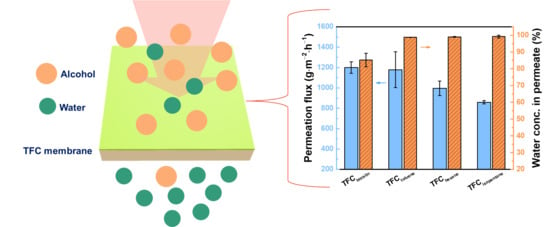
Surface Properties, Free Volume, and Performance for Thin-Film Composite Pervaporation Membranes Fabricated through Interfacial Polymerization Involving Different Organic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
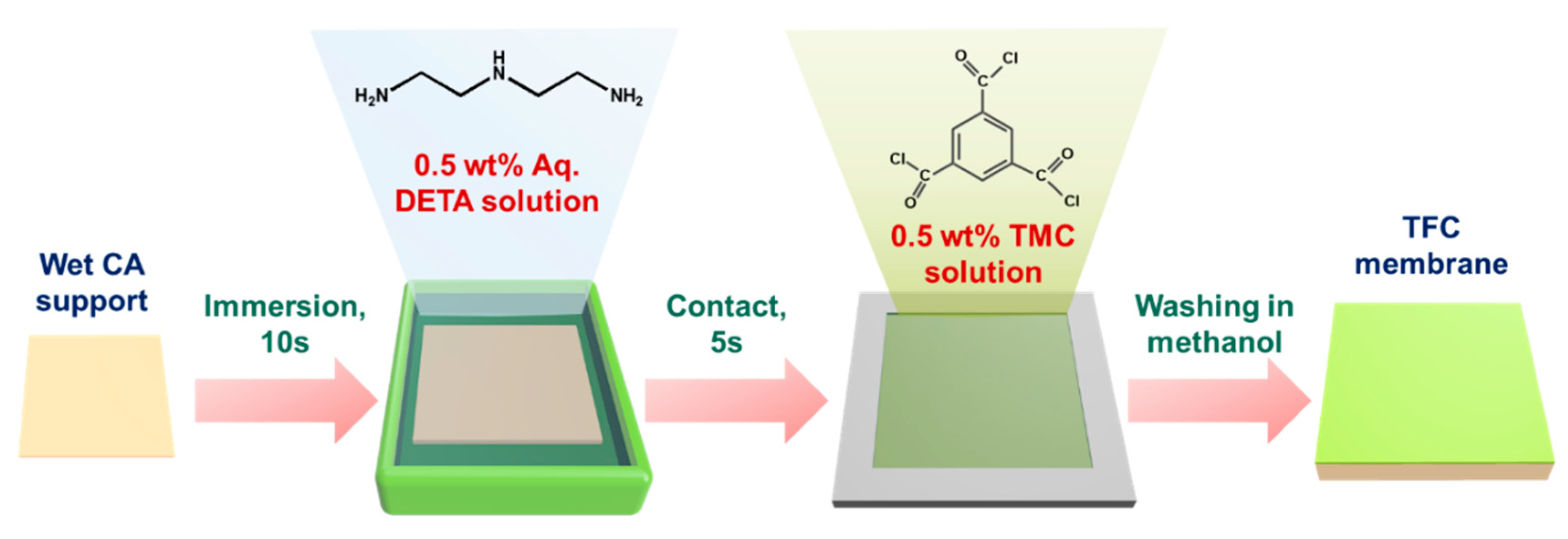
