. Res. 2012, 51, 12009–12016. [Google Scholar] [CrossRef]
- Zhou, X.; Li, J.; Wu, Y. Synergistic effect of aluminum hypophosphite and intumescent flame retardants in polylactide. Polymers Adv. Technol. 2015, 26, 255–265. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Qi, X.Z.; Wang, X.L. Study on Preparation and Thermal Degradation Behavior of Intumescent Flame Retardant PLA Reinforced with BPC. Plast. Sci. Technol. 2018, 46, 73–77. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, W.B.; Li, W.Z.; Zhou, J.Z.; Fu, J.H.; Zhou, G.Y. Research on the bamboo charcoal properties of Bambusa blumeana Schult. f and Dinochloa puberula. World Bamboo Rattan 2009, 5. [Google Scholar] [CrossRef]
- Li, H.Z.; Chen, S.C.; Wang, Y.Z. Preparation and characterization of nanocomposites of polyvinyl alcohol/cellulose nanowhiskers/chitosan. Compos. Sci. Technol. 2015, 115, 60–65. [Google Scholar] [CrossRef]
- Asada, T.; Ishihara, S.; Yamane, T.; Toba, A.; Yamada, A.; Oikawa, K. Science of bamboo charcoal: Study on carbonizing temperature of bamboo charcoal and removal capability of harmful gases. J. Health Sci. 2002, 48, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.J.; Gou, Z.; Fujita, H.; Fujii, T.; Sakoda, A. Simple fabrication of molecular sieving carbon for biogas upgrading via a temperature controlled carbonization of Phyllostachys pubescens. Renew. Energy 2016, 86, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Tsai, M.H.; Lo, S.F.; Tsai, M.J. Effects of manufacturing conditions on the adsorption capacity of heavy metal ions by Makino bamboo charcoal. Bioresour. Technol. 2008, 99, 7027–7033. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, J.; Kwong, F.L.; Ng, D.H.L.; Tjong, S.C. Synthesis of multiwalled carbon nanotubes from bamboo charcoal and the roles of minerals on their growth. Biomass Bioenergy 2012, 36, 12–19. [Google Scholar] [CrossRef]
- Qian, S.; Sheng, K.; Yao, W.; Yu, H. Poly (lactic acid) biocomposites reinforced with ultrafine bamboo-char: Morphology, mechanical, thermal, and water absorption properties. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, W.; Qian, S.; Sheng, K. Fabrication and reinforcement of ternary composites based on polypropylene matrix with bamboo particle/ultrafine bamboo-char. Polym. Compos. 2018, 39, 4364–4371. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Botta, L.; Fortunato Gulino, E.; Gulli, D. Tunable release of Chlorhexidine from Polycaprolactone-based filaments containing graphene nanoplatelets. Eur. Polym. J. 2018, 110, 221–232. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Pinheiro, I.F.; Mariano, M.; Cividanes, L.S.; Costa, J.C.M.; Nascimento, N.R.; Lona, L.M.F. Environmentally friendly polymer composites based on PBAT reinforced with natural fibers from the amazon forest. Polym. Compos. 2019, 40, 3351–3360. [Google Scholar] [CrossRef]
- Pinheiro, I.F.; Ferreira, F.V.; Alves, G.F.; Rodolfo, A.; Morales, A.R.; Mei, L.H.I. Biodegradable PBAT-Based Nanocomposites Reinforced with Functionalized Cellulose Nanocrystals from Pseudobombax munguba: Rheological, Thermal, Mechanical and Biodegradability Properties. J. Polym. Environ. 2019, 27, 757–766. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Trindade, G.N.; Lona, L.M.F.; Bernardes, J.S.; Gouveia, R.F. LDPE-based composites reinforced with surface modified cellulose fibres: 3D morphological and morphometrical analyses to understand the improved mechanical performance. Eur. Polym. J. 2019, 117, 105–113. [Google Scholar] [CrossRef]
