Design of pH Responsive Textile as a Sensor Material for Acid Rain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabric
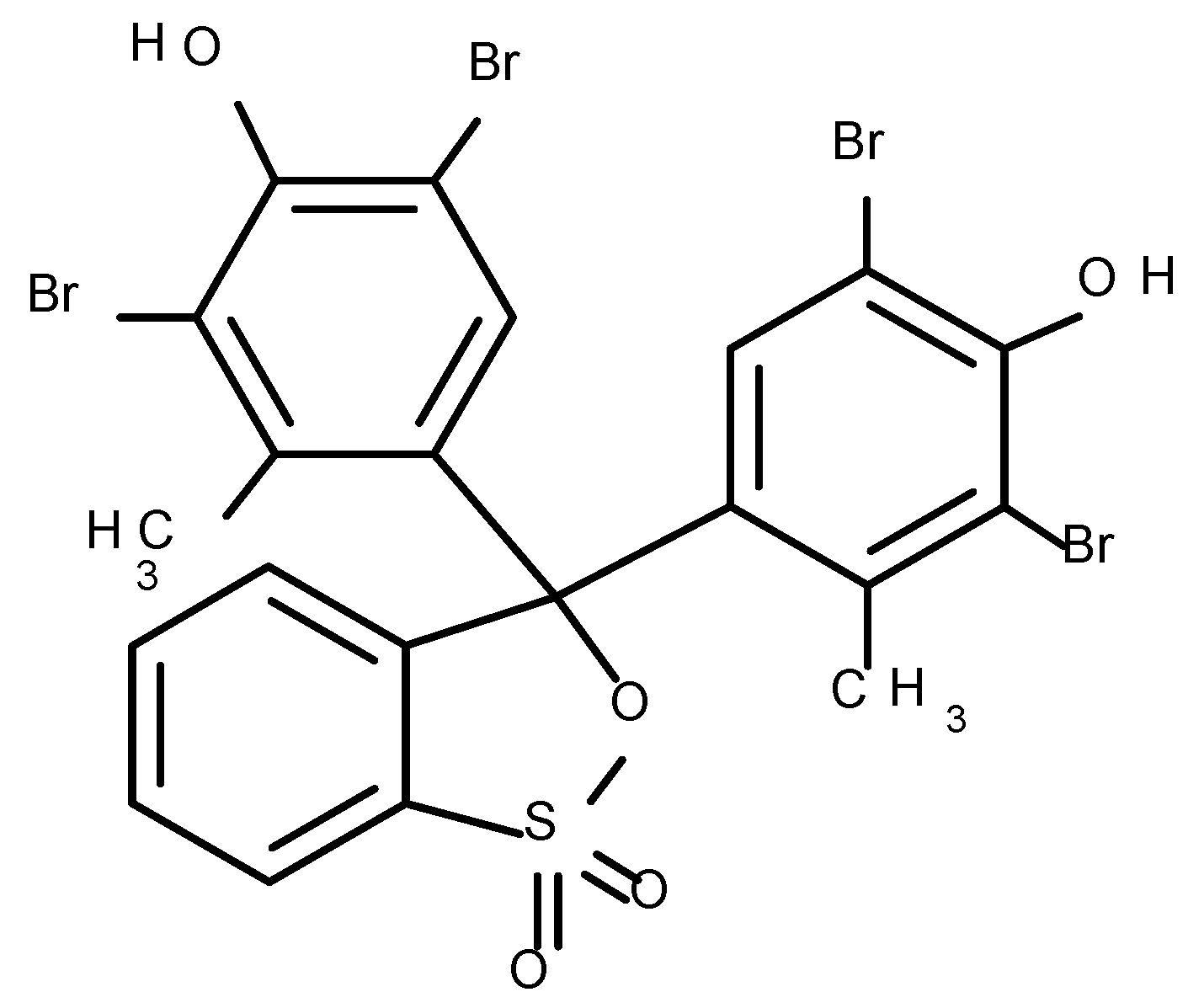
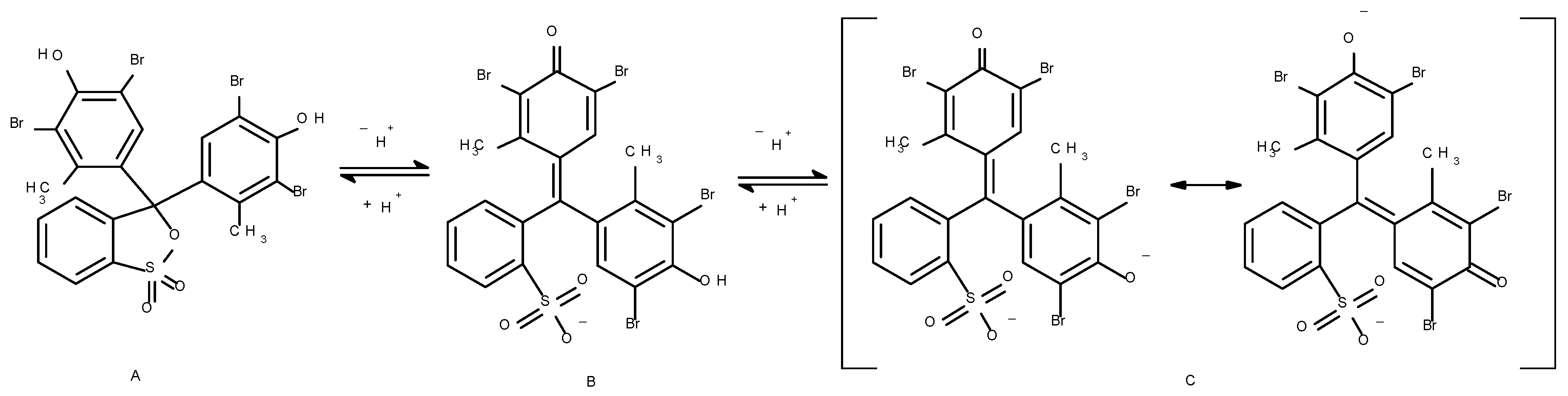
2.2. Dye
2.3. Auxiliaries
2.4. Finishing Agent
2.5. Dyeing
2.6. Finishing
