Synthesis of Dimethyl Octyl Aminoethyl Ammonium Bromide and Preparation of Antibacterial ABS Composites for Fused Deposition Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Octyl-QDED
2.3. Preparation of ABS/Octyl-QDED
2.4. Characterization of Octyl-QDED
2.5. FT-IR Characterization of ABS/Octyl-QDED
2.6. Rheology Performance of ABS/Octyl-QDED
2.7. Fabrication of FDM Filaments and FDM of Specimens
2.8. Mechanical Properties of ABS/Octyl-QDED
2.9. Cross-Section Morphology of Printed ABS/Octyl-QDED
2.10. Antibacterial Performance of Octyl-QDED and ABS/Octyl-QDED
3. Results and Discussion
3.1. Characterization of Octyl-QDED
3.2. Preparation of ABS/Octyl-QDED
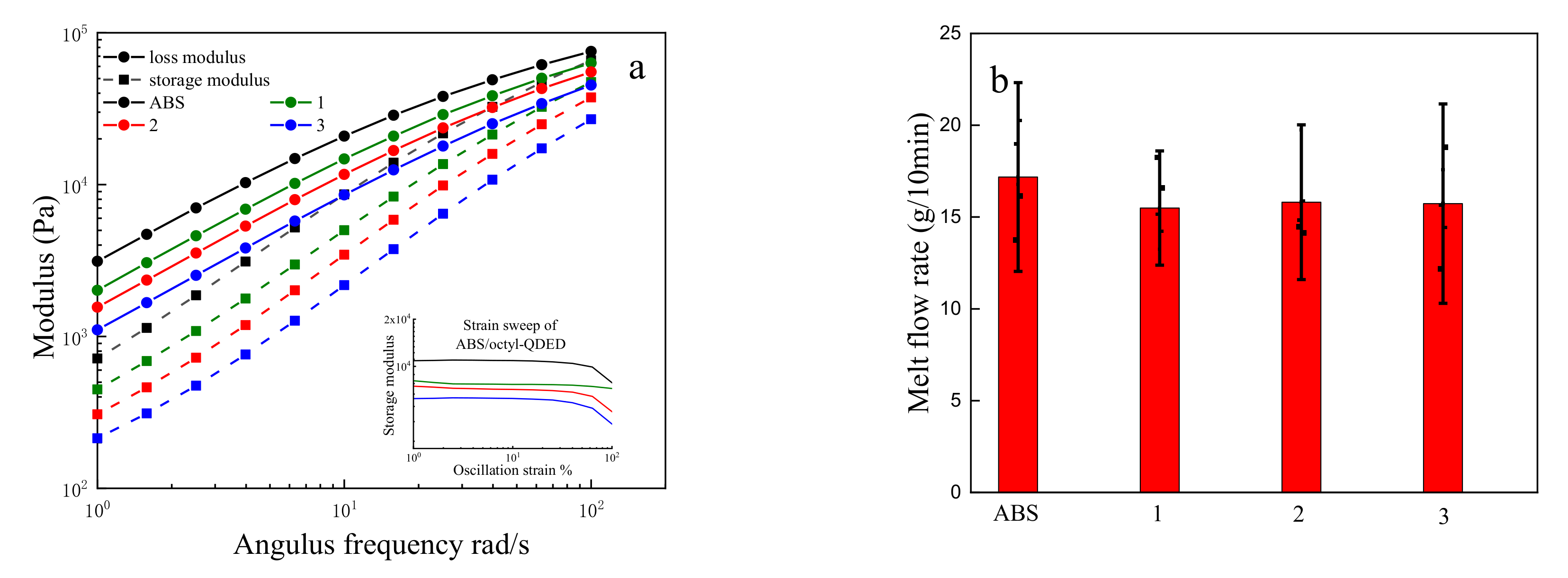
3.3. Rheology Analysis of ABS/Octyl-QDED
3.4. Specimen Fabrication and Mechanical Properties of ABS/Octyl-QDED
3.5. Cross-Section Morphology of the Printed ABS/Octyl-QDED
3.6. Antibacterial Performance of Octyl-QDED and ABS/Octyl-QDED
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing, 2nd ed.; Springer: New York, NY, USA, 2015; p. 498. [Google Scholar]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Carneiro, O.S.; Silva, A.F.; Gomes, R. Fused deposition modeling with polypropylene. Mater. Des. 2015, 83, 768–776. [Google Scholar] [CrossRef]
- Tanikella, N.G.; Wittbrodt, B.; Pearce, J.M. Tensile strength of commercial polymer materials for fused filament fabrication 3D printing. Addit. Manuf. 2017, 15, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, F.A.M.M.; Fonseca, A.C.; Domingos, M.; Gloria, A.; Serra, A.C.; Coelho, J.F.J. The potential of unsaturated polyesters in biomedicine and tissue engineering: Synthesis, structure-properties relationships and additive manufacturing. Prog. Polym. Sci. 2017, 68, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Guvendiren, M.; Molde, J.; Soares, R.M.D.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef]
- Salentijn, G.I.J.; Oomen, P.E.; Grajewski, M.; Verpoorte, E. Fused Deposition Modeling 3D Printing for (Bio)analytical Device Fabrication: Procedures, Materials, and Applications. Anal. Chem. 2017, 89, 7053–7061. [Google Scholar] [CrossRef] [Green Version]
- Browne, M.P.; Redondo, E.; Pumera, M. 3D Printing for Electrochemical Energy Applications. Chem. Rev. 2020, 120, 2783–2810. [Google Scholar] [CrossRef]
- Nikzad, M.; Masood, S.H.; Sbarski, I. Thermo-mechanical properties of a highly filled polymeric composites for Fused Deposition Modeling. Mater. Des. 2011, 32, 3448–3456. [Google Scholar] [CrossRef]
- Rosenzweig, D.H.; Carelli, E.; Steffen, T.; Jarzem, P.; Haglund, L. 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int. J. Mol. Sci. 2015, 16, 15118–15135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Mangadlao, J.D.; Wallat, J.; Leon, A.D.; Pokorski, J.K.; Advincula, R.C. 3D Printing Biocompatible Polyurethane/Poly(lactic acid)/Graphene Oxide Nanocomposites: Anisotropic Properties. ACS Appl. Mater. Interfaces. 2017, 9, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Alfredo, N.; Dorronsoro, A.; Cortajarena, A.L.; Rodríguez-Hernández, J. Antimicrobial 3D porous scaffolds prepared by additive manufacturing and breath figures. ACS Appl. Mater. Interfaces. 2017, 9, 37454–37462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valino, A.D.; Dizon, J.; Espera, A.H., Jr.; Chen, Q.; Messman, J.; Advincula, R.C. Advances in 3D printing of thermoplastic polymer composites and nanocomposites. Prog. Polym. Sci. 2019, 98, 101162. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T. Polyester-based green composites for three-dimensional printing strips: Preparation, characterization and antibacterial properties. Polym. Bull. 2016, 74, 2277–2295. [Google Scholar] [CrossRef]