- Chinh, N.T.; Trang, N.T.; Thanh, D.T.; Hang, T.T.; Giang, N.V.; Quan, P.M.; Hoang, T. Thermal property, morphology, and hydrolysis ability of poly (lactic acid)/chitosan nanocomposites using polyethylene oxide. J. Appl. Polymer Sci. 2014, 132. [Google Scholar] [CrossRef]
- Wei, L.L.; Wang, D.Y.; Chen, H.B.; Chen, L.; Wang, X.L.; Wang, Y.Z. Effect of a phosphorus-containing flame retardant on the thermal properties and ease of ignition of poly (lactic acid). Polymer Degrad. Stab. 2011, 96, 1557–1561. [Google Scholar] [CrossRef]
- Liu, C.Y.; Fang, W.; Qian, K.; Liu, X.Q.; Liu, J.Y. Study on flame retardant system of polylactic acid/aluminum hypophosphite/silica husk. Chinaplastics Ind. 2016, 44, 72–75. (In Chinese) [Google Scholar] [CrossRef]
- Lewin, M.; Weil, E.D. Mechanisms and modes of action in flame retardancy of polymers. Fire-Retardant Mater. 2001, 1, 31–68. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Smart, G.; Nazare, S.; Kandola, B.; Price, D. Quantification of zinc hydroxystannate** and stannate** synergies in halogen-containing flame-retardant polymeric formulations. J. Fire Sci. 2010, 28, 217–248. [Google Scholar] [CrossRef]
- Schartel, B.; Bartholmai, M.; Knoll, U. Some comments on the use of cone calorimeter data. Polymer Degrad. Stab. 2005, 88, 540–547. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. Int. J. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Park, W.H.; Yoon, K.B. Optimization of pyrolysis properties using TGA and cone calorimeter test. J. Therm. Sci. 2013, 22, 168–173. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent developments in the fire retardancy of polymeric materials. Prog. Polymer Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Morin, M.; Pécate, S.; Hemati, M. Kinetic study of biomass char combustion in a low temperature fluidized bed reactor. Chem. Eng. J. 2018, 331, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Hirschler, M.M.; Shakir, S. Measurements of cable fire properties by using heat release equipment. In Proceedings of Flame Retardants 92; Elsevier Applied Science: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Marney, D.C.O.; Russell, L.J.; Mann, R. Fire performance of wood (Pinus radiata) treated with fire retardants and a wood preservative. Fire Mater. Int. J. 2008, 32, 357–370. [Google Scholar] [CrossRef]
- Morgan, A.B.; Bundy, M. Cone calorimeter analysis of UL-94 V-rated plastics. Fire Mater. Int. J. 2007, 31, 257–283. [Google Scholar] [CrossRef]
- Cardelli, A.; Ruggeri, G.; Calderisi, M.; Lednev, O.; Cardelli, C.; Tombari, E. Effects of poly (dimethylsiloxane) and inorganic fillers in halogen free flame retardant poly (ethylene-co-vinyl acetate) compound: A chemometric approach. Polymer Degrad. Stab. 2012, 97, 2536–2544. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, J.; Qiu, C.; Yao, Y.; Sakai, E.; Yang, L. Mechanical properties and degrading behaviors of aluminum hypophosphite-polylactic acid (PLA) nanocomposites. Polymer-Plast. Technol. Mater. 2019, 58, 126–138. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, Y.; Du, X.; Song, P.; Fang, Z. Green and scalable fabrication of core–shell biobased flame retardants for reducing flammability of polylactic acid. ACS Sustain. Chem. Eng. 2019, 7, 8954–8963. [Google Scholar] [CrossRef]






| Sample | Composition (wt. %) | |||
|---|---|---|---|---|
| PLA | BP | BC | AHP | |
| PLA | 100 | 0 | 0 | 0 |
| BP/PLA (25/75) | 75 | 25 | 0 | 0 |
| 20BC/PLA (20/80) | 80 | 0 | 20 | 0 |
| 25BC/PLA (25/75) | 75 | 0 | 25 | 0 |
| 30BC/PLA (30/70) | 70 | 0 | 30 | 0 |
| 35BC/PLA (35/65) | 65 | 0 | 35 | 0 |