2.7. Methods of Testing
2.7.1. Spectrophotometric Measurements
2.7.2. Colour Fastness to Rubbing
2.7.3. Colour Fastness to Domestic and Commercial Laundering
2.7.4. Colour Fastness to Light
2.7.5. Determination of pH Responsiveness of Unfinished and Finished Dyed Fabric
2.7.6. Determination of Response Time
2.7.7. Water and Oil Repellency
3. Results and Discussion
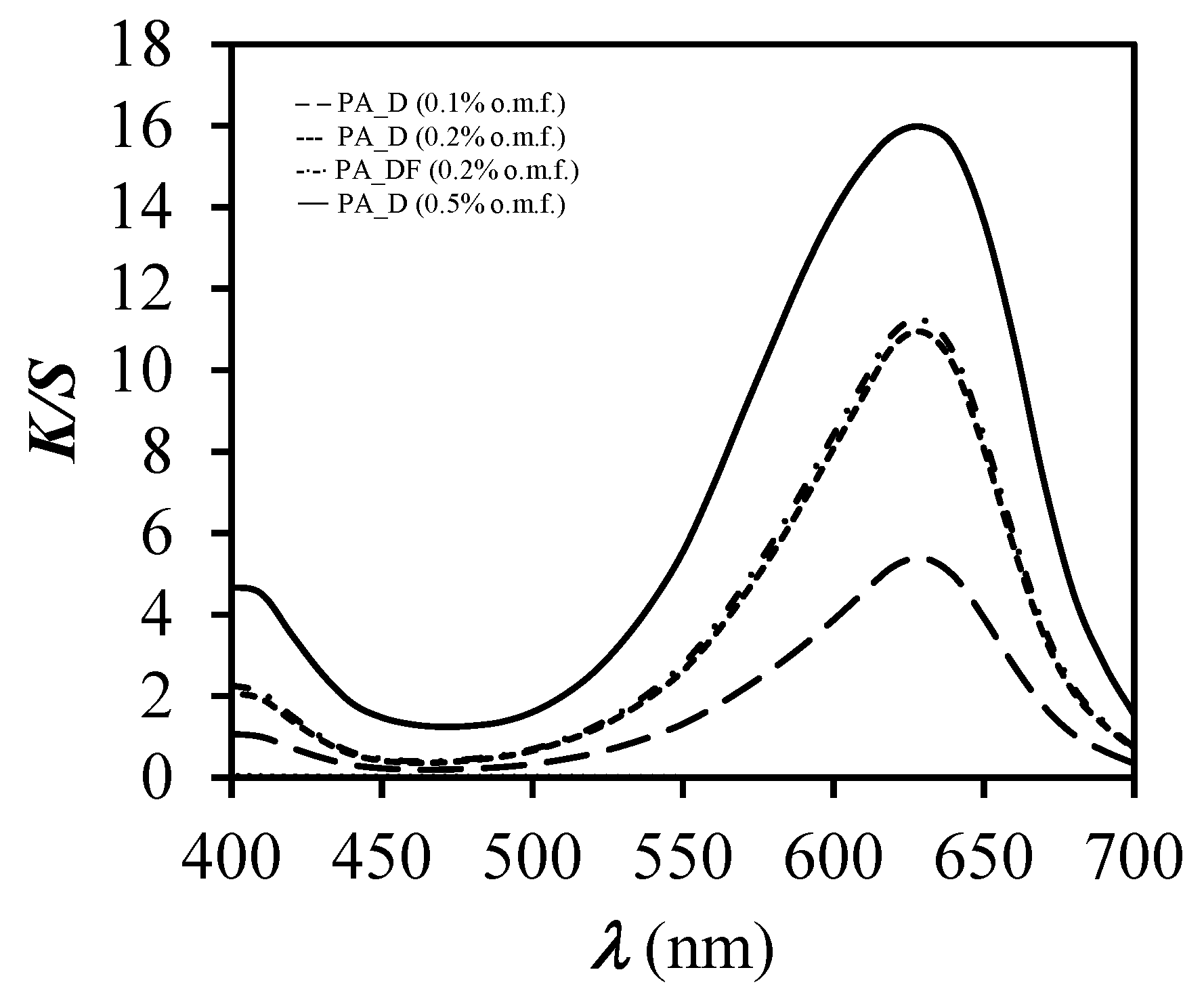
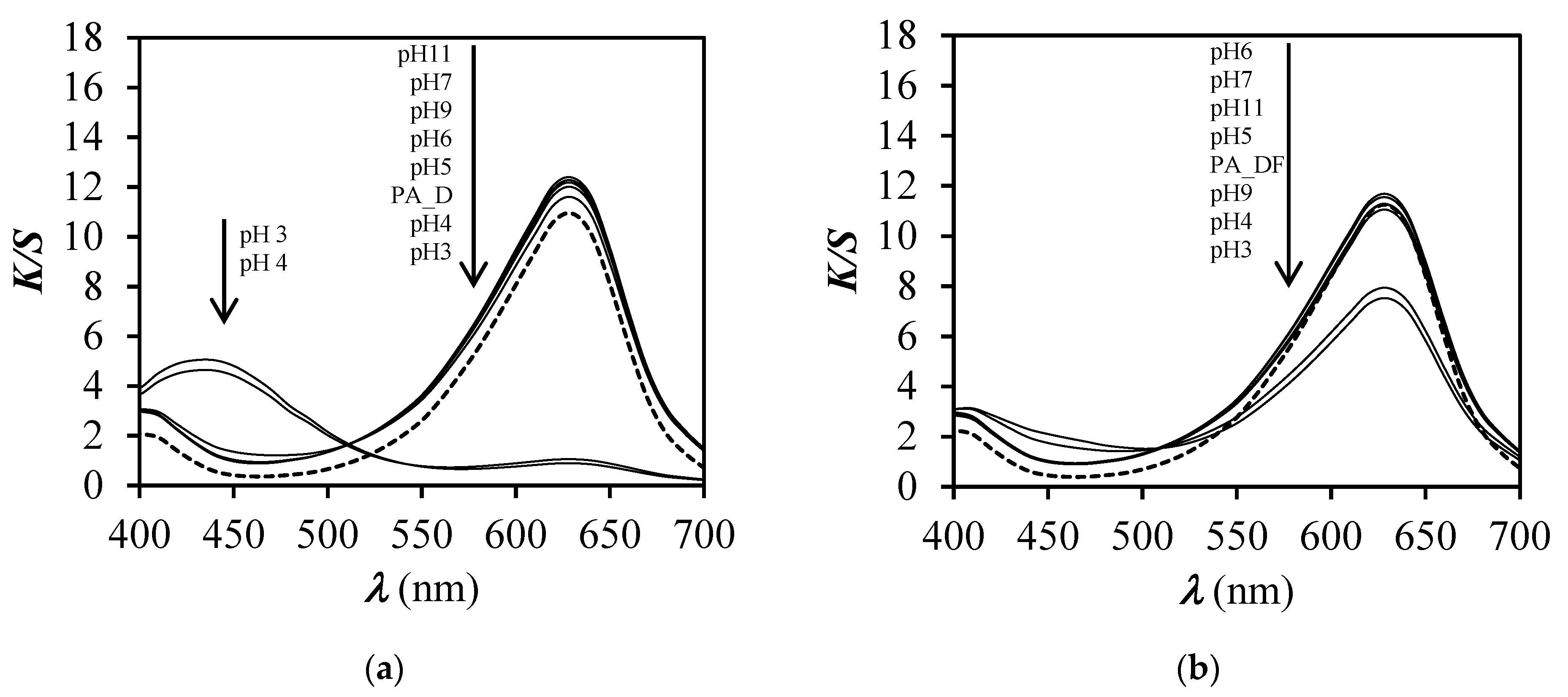
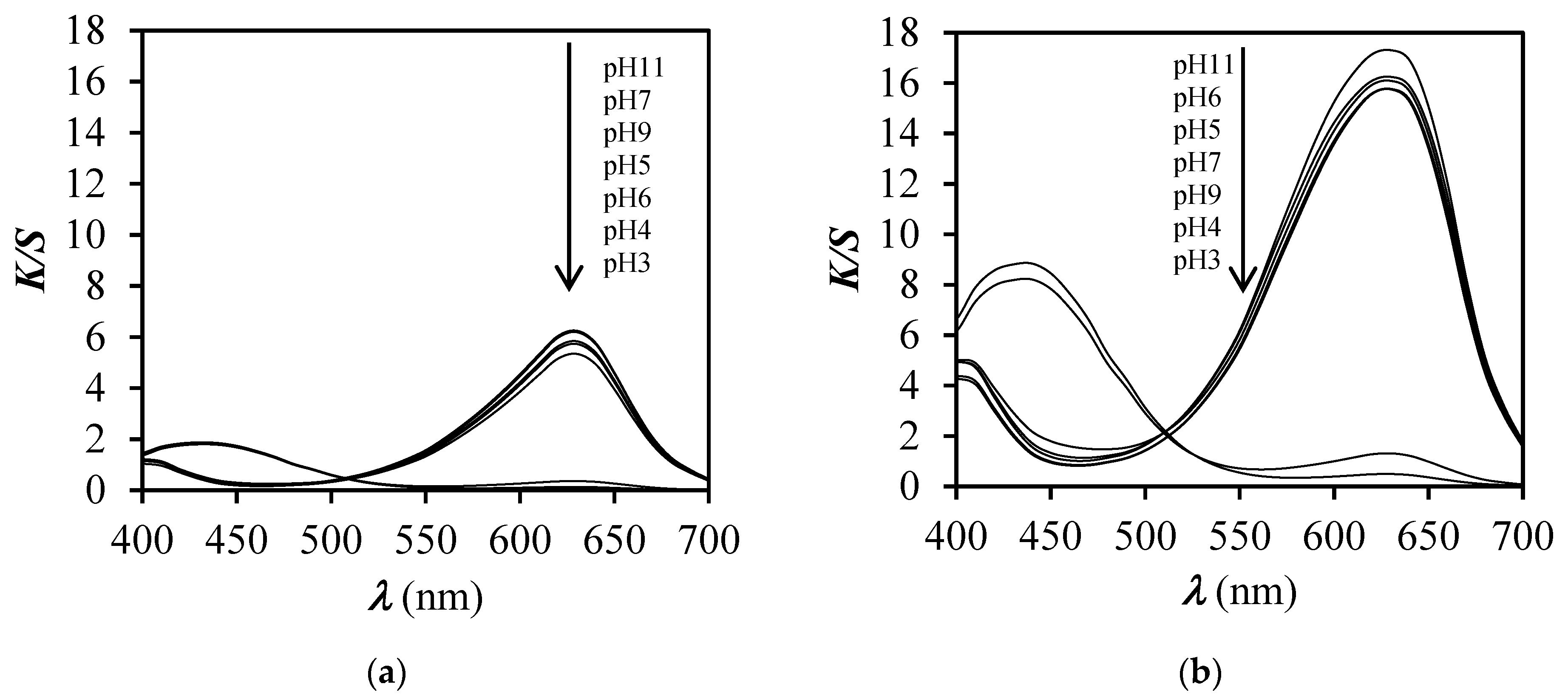
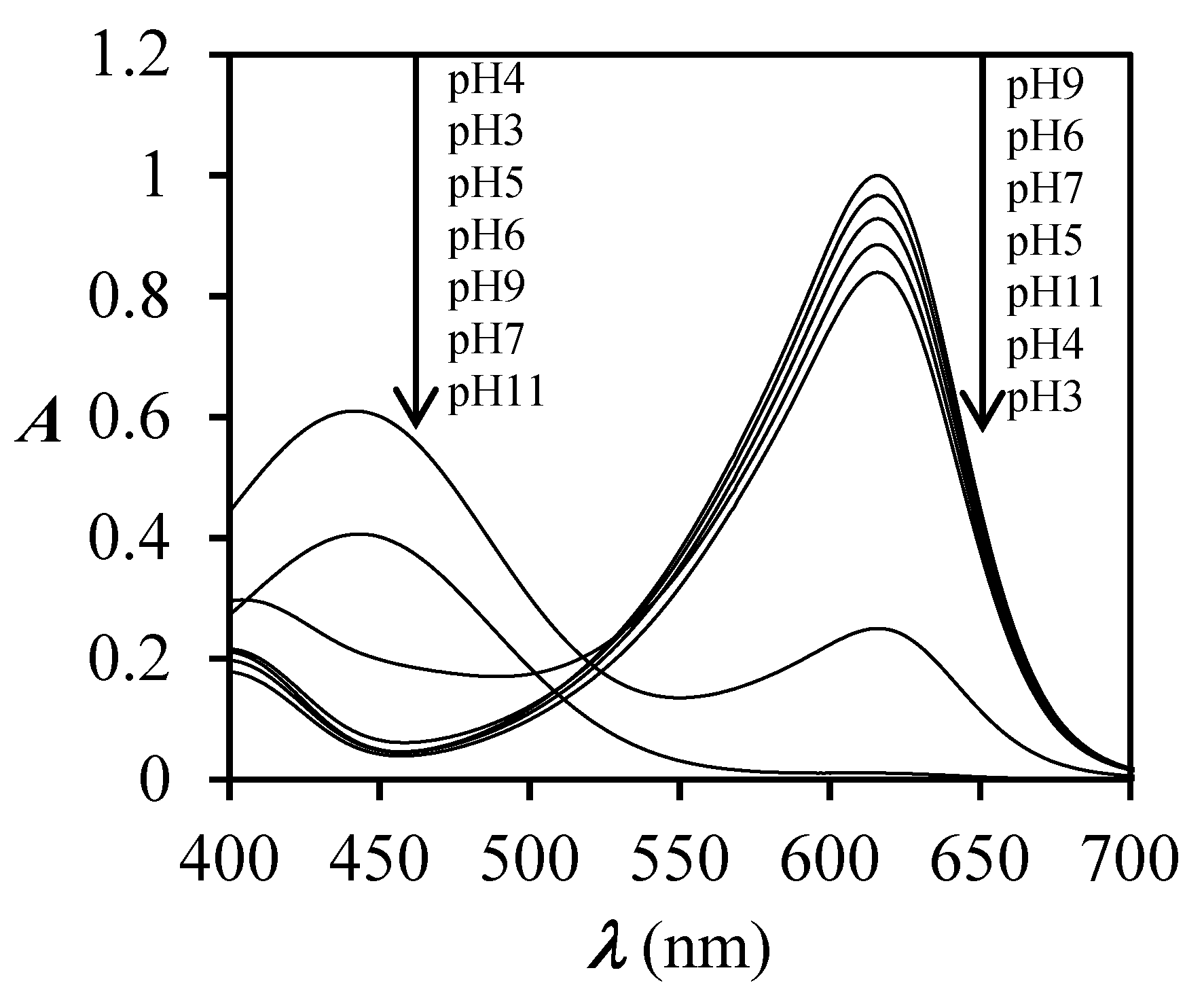
3.1. Spectrophotometric Measurements
