Bifunctional Microcapsules with n-Octadecane/Thyme Oil Core and Polyurea Shell for High-Efficiency Thermal Energy Storage and Antibiosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Antibacterial MicroPCMs
2.3. Characterization
2.3.1. Morphology Analysis
2.3.2. Chemical Structure Analysis
2.3.3. Thermal Stability Test
2.3.4. Thermal Storage Property
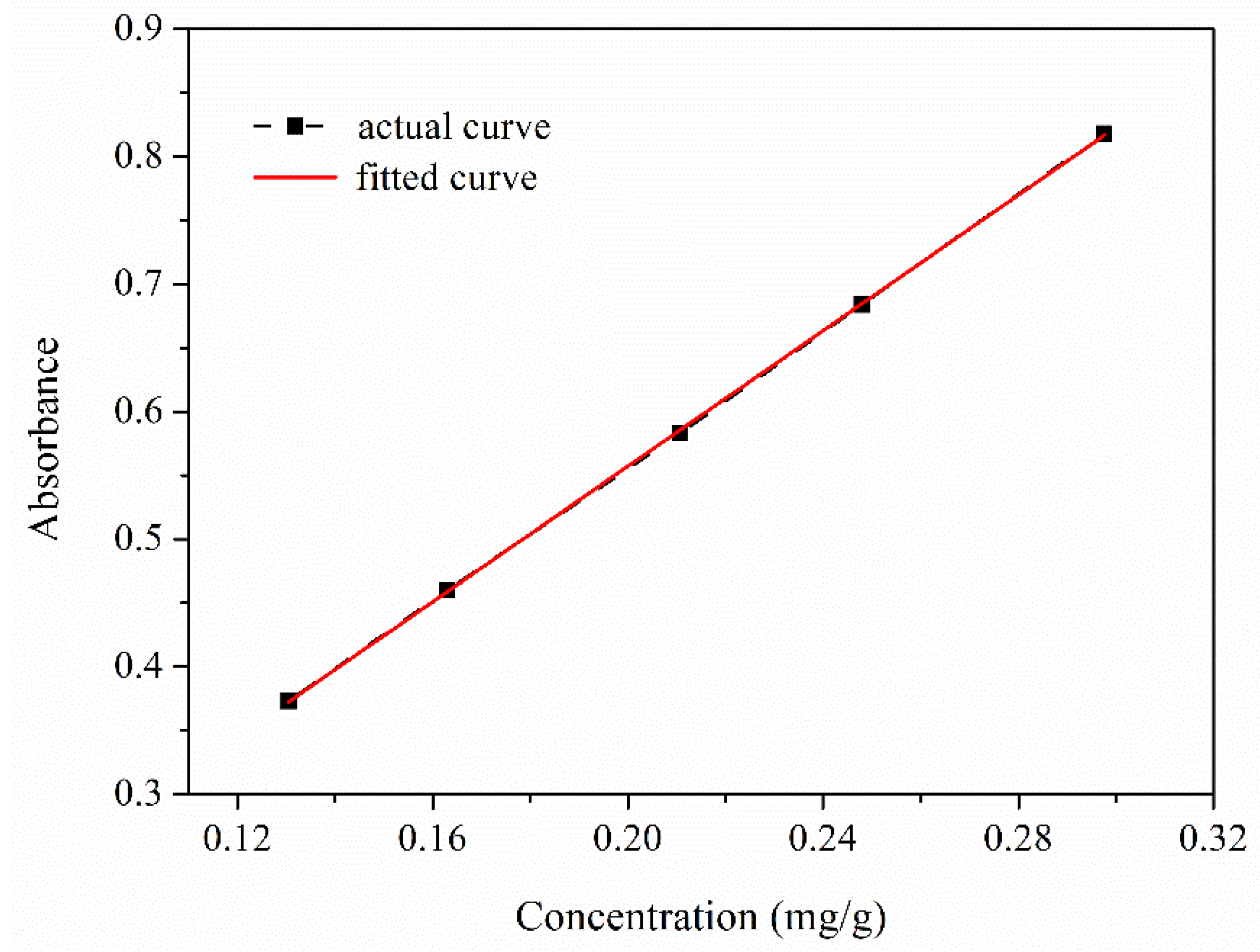
2.3.5. Encapsulation Efficiency and Release Behavior of TO from Microcapsules
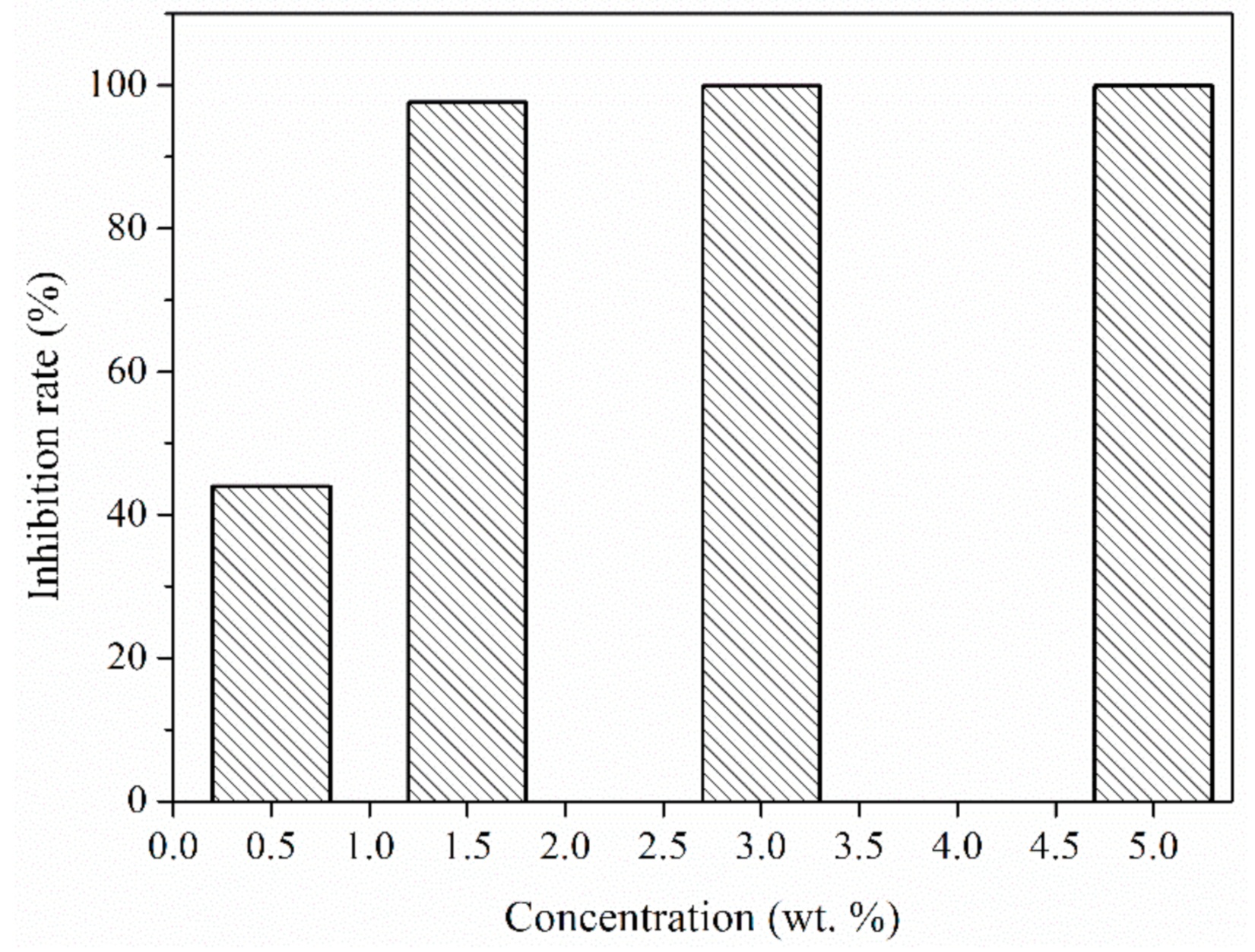
2.3.6. Antibacterial Test
3. Results and Discussion
3.1. Preparation and Formation Mechanism of Antibacterial microPCMs
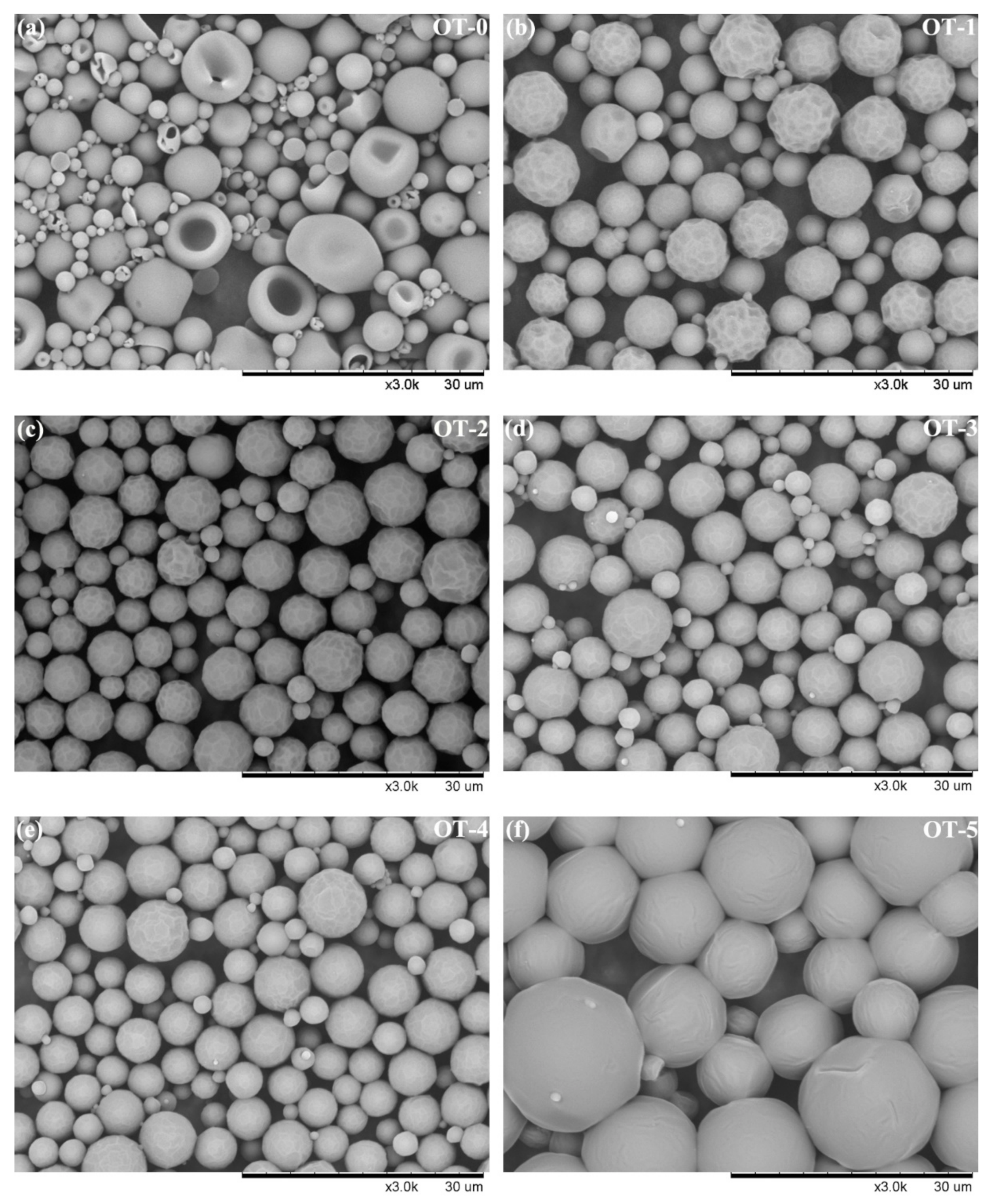
3.2. Morphology of the Microcapsules
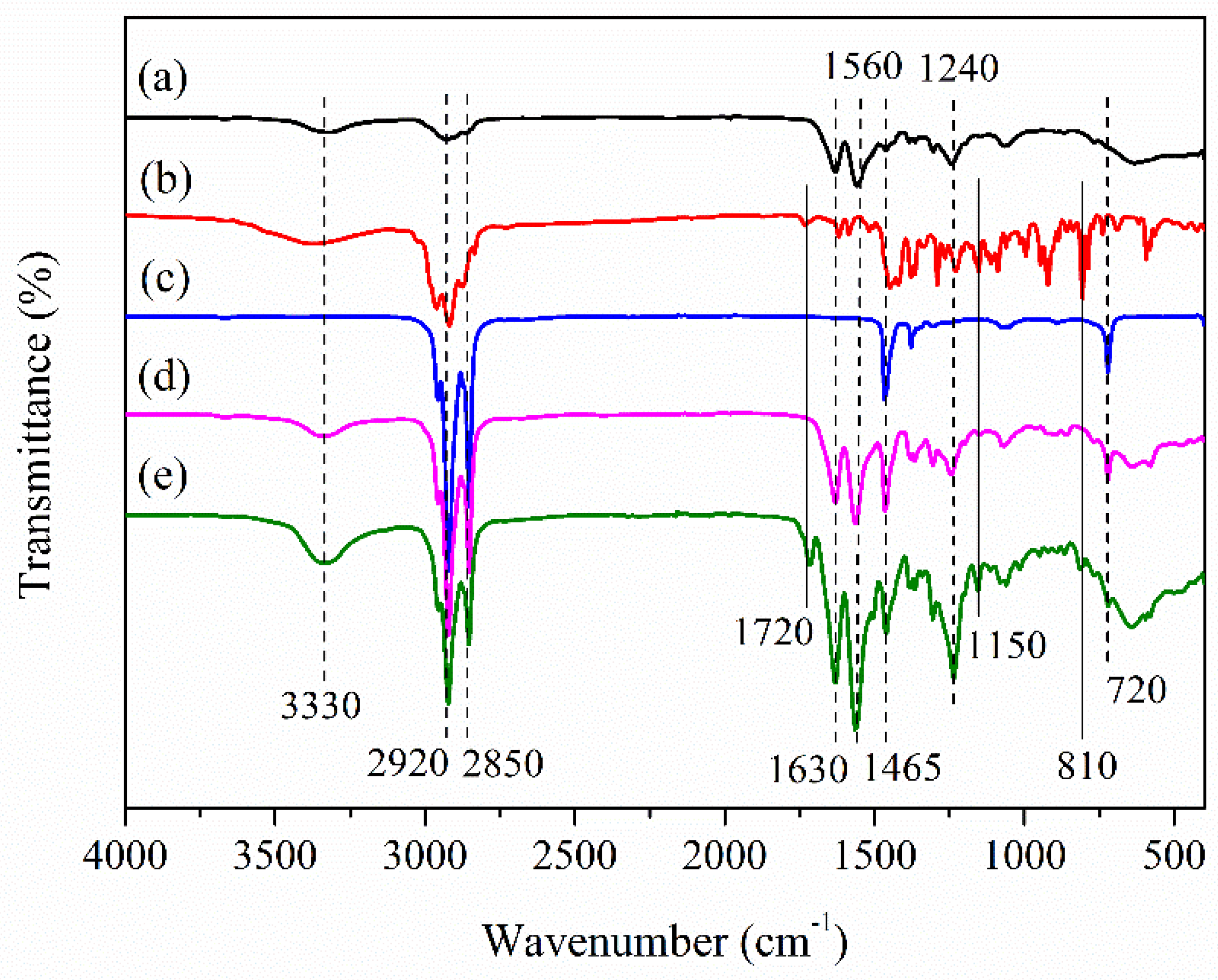
3.3. Chemical Structure of the Microcapsules
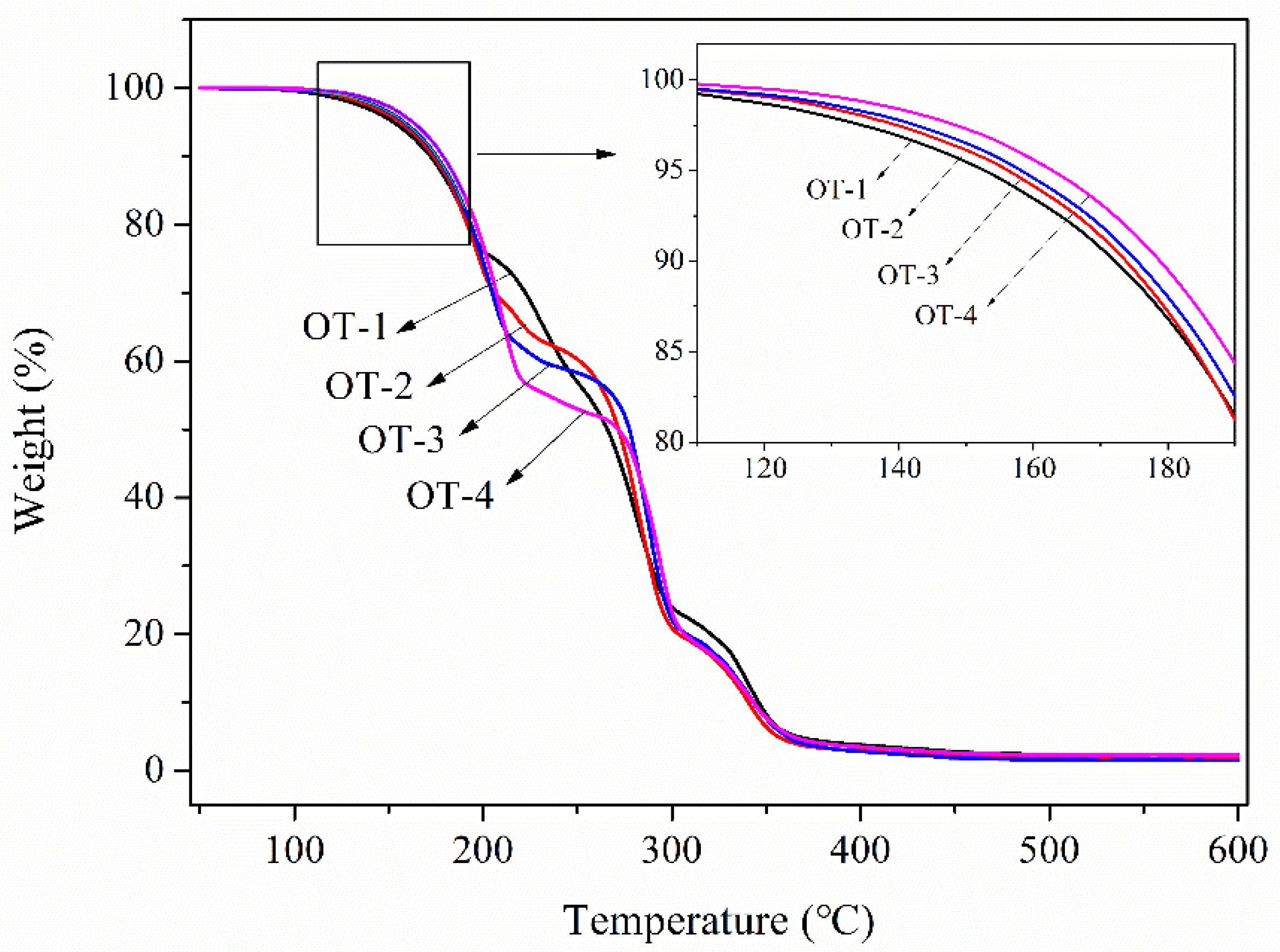
3.4. Thermal Stability of the Microcapsules
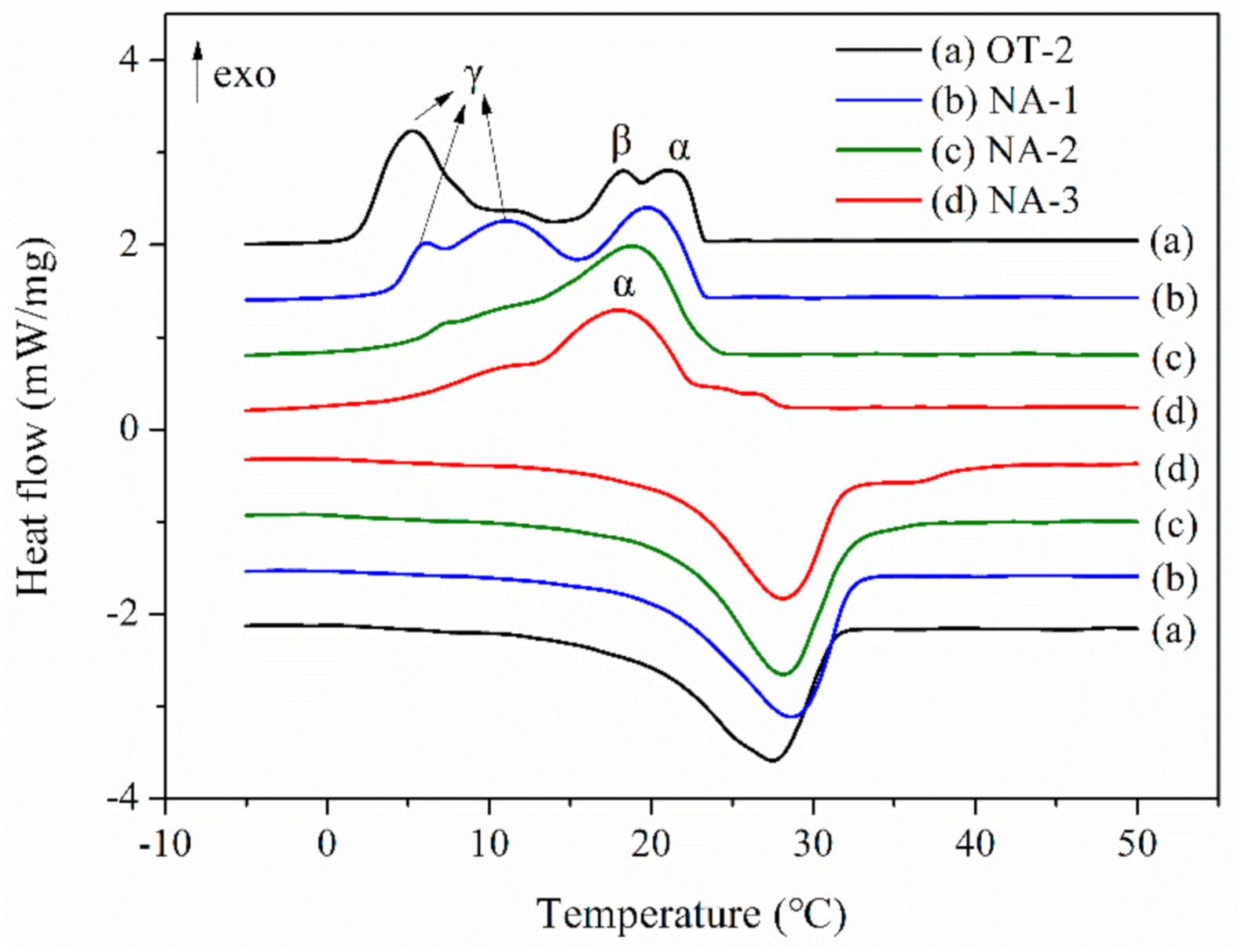
3.5. Heat Storage Performance of the Microcapsules
3.6. Encapsulation Efficiency and Release Behavior of TO from Microcapsules
3.7. Antibacterial Activity of the Microcapsules
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khadiran, T.; Hussein, M.; Zainal, Z.; Rusli, R. Encapsulation techniques for organic phase change materials as thermal energy storage medium: A review. Sol. Energy Mater. Sol. Cells 2015, 143, 78–98. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, G. Review on microencapsulated phase change materials (MEPCMs): Fabrication, characterization and applications. Renew. Sustain. Energy Rev. 2011, 15, 3813–3832. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Hyun, D.C.; Levinson, N.S.; Jeong, U.; Xia, Y. Emerging applications of phase-change materials (PCMs): Teaching an old dog new tricks. Angew. Chem. Int. Ed. 2014, 53, 3780–3795. [Google Scholar] [CrossRef] [PubMed]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Mahlia, T.; Metselaar, H. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Chai, L.; Wang, X.; Wu, D. Development of bifunctional microencapsulated phase change materials with crystalline titanium dioxide shell for latent-heat storage and photocatalytic effectiveness. Appl. Energy 2015, 138, 661–674. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Y.; Huang, J.; Wang, S. High thermal conductive paraffin/calcium carbonate phase change microcapsules based composites with different carbon network. Appl. Energy 2018, 218, 184–191. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, T. Effects of parameters of the shell formation process on the performance of microencapsulated phase change materials based on melamine-formaldehyde. Text. Res. J. 2016, 87, 1848–1859. [Google Scholar] [CrossRef]
- Alkan, C.; Sarı, A.; Karaipekli, A.; Uzun, O. Preparation, characterization, and thermal properties of microencapsulated phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 143–147. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Synthesis and properties of microencapsulated n-octadecane with polyurea shells containing different soft segments for heat energy storage and thermal regulation. Sol. Energy Mater. Sol. Cells 2009, 93, 1366–1376. [Google Scholar] [CrossRef]
- Fathi, Z.; Doustkhah, E.; Rostamniab, S.; Darvishi, F.; Ghodsi, A.; Ide, Y. Interaction of Yarrowia lipolytica lipase with dithiocarbamate modified magnetic carbon Fe3O4@C-NHCS2H core-shell nanoparticles. Int. J. Biol. Macromol. 