2.2. Fabrication of Thin-Film Composite Membrane
2.3. Characterization
2.4. Pervaporation Experiment
3. Results and Discussion
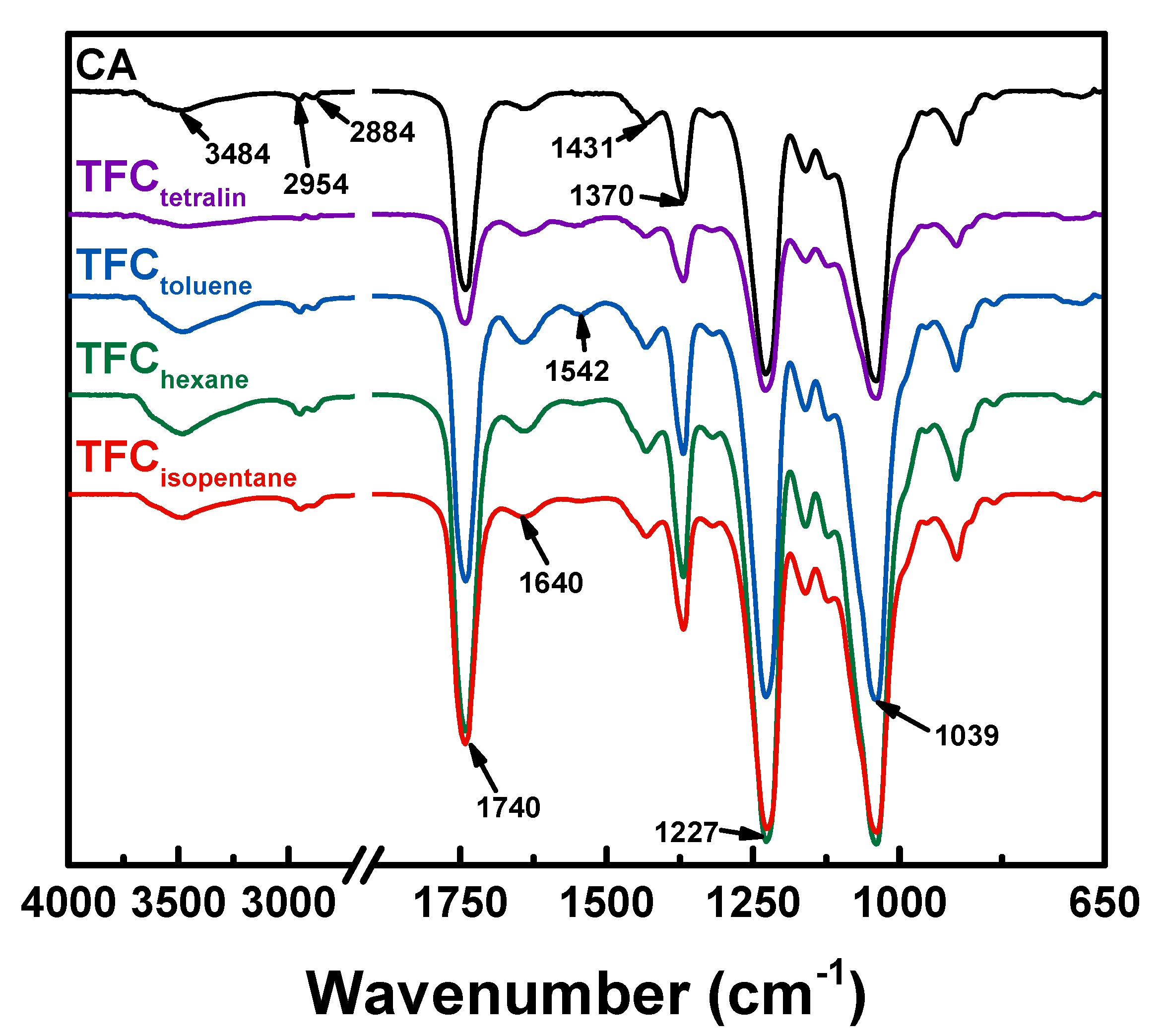
3.1. Surface Chemical Analysis
3.2. Morphology, Surface Roughness and Hydrophilicity
3.3. Free Volume Analysis Using Variable