3.2. Colour Fastness to Rubbing
3.3. Colour Fastness to Domestic and Commercial Laundering
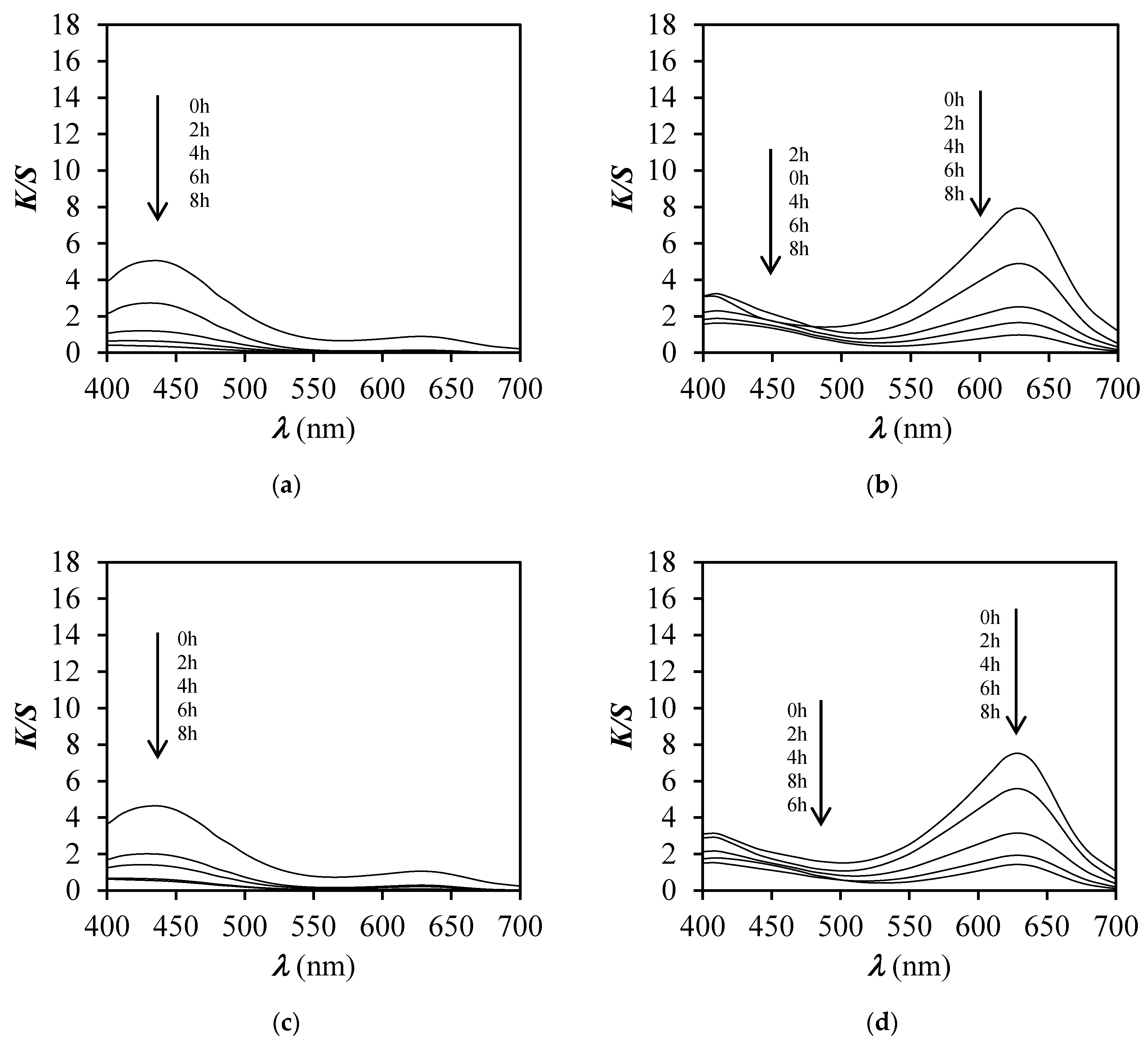
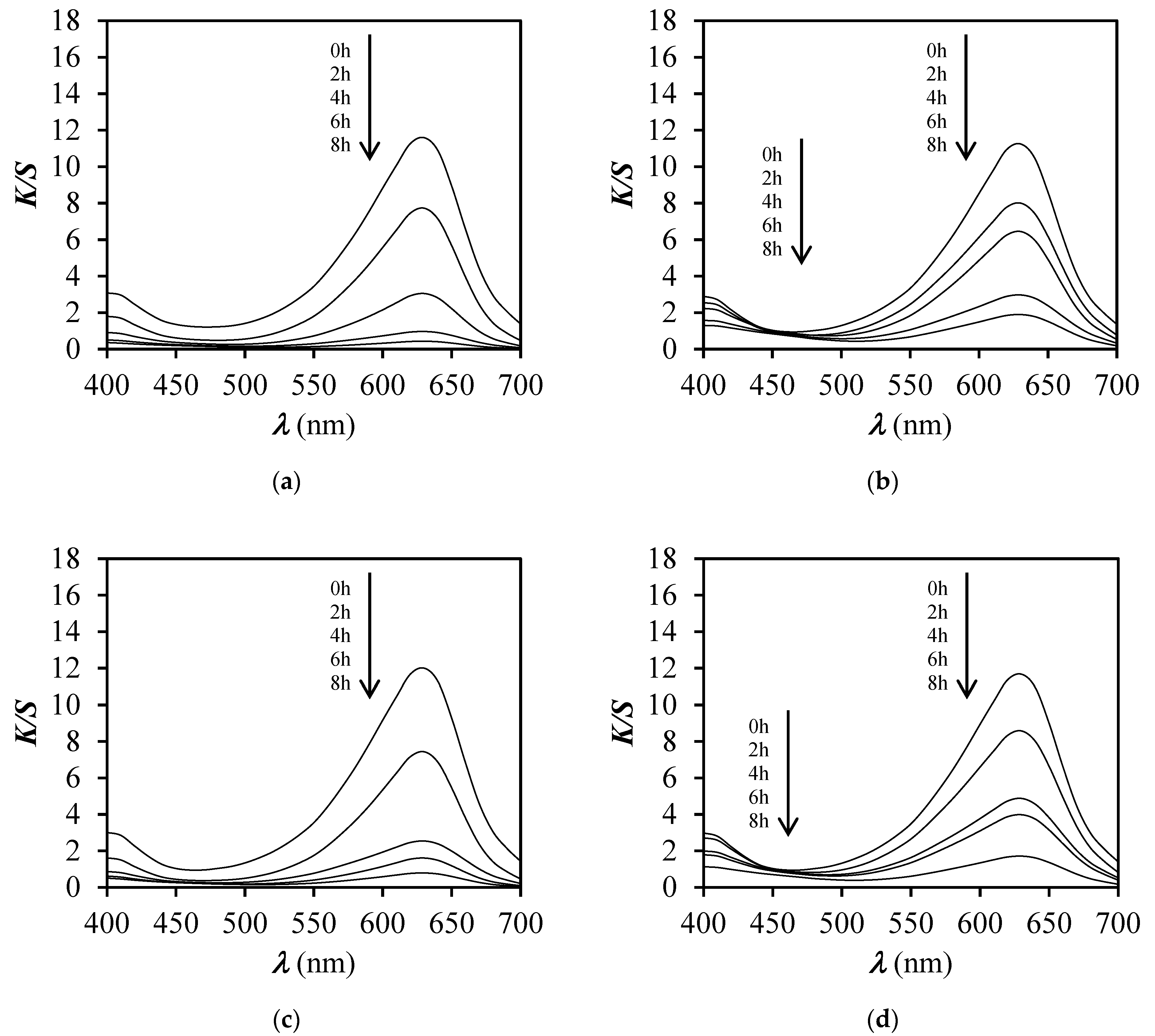
3.4. Colour Fastness to Light
3.5. Response Time and Reversibility Results
3.6. Water and Oil Repellency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grennfelt, P.; Engleryd, A.; Forsium, M.; Hov, Ø.; Rodhe, H.; Cowling, E. Acid rain and air pollution: 50 years of progress in environmental science and policy. Ambio 2020, 49, 849–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourth Report for Policy Makers: Towards Clean Air for Sustainable Future in East Asia through Collaborative Activities; Acid Deposition Monitoring Network in East Asia (EANET): Bangkok, Thailand, 2019; Available online: https://www.eanet.asia (accessed on 7 January 2020).