- Bayraktar, I.; Doganay, D.; Coskun, S.; Kaynak, C.; Akca, G.; Unalan, H.E. 3D printed antibacterial silver nanowire/polylactide nanocomposites. Compos. Part B Eng. 2019, 172, 671–678. [Google Scholar] [CrossRef]
- Zeng, W.; He, J.; Liu, F. Preparation and properties of antibacterial ABS plastics based on polymeric quaternary phosphonium salts antibacterial agents. Polym. Adv. Technol. 2019, 30, 2515–2522. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K.D. Antibacterial Action of Chitosan and Carboxymethylated Chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Simoncic, B.; Tomsic, B. Structures of Novel Antimicrobial Agents for Textiles - A Review. Text. Res. J. 2010, 80, 1721–1737. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Aberasturi, D.J.; Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- León-Cabezas, M.A.; Martínez-García, A.; Varela-Gandía, F.J. Innovative functionalized monofilaments for 3D printing using fused deposition modeling for the toy industry. Procedia Manufacturing. 2017, 13, 738–745. [Google Scholar] [CrossRef]
- Mania, S.; Ryl, S.; Jinn, J.R.; Wang, Y.J. The Production possibility of the antimicrobial filaments by co-extrusion of the PLA pellet with chitosan powder for FDM 3D printing technology. Polymers 2019, 11, 1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhu, H.; Fu, W.; Zhang, Y.; Xu, B.; Gao, F.; Cao, Z.; Liu, W. A High Strength Self-Healable Antibacterial and Anti-Inflammatory Supramolecular Polymer Hydrogel. Macromol. Rapid. Commun. 2017, 38, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Tezel, U.; Pavlostathis, S.G. Quaternary ammonium disinfectants: Microbial adaptation, degradation and ecology. Curr. Opin. Biotechnol. 2015, 33, 296–304. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Yu, Z.G.; Zhu, M.Y.; Shen, L.Q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total. Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Xu, H.H. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H.K. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent. Mater. 2014, 30, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, N.; Sugaya, S.; Nakamura, T.; Yamaguchi, Y.; Kondo, Y.; Kawada, K.; Teranaka, T. Synthesis and antimicrobial activity of quaternary ammonium silane coupling agents. J. Oleo Sci. 2011, 60, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.J.; Chai, M.Y.; Li, W.B.; Ping, Y.; Tang, G.P.; Yang, W.T.; Ma, J.; Liu, F.S. Well-Defined Poly(2-hydroxyl-3-(2-hydroxyethylamino)propyl methacrylate) Vectors with Low Toxicity and High Gene Transfection Efficiency. Biomacromolecules 2010, 11, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.J.; Zhu, Y.; Chai, M.Y.; Liu, F.S. Comparison of ethanolamine/ethylenediamine-functionalized poly(glycidylmethacrylate) for efficient gene delivery. Acta Biomater. 2011, 7, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.B.; Chai, M.Y.; Zhu, Y.; Yang, W.T.; Xu, F.J. Aminated poly(glycidyl methacrylate)s for constructing efficient gene carriers. ACS Appl. Mater. Interf. 2013, 5, 3212–3218. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marini, M.; Bondi, M.; Iseppi, R.; Toselli, M.; Pilati, F. Preparation and antibacterial activity of hybrid materials containing quaternary ammonium salts via sol–gel process. Eur. Polym. J. 2007, 43, 3621–3628. [Google Scholar] [CrossRef]
- Imazato, S.; Chen, J.; Ma, S.; Izutani, N.; Li, F. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jpn. Dent. Sci. Rev. 2012, 48, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Liu, S. Antibacterial surface design—Contact kill. Prog. Surf. Sci. 2016, 91, 136–153. [Google Scholar] [CrossRef]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weiss, E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef]