2018, 117, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Bayés-García, L.; Ventolà, L.; Cordobilla, R.; Benages-Vilau, R.; Calvet, T.; Cuevas-Diarte, M. Phase change materials (PCM) microcapsules with different shell compositions: Preparation, characterization and thermal stability. Sol. Energy Mater. Sol. Cells 2010, 94, 1235–1240. [Google Scholar] [CrossRef]
- Mondal, S. Phase change materials for smart textiles—An overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Pomianowski, M.; Heiselberg, P.; Zhang, Y. Review of thermal energy storage technologies based on PCM application in buildings. Energy Build. 2013, 67, 56–69. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.; Chen, C.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Wu, D. Fabrication of multifunctional microcapsules containing n-eicosane core and zinc oxide shell for low-temperature energy storage, photocatalysis, and antibiosis. Energy Convers. Manag. 2015, 106, 873–885. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wu, D. Design and synthesis of multifunctional microencapsulated phase change materials with silver/silica double-layered shell for thermal energy storage, electrical conduction and antimicrobial effectiveness. Energy 2016, 111, 498–512. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Zhao, L.; Shi, X.; Song, G.; Tang, G. A facile approach to synthesize microencapsulated phase change materials embedded with silver nanoparicle for both thermal energy storage and antimicrobial purpose. Energy 2018, 158, 1052–1059. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wang, B.; Feng, X.; Mao, Z.; Sui, X. Antibacterial phase change microcapsules obtained with lignin as the Pickering stabilizer and the reducing agent for silver. Int. J. Biol. Macromol. 