- Rafaj, P.; Kiesewetter, G.; Gül, T.; Schöpp, W.; Cofala, J.; Klimont, Z.; Purohit, P.; Heyes, C.; Amann, M.; Borken-Kleefeld, J.; et al. Outlook for clean air in the context of sustainable development goals. Glob. Environ. Change 2018, 53, 1–11. [Google Scholar] [CrossRef]
- Honarvar, M.G.; Latifi, M. Overview of wearable electronics and smart textiles. J. Text. Inst. 2017, 108, 631–652. [Google Scholar] [CrossRef]
- Wu, J.X.; Li, L. An introduction to wearable technology and smart textiles and apparel: Terminology, statistics, evolution, and challenges. In Smart and Functional Soft Materials; Dong, X., Ed.; Intech Open: London, UK, 2019; p. 11. [Google Scholar]
- Vik, M.; Periyasamy, A.P. Chromic materials: Fundamentals, Measurements, and Applications; Viková, M., Ed.; Apple Academic Press: New York, NY, USA, 2018; p. 422. [Google Scholar]
- Christie, R.M. Chromic materials for technical textile applications. In Advances in the Dyeing and Finishing of Technical Textiles; Woodhead Publishing: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2013; pp. 3–36. [Google Scholar]
- Bamfield, P. Chromic Phenomena: Technological Applications of Colour Chemistry; Royal Society of Chemistry: Cambridge, UK, 2001; pp. 1–8. [Google Scholar]
- Van der Schueren, L.; De Clerck, K.; Brancatelli, G.; Rosace, G.; Van Damme, E.; De Vos, W. Novel cellulose and polyamide halochromic textile sensors based on the encapsulation of Methyl Red into a sol-gel matrix. Sens. Actuator B Chem. 2012, 162, 27–34. [Google Scholar] [CrossRef]
- Steyaert, I.; Vancoillie, G.; Hoogenboom, R.; De Clerck, K. Dye immobilization in halochromic nanofibers through blend electrospinning of a dye-containing copolymer and polyamide-6. Polym. Chem. 2015, 6, 2685–2694. [Google Scholar] [CrossRef]
- De Meyer, T.; Steyaert, I.; Hemelsoet, K.; Hoogenboom, R.; Van Speybroeck, V.; De Clerck, K. Halochromic properties of sulfonphthaleine dyes in a textile environment: The influence of substituents. Dye. Pigment. 2016, 124, 249–257. [Google Scholar] [CrossRef]
- De Clerck, K.; Geltmeyer, J.; Steyaert, I.; Van der Schueren, L. Halochromic textile materials as innovative pH-sensors. Adv. Sci. Technol. 2012, 80, 47–52. [Google Scholar]
- Van der Schueren, L.; De Clerck, K. Coloration and application of pH-sensitive dyes on textile materials. Coloration Technol. 2012, 128, 82–90. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Clerck, K. The use of pH-indicator dyes for pH-sensitive textile materials. Text. Res. J. 2010, 80, 590–603. [Google Scholar] [CrossRef]
- Mohr, G.J.; Müller, H. Tailoring colour changes of optical sensor materials by combining indicator and inert dyes and their use in sensor layers, textiles and non-wovens. Sens. Actuator B Chem. 2015, 206, 788–793. [Google Scholar] [CrossRef]
- Kassal, P.; Zubak, M.; Scheipl, G.; Mohr, G.J.; Steinberg, M.D.; Murković Steinberg, I. Smart bandage with wireless connectivity for optical monitoring of pH. Sens. Actuator B Chem. 2017, 246, 455–460. [Google Scholar] [CrossRef]
- Agarwal, A.; Raheja, A.; Natarajan, T.S.; Chandra, T.S. Development of universal pH sensing electrospun nanofibers. Sens. Actuators B Chem. 2012, 161, 1097–1101. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Clerck, K. Textile materials with a pH-sensitive function. Int. J. Cloth. Sci. Technol. 2011, 23, 269–274. [Google Scholar] [CrossRef]
- Pallás, I.; Marcos, M.D.; Martínez-Máñez, R.; Ros-Lis, J.V. Development of a textile nanocomposite as naked eye indicator of the exposition to strong acids. Sensors 2017, 17, 2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, J.G. Polyamide fibers. In Handbook of Textile Fibres; Woodhead Publishing: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2001; pp. 194–326. [Google Scholar]
- Vasanthan, N. 7–Polyamide fiber formation: Structure, properties and characterization. In Handbook of Textile Fibre Structure; Woodhead Publishing: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2009; pp. 232–256. [Google Scholar]
- Kothari, V.K. Polyester and polyamide fibres–apparel applications. In Polyesters and Polyamides; Woodhead Publishing: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2008; pp. 419–440. [Google Scholar]
- Textile Exchange. Preferred Fiber and Materials Market Report [Online]. 2019. Available online: https://store.textileexchange.org/product/2019-preferred-fiber-materials-report/ (accessed on 20 February 2020).