| 40BC/PLA (40/60) | 60 | 0 | 40 | 0 |
| BC/PLA/AHP (25/70/5) | 70 | 0 | 25 | 5 |
| BC/PLA/AHP (25/65/10) | 65 | 0 | 25 | 10 |
| BC/PLA/AHP (25/60/15) | 60 | 0 | 25 | 15 |
| BC/PLA/AHP (25/55/20) | 55 | 0 | 25 | 20 |
| BC/PLA/AHP (25/50/25) | 50 | 0 | 25 | 25 |
| BC/PLA/AHP (25/45/30) | 45 | 0 | 25 | 30 |
| Content (wt. %) | C | H | N | S | O | Fixed Carbon | Volatile Component | Ash |
|---|---|---|---|---|---|---|---|---|
| BC | 75.7 | 2.9 | 0.7 | 0.2 | 11.0 | 75.7 | 14.8 | 9.5 |
| BP | 47.2 | 6.2 | 0.1 | 0.3 | 45.1 | 16.5 | 82.5 | 1.1 |
| Material | Dx (10)/μm | Dx (50)/μm | Dx (90)/μm |
|---|---|---|---|
| BC | 5.6 | 22.7 | 156.7 |
| BP | 7. | 54.8 | 192.5 |
| Sample | w(AHP)/% | LOI/% | UL-94 | ||
|---|---|---|---|---|---|
| t1/t2 | Dripping | Rating | |||
| 1 | 0 | 23.8 | 3.3/2.3 | Yes | V-2 |
| 2 | 5 | 24.0 | 5.1/1.4 | Yes | V-2 |
| 3 | 10 | 26.0 | 3.1/1.6 | No | V-0 |
| 4 | 15 | 27.1 | 1.0/1.2 | No | V-0 |
| 5 | 20 | 29.3 | 1.0/1.1 | No | V-0 |
| 6 | 25 | 30.0 | 1.0/1.6 | No | V-0 |
| 7 | 30 | 31.0 | 1.2/1.1 | No | V-0 |
| Sample | LOI/vol% | Syn Effectivity |
|---|---|---|
| PLA | 22.0 | - |
| BC/PLA (25/75) | 23.8 | - |
| PLA/AHP (75/15) | 25.2 | - |
| PLA/AHP (75/20) | 26.4 | - |
| PLA/AHP (75/25) | 26.8 | - |
| PLA/AHP (75/30) | 27.3 | - |
| BC/PLA/AHP (25/60/15) | 27.1 | 1.02 |
| BC/PLA/AHP (25/55/20) | 29.3 | 1.18 |
| BC/PLA/AHP (25/50/25) | 30.0 | 1.21 |
| BC/PLA/AHP (25/45/30) | 31.0 | 1.27 |
| Sample | TTI (s) | pHRR (kW/m2) | THR (MJ/m2) | pMLR (g/s) | RM (%) | pEHC (KJ/kg) | pSEA (m2/kg) | Peak COY/CO2Y kg/kg | FPI | FGI |
|---|---|---|---|---|---|---|---|---|---|---|
| PLA | 51 | 484.27 | 68.97 | 0.47 | 4.37 | 73.41 | 79.53 | 0.19/1420.79 | 0.11 | 2.56 |
| BP/PLA (25/75) | 38 | 330.05 | 78.01 | 0.77 | 3.46 | 79.52 | 333.43 | 0.33/2181.69 | 0.12 | 3.05 |
| BC/PLA (20/80) | 38 | 342.47 | 66.78 | 0.56 | 12.18 | 78.54 | 747.55 | 0.10/242.21 | 0.11 | 2.25 |
| BC/PLA (25/75) | 33 | 320.32 | 59.94 | 0.57 | 19.46 | 79.84 | 1538.26 | 0.01/105.59 | 0.10 | 2.44 |
| BC/PLA (30/70) | 27 | 298.66 | 57.77 | 0.58 | 24.69 | 77.18 | 500.54 | 2.77/6327.12 | 0.09 | 2.99 |
| BC/PLA (35/65) | 27 | 296.09 | 55.94 | 0.54 | 26.80 | 77.06 | 740.01 | 0.03/177.54 | 0.09 | 3.12 |
| BC/PLA (40/60) | 27 | 267.07 | 53.89 | 0.43 | 32.38 | 79.17 | 1159.29 | 0.18/478.02 | 0.10 | 2.90 |
| BC/PLA/AHP (25/60/15) | 27 | 155.01 | 47.96 | 0.40 | 30.08 | 78.24 | 330.52 | 0.01/245.41 | 0.17 | 2.54 |
| BC/PLA/AHP (25/55/20) | 27 | 144.14 | 49.73 | 0.33 | 37.70 | 70.83 | 649.82 | 0.17/308.66 | 0.18 | 2.25 |
| BC/PLA/AHP (25/50/25) | 27 | 130.56 | 48.19 | 0.29 | 43.03 | 72.52 | 369.71 | 0.26/409.17 | 0.21 | 2.07 |
| BC/PLA/AHP (25/45/30) | 27 | 134.77 | 45.11 | 0.33 | 46.59 | 76.94 | 977.52 | 0.01/258.21 | 0.20 | 2.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, L.; Semple, K.; Zhang, M.; Zhang, W.; Dai, C. Development of Biodegradable Flame-Retardant Bamboo Charcoal Composites, Part I: Thermal and Elemental Analyses. Polymers 2020, 12, 2217. https://doi.org/10.3390/polym12102217
Wang S, Zhang L, Semple K, Zhang M, Zhang W, Dai C. Development of Biodegradable Flame-Retardant Bamboo Charcoal Composites, Part I: Thermal and Elemental Analyses. Polymers. 2020; 12(10):2217. https://doi.org/10.3390/polym12102217
Chicago/Turabian StyleWang, Shanshan, Liang Zhang, Kate Semple, Min Zhang, Wenbiao Zhang, and Chunping Dai. 2020. "Development of Biodegradable Flame-Retardant Bamboo Charcoal Composites, Part I: Thermal and Elemental Analyses" Polymers 12, no. 10: 2217. https://doi.org/10.3390/polym12102217