2020, 144, 624–631. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; Wu, D. Design and synthesis of magnetic microcapsules based on n-eicosane core and Fe3O4/SiO2 hybrid shell for dual-functional phase change materials. Appl. Energy 2014, 134, 456–468. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Kim, Y.S.; Lee, H.M.; Kim, J.H.; Cheong, I.W.; Koh, W.-G. Magnetic nanoparticle-embedded PCM nanocapsules based on paraffin core and polyurea shell. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 46–51. [Google Scholar] [CrossRef]
- Gao, F.; Wang, X.; Wu, D. Design and fabrication of bifunctional microcapsules for solar thermal energy storage and solar photocatalysis by encapsulating paraffin phase change material into cuprous oxide. Sol. Energy Mater. Sol. Cells 2017, 168, 146–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, J.; Ma, C.; Guo, L.; Meng, X. Solar-driven phase change microencapsulation with efficient Ti4O7 nanoconverter for latent heat storage. Nano Energy 2018, 53, 579–586. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Zhao, T. Fabrication and characterization of poly(melamine-formaldehyde)/silicon carbide hybrid microencapsulated phase change materials with enhanced thermal conductivity and light-heat performance. Sol. Energy Mater. Sol. Cells 2018, 183, 82–91. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, H.; Liu, J.; Fang, X.; Zhang, Z. Novel slurry containing graphene oxide-grafted microencapsulated phase change material with enhanced thermo-physical properties and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2015, 143, 29–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wu, D. Microencapsulation of n-dodecane into zirconia shell doped with rare earth: Design and synthesis of bifunctional microcapsules for photoluminescence enhancement and thermal energy storage. Energy 2016, 97, 113–126. [Google Scholar] [CrossRef]