- Zhang, F.; Chen, Y.; Lin, H.; Lu, Y. Synthesis of an amino-terminated hyperbranched polymer and its application in reactive dyeing on cotton as a salt-free dyeing auxiliary. Coloration Technol. 2007, 123, 351–357. [Google Scholar] [CrossRef]
- Weather Atlas. Monthly Weather Forecast and Climate: Ljubljana, Slovenia. Available online: https://www.weather-atlas.com/en/slovenia/ljubljana-climate (accessed on 10 March 2020).
- Slovenian Environment Agency. Online Data of Ljubljana Rainfall Obtained from for the Year 2019. Available online: https://meteo.arso.gov.si/met/sl/app/webmet/ (accessed on 20 February 2020).


| Sample | cd (% o.m.f.) | pH | L* | a* | b* |
|---|---|---|---|---|---|
| PA | / | / | 93.73 | −0.01 | 2.37 |
| 0.1 | / | 57.26 | −21.08 | −29.41 | |
| 3 | 79.14 | −0.37 | 44.16 | ||
| 4 | 74.78 | −8.02 | 37.62 | ||
| 5 | 56.07 | −22.05 | −27.46 | ||
| 6 | 57.45 | −19.96 | −31.54 | ||
| 7 | 55.74 | −19.69 | −32.45 | ||
| 9 | 56.16 | −19.52 | −32.08 | ||
| 11 | 55.21 | −19.53 | −31.99 | ||
| PA_D | 0.2 | / | 48.27 | −19.23 | −32.76 |
| 3 | 70.53 | 1.24 | 50.92 | ||
| 4 | 69.58 | −2.63 | 46.72 | ||
| 5 | 47.40 | −23.00 | −25.53 | ||
| 6 | 47.91 | −19.65 | −32.22 | ||
| 7 | 47.77 | −18.94 | −33.35 | ||
| 9 | 47.91 | −18.99 | −33.14 | ||
| 11 | 47.78 | −18.54 | −34.16 | ||
| 0.5 | / | 37.23 | −18.35 | −24.10 | |
| 3 | 62.98 | 8.74 | 56.89 | ||
| 4 | 56.95 | −3.90 | 44.81 | ||
| 5 | 36.24 | −19.08 | −21.45 | ||
| 6 | 36.53 | −16.09 | −26.76 | ||
| 7 | 38.28 | −15.38 | −30.11 | ||
| 9 | 38.52 | −14.97 | −30.70 | ||
| 11 | 36.62 | −15.28 | −28.94 | ||
| PA_DF | 0.2 | / | 47.47 | −19.31 | −32.23 |
| 3 | 49.57 | −24.67 | −14.51 | ||
| 4 | 50.20 | −26.90 | −8.03 | ||
| 5 | 48.82 | −20.33 | −31.20 | ||
| 6 | 48.16 | −19.71 | −31.92 | ||
| 7 | 48.22 | −19.50 | −32.03 | ||
| 9 | 48.83 | −19.95 | −31.19 | ||
| 11 | 48.91 | −19.68 | −32.44 |
| Sample | Visual Assessment Using Grey Scale | |
|---|---|---|
| Staining of Dry Cotton Fabric | Staining of Wet Cotton Fabric | |
| PA_D–warp direction | 5 | 5 |
| PA_D–weft direction | 5 | 5 |
| PA_DF–warp direction | 5 | 5 |
| PA_DF–weft direction | 5 | 5 |
| Sample | Visual Assessment Using Grey Scale | ||
|---|---|---|---|
| Change of Colour | Staining of Polyamide Fabric | Staining of Cotton Fabric | |
| PA_D_1w | 4/5 | 4/5 | 5 |
| PA_D_10w | 4/5 | 4/5 | 5 |
| PA_DF_1w | 5 | 4/5 | 5 |
| PA_DF_10w | 4/5 | 4 | 4/5 |
| Sample | Test Number of Liquid Hydrocarbon * |
|---|---|
| PA_D | 1 |
| PA_DF | 6 |
| PA_T_1w | 4 |
| PA_DF_10w | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojkoski, V.; Kert, M. Design of pH Responsive Textile as a Sensor Material for Acid Rain. Polymers 2020, 12, 2251. https://doi.org/10.3390/polym12102251
Stojkoski V, Kert M. Design of pH Responsive Textile as a Sensor Material for Acid Rain. Polymers. 2020; 12(10):2251. https://doi.org/10.3390/polym12102251
Chicago/Turabian StyleStojkoski, Viktor, and Mateja Kert. 2020. "Design of pH Responsive Textile as a Sensor Material for Acid Rain" Polymers 12, no. 10: 2251. https://doi.org/10.3390/polym12102251