Monoenergy Slow Positron Beam
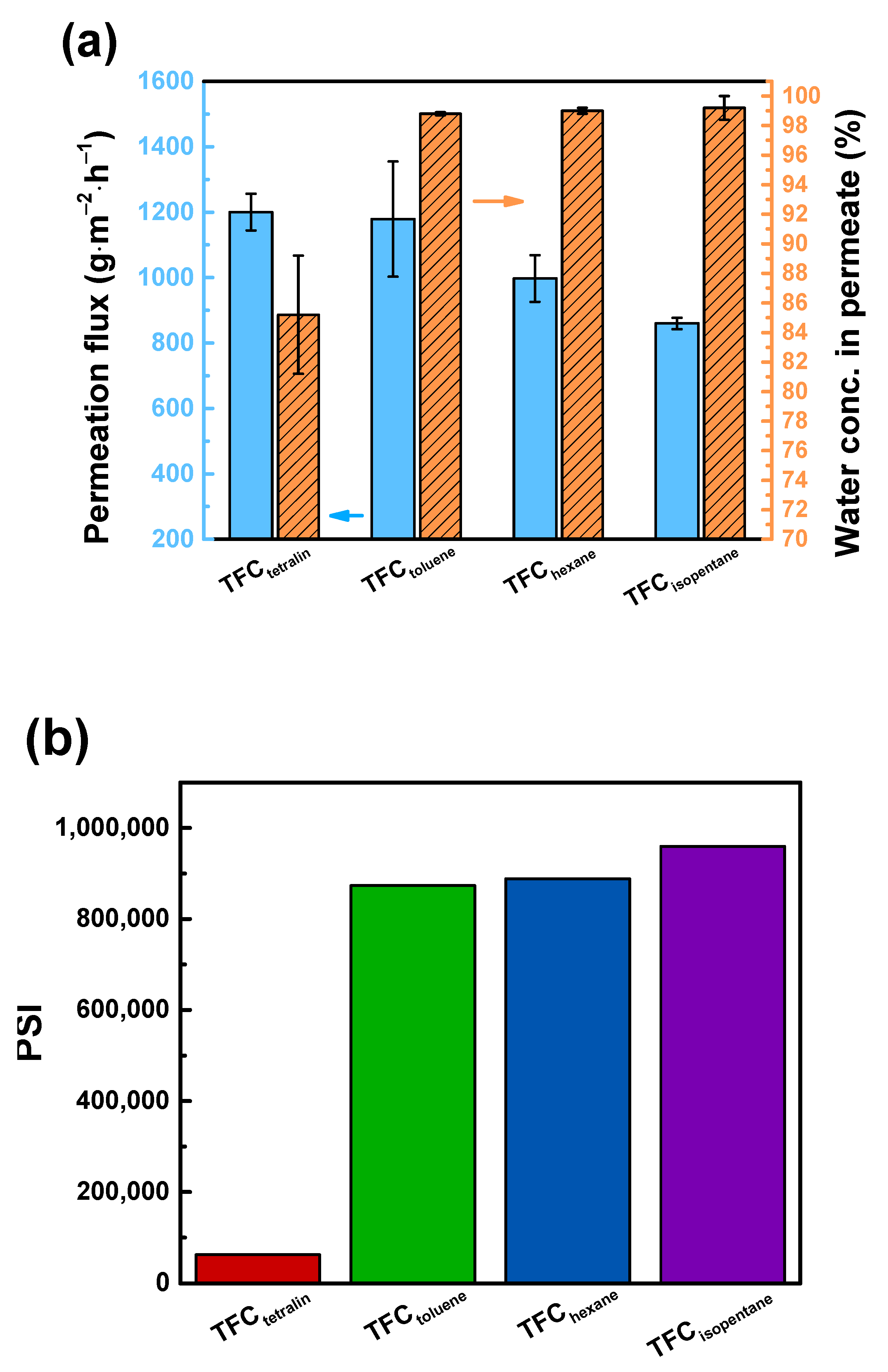
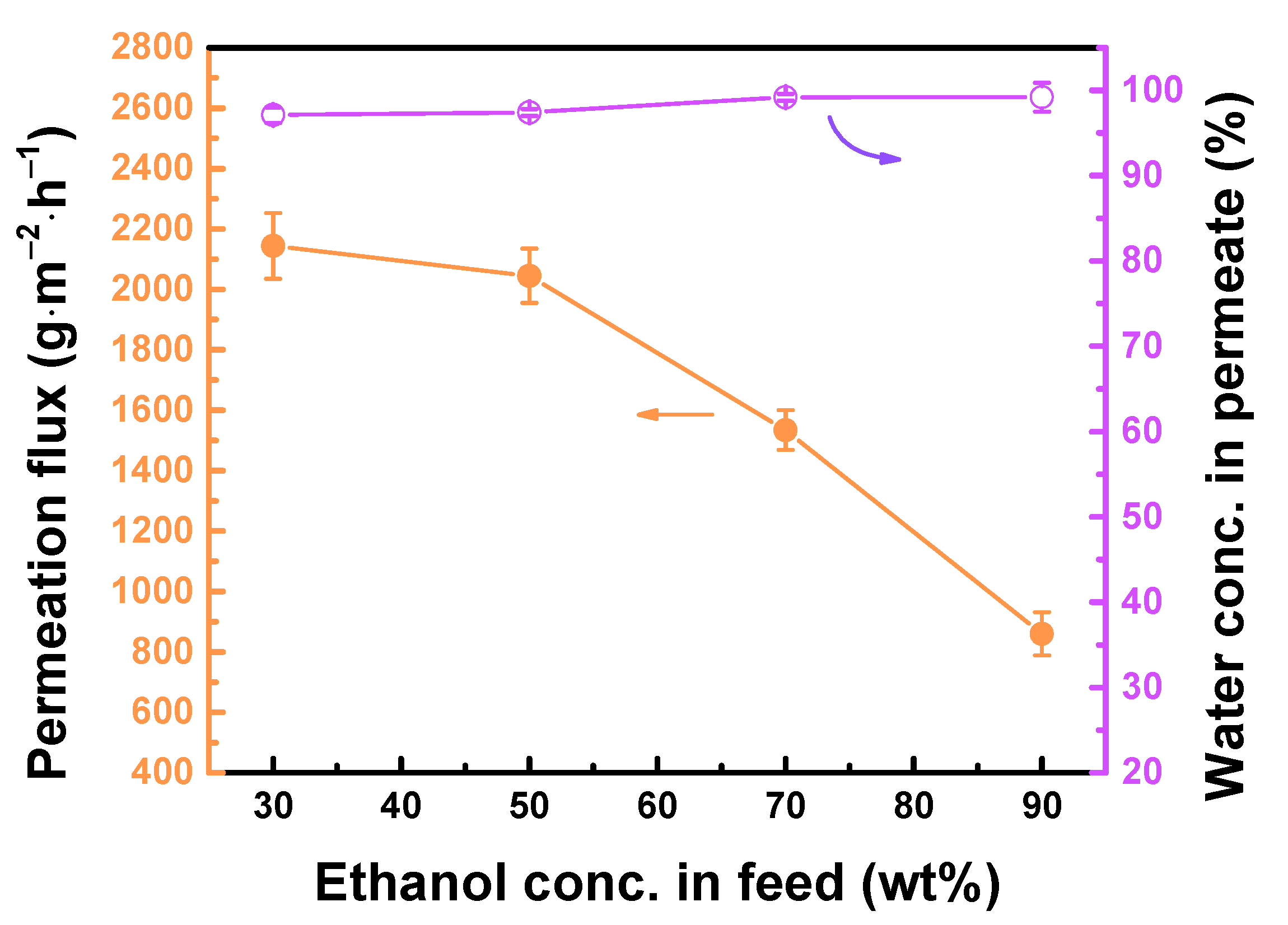
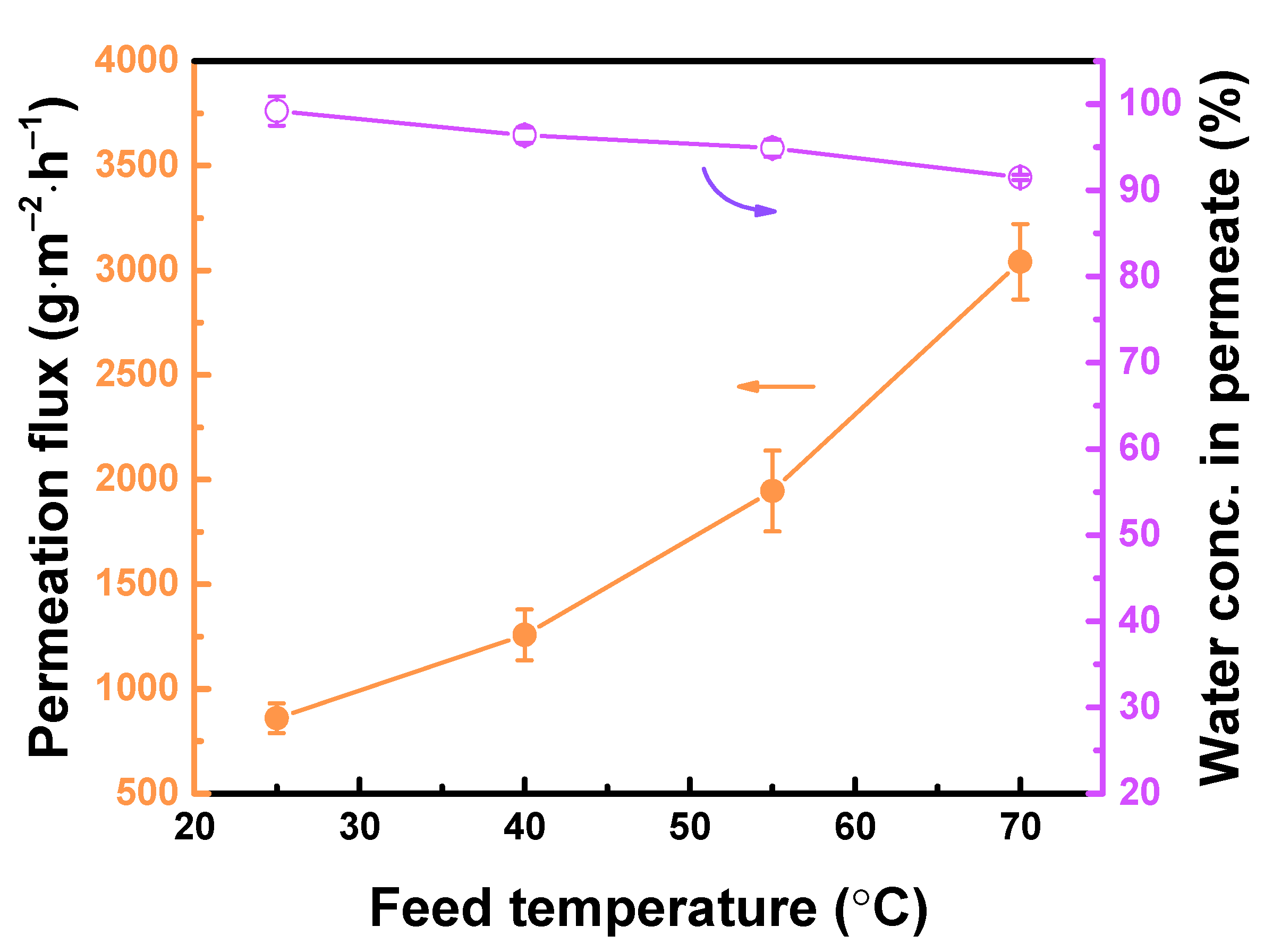