- Scacchetti, F.A.P.; Pinto, E.; Soares, G.M.B. Functionalization and characterization of cotton with phase change materials and thyme oil encapsulated in beta-cyclodextrins. Prog. Org. Coat. 2017, 107, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.-U.; Shahid, M.; Mohammad, F. Green chemistry approaches to develop antimicrobial textiles based on sustainable biopolymers—A Review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. [Google Scholar] [CrossRef]
- Van Der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Rivera, Z.H.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.B.; Hollman, P.C.H.; et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Ferreira-Grosso, C.R.; Aldapa, C.A.G.; Rangel-Vargas, E.; Rodriguez-Marin, M.L.; Guzmán-Ortiz, F.A.; Falfan-Cortes, R.N. Recent advances in microencapsulation of natural sources of antimicrobial compounds used in food—A review. Food Res. Int. 2017, 102, 575–587. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant. Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Luo, Y.; Wang, M.; Chen, F.; Liu, J.; Meng, K.; Zhao, H. Double-layered microcapsules significantly improve the long-term effectiveness of essential oil. Polymers 2020, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.M.; Rodrigues, S.N.; Barreiro, M.F.; Rodrigues, A.E. Release of thyme oil from polylactide microcapsules. Ind. Eng. Chem. Res. 2011, 50, 13752–13761. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Xiao, Z.; Wang, X. Preparation and properties of cinnamon-thyme-ginger composite essential oil nanocapsules. Ind. Crop. Prod. 2018, 122, 85–92. [Google Scholar] [CrossRef]