- Wiarachai, O.; Thongchul, N.; Kiatkamjornwong, S.; Hoven, V.P. Surface-quaternized chitosan particles as an alternative and effective organic antibacterial material. Colloid. Surface. B. 2012, 92, 121–129. [Google Scholar] [CrossRef]
- Gadkari, R.R.; Ali, W.; Das, A.; Alagirusamy, R. Configuration of a unique antibacterial needle-punched nonwoven fabric from silver impregnated polyester nanocomposite fibres. J. Ind. Text. 2020, 1–17. [Google Scholar] [CrossRef]
- Rodríguez, H.S.; Hinestroza, J.P.; Ochoa-Puentes, C.; Sierra, C.A.; Soto, C.Y. Antibacterial activity against Escherichia coli of Cu-BTC (MOF-199) metal-organic framework immobilized onto cellulosic fibers. J. Appl. Polym. Sci. 2014, 131, 40815. [Google Scholar] [CrossRef]
- Ziabka, M.; Menaszek, E.; Tarasiuk, J.; Wronski, S. Biocompatible Nanocomposite Implant with Silver Nanoparticles for Otology—In Vivo Evaluation. Nanomaterials 2018, 8, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanat, N.; James, N.L.; McKenzie, D.R. Welding methods for joining thermoplastic polymers for the hermetic enclosure of medical devices. Med. Eng. Phys. 2010, 32, 690–699. [Google Scholar] [CrossRef] [PubMed]
- McCullough, E.J.; Yadavalli, V.K. Surface modification of fused deposition modeling ABS to enable rapid prototyping of biomedical microdevices. J. Mater. Process. Tech. 2013, 213, 947–954. [Google Scholar] [CrossRef]
- Jang, S.P.; Kim, D. Thermal, mechanical, and diffusional properties of nylon 6/ABS polymer blends: Compatibilizer effect. Polym. Eng. Sci. 2000, 40, 1635–1642. [Google Scholar] [CrossRef]
- Guo, T.; Ding, X.; Han, H.; Zhang, L.; Zhang, Y.; Zhou, K. Wide-angle X-ray diffraction investigation on crystallization behavior of PA6/PS/SEBS-g-MA blends. J. Polym. Res. 2012, 19, 9813. [Google Scholar] [CrossRef]
- Bonda, S.; Mohanty, S.; Nayak, S.K. Influence of compatibilizer on mechanical, morphological and rheological properties of PP/ABS blends. Iran. Polym. J. 2014, 23, 415–425. [Google Scholar] [CrossRef]
- Elmaghor, F.; Zhang, L.; Fan, R.; Li, H. Recycling of polycarbonate by blending with maleic anhydride grafted ABS. Polymer 2004, 45, 6719–6724. [Google Scholar] [CrossRef]
- Tejero, R.; López, D.; Lópezfabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. Antimicrobial polymethacrylates based on quaternized 1,3-thiazole and 1,2,3-triazole side-chain groups. Polym. Chem. 2015, 6, 3449–3459. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.B.; He, J.W.; Liu, F. Synthesis of imidazolium-containing mono-methacrylates as polymerizable antibacterial agents for acrylic bone cements. J. Mech. Behav. Biomed. Mater. 2017, 74, 176–182. [Google Scholar] [CrossRef]








| Designation | Content of Octyl-QDED (phr 1) |
|---|---|
| ABS | 0 |
| 1 | 1 |
| 2 | 2 |
| 3 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, S.; Zhang, Y.; Mi, J.; Ding, X. Synthesis of Dimethyl Octyl Aminoethyl Ammonium Bromide and Preparation of Antibacterial ABS Composites for Fused Deposition Modeling. Polymers 2020, 12, 2229. https://doi.org/10.3390/polym12102229
Wang Y, Wang S, Zhang Y, Mi J, Ding X. Synthesis of Dimethyl Octyl Aminoethyl Ammonium Bromide and Preparation of Antibacterial ABS Composites for Fused Deposition Modeling. Polymers. 2020; 12(10):2229. https://doi.org/10.3390/polym12102229
Chicago/Turabian StyleWang, Yue, Sen Wang, Yaocheng Zhang, Jianguo Mi, and Xuejia Ding. 2020. "Synthesis of Dimethyl Octyl Aminoethyl Ammonium Bromide and Preparation of Antibacterial ABS Composites for Fused Deposition Modeling" Polymers 12, no. 10: 2229. https://doi.org/10.3390/polym12102229