- Piletti, R.; Zanetti, M.; Jung, G.; De Mello, J.M.M.; Dalcanton, F.; Soares, C.; Riella, H.G.; Fiori, M.A. Microencapsulation of garlic oil by betacyclodextrin as a thermal protection method for antibacterial action. Mater. Sci. Eng. C 2019, 94, 139–149. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Z.; Zhou, J.; Xie, Y.; Wu, H.; Yuan, T.; Yang, Z. Facile fabrication of tea tree oil-loaded antibacterial microcapsules by complex coacervation of sodium alginate/quaternary ammonium salt of chitosan. RSC Adv. 2016, 6, 13032–13039. [Google Scholar] [CrossRef]
- Leimann, F.V.; Gonçalves, O.H.; Machado, R.; Bolzan, A. Antimicrobial activity of microencapsulated lemongrass essential oil and the effect of experimental parameters on microcapsules size and morphology. Mater. Sci. Eng. C 2009, 29, 430–436. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Lu, D. Preparation and antimicrobial activity of thyme essential oil microcapsules prepared with gum arabic. RSC Adv. 2019, 9, 19740–19747. [Google Scholar] [CrossRef] [Green Version]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- Moreira, A.C.G.; Martins, I.M.; Fernandes, I.; Barreiro, M.F.; Rodrigues, A.E. Microencapsulation of red and white thyme oil in poly(lactic-co-glycolic) acid: Assessment of encapsulation efficiency and antimicrobial capacity of the produced microcapsules. Can. J. Chem. Eng. 2016, 94, 469–475. [Google Scholar] [CrossRef]
- Ye, K.; Zhao, D.; Shi, X.; Lu, X. Use of caprylic/capric triglyceride in the encapsulation of dementholized peppermint fragrance leading to smaller and better distributed nanocapsules. RSC Adv. 2016, 6, 84119–84126. [Google Scholar] [CrossRef]
- Zhao, D.; Jiao, X.; Zhang, M.; Ye, K.; Shi, X.; Lu, X.; Qiu, G.; Shea, K.J. Preparation of high encapsulation efficiency fragrance microcapsules and their application in textiles. RSC Adv. 2016, 6, 80924–80933. [Google Scholar] [CrossRef]
- Jiao, X.; Zhao, D.; Zhang, Y.; Wu, Q.; Qiu, G.; Lu, X.; Shi, X. Synthesis and studies of poly(ethylene glycol dimethacrylate) microcapsule. Colloid Polym. Sci. 2015, 294, 639–646. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Fabrication and performances of microencapsulated phase change materials based on n-octadecane core and resorcinol-modified melamine-formaldehyde shell. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 129–138. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, Y.-F.; Tao, X.; Yick, K.-L. Crystallization and prevention of supercooling of microencapsulated n-alkanes. J. Colloid Interface Sci. 2005, 281, 299–306. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, X.; Wang, X.; Li, J.; Zhu, Q. Super-cooling prevention of microencapsulated phase change material. Thermochim. Acta 2004, 413, 1–6. [Google Scholar] [CrossRef]
- Juven, B.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [Green Version]





| Sample | GA (g) | OD (g) | TO (g) | IPDI (g) | HMDA (g) |
|---|---|---|---|---|---|
| OT-0 | 1.00 | 0.00 | 12.00 | 1.89 | 1.11 |
| OT-1 | 1.00 | 9.00 | 3.00 | 1.89 | 1.11 |
| OT-2 | 1.00 | 9.60 | 2.40 | 1.89 | 1.11 |
| OT-3 | 1.00 | 10.00 | 2.00 | 1.89 | 1.11 |
| OT-4 | 1.00 | 10.29 | 1.71 | 1.89 | 1.11 |
| OT-5 | 1.00 | 12.00 | 0.00 | 1.89 | 1.11 |
| Sample Code | Td5 (°C) | Td10 (°C) | First-Stage Weight Loss Rate (%) |
|---|---|---|---|
| TO | 94.8 | 111.2 | 96.12 |
| OD | 145.4 | 166.4 | 96.75 |
| OT-1 | 152.7 | 172.2 | 22.90 |
| OT-2 | 156.3 | 173.8 | 28.74 |
| OT-3 | 158.2 | 175.5 | 35.32 |
| OT-4 | 162.9 | 178.9 | 41.83 |
| Sample Code | Melting Process | Crystallization Process | EOD (%) | ||||
|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tα (°C) | Tβ (°C) | Tγ (°C) | ΔHc (J/g) | ||
| OD | 28.6 | 214.2 | 20.9 | - | 19.7 | 215.0 | - |
| OT-1 | 26.5 | 133.1 | 21.0 | 17.4 | 5.7 | 138.0 | 85.3 |
| OT-2 | 27.5 | 138.7 | 21.0 | 18.3 | 5.3 | 145.1 | 83.7 |
| OT-3 | 28.0 | 146.1 | 21.2 | 18.4 | 5.6 | 149.0 | 82.6 |
| OT-4 | 28.0 | 154.2 | - | 18.1 | 6.8 | 156.7 | 82.0 |
| Model Type | Equation of Model | R2 |
|---|---|---|
| Zero-order dynamical model | Q = 0.7872 t + 16.2661 | 0.9438 |
| First-order dynamical model | ln(100-Q) = −0.0252 t + 4.6355 | 0.9470 |
| Higuchi dynamical model | Q = 9.0098 t1/2 − 1.0517 | 0.9944 |
| Korsmeyer–Peppas dynamical model | Q = 9.7521 t0.4476 | 0.9853 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, C.; Wang, M.; Zhao, T.; Li, W. Bifunctional Microcapsules with n-Octadecane/Thyme Oil Core and Polyurea Shell for High-Efficiency Thermal Energy Storage and Antibiosis. Polymers 2020, 12, 2226. https://doi.org/10.3390/polym12102226
Wang X, Li C, Wang M, Zhao T, Li W. Bifunctional Microcapsules with n-Octadecane/Thyme Oil Core and Polyurea Shell for High-Efficiency Thermal Energy Storage and Antibiosis. Polymers. 2020; 12(10):2226. https://doi.org/10.3390/polym12102226
Chicago/Turabian StyleWang, Xianfeng, Chunhong Li, Meihui Wang, Tao Zhao, and Wenyao Li. 2020. "Bifunctional Microcapsules with n-Octadecane/Thyme Oil Core and Polyurea Shell for High-Efficiency Thermal Energy Storage and Antibiosis" Polymers 12, no. 10: 2226. https://doi.org/10.3390/polym12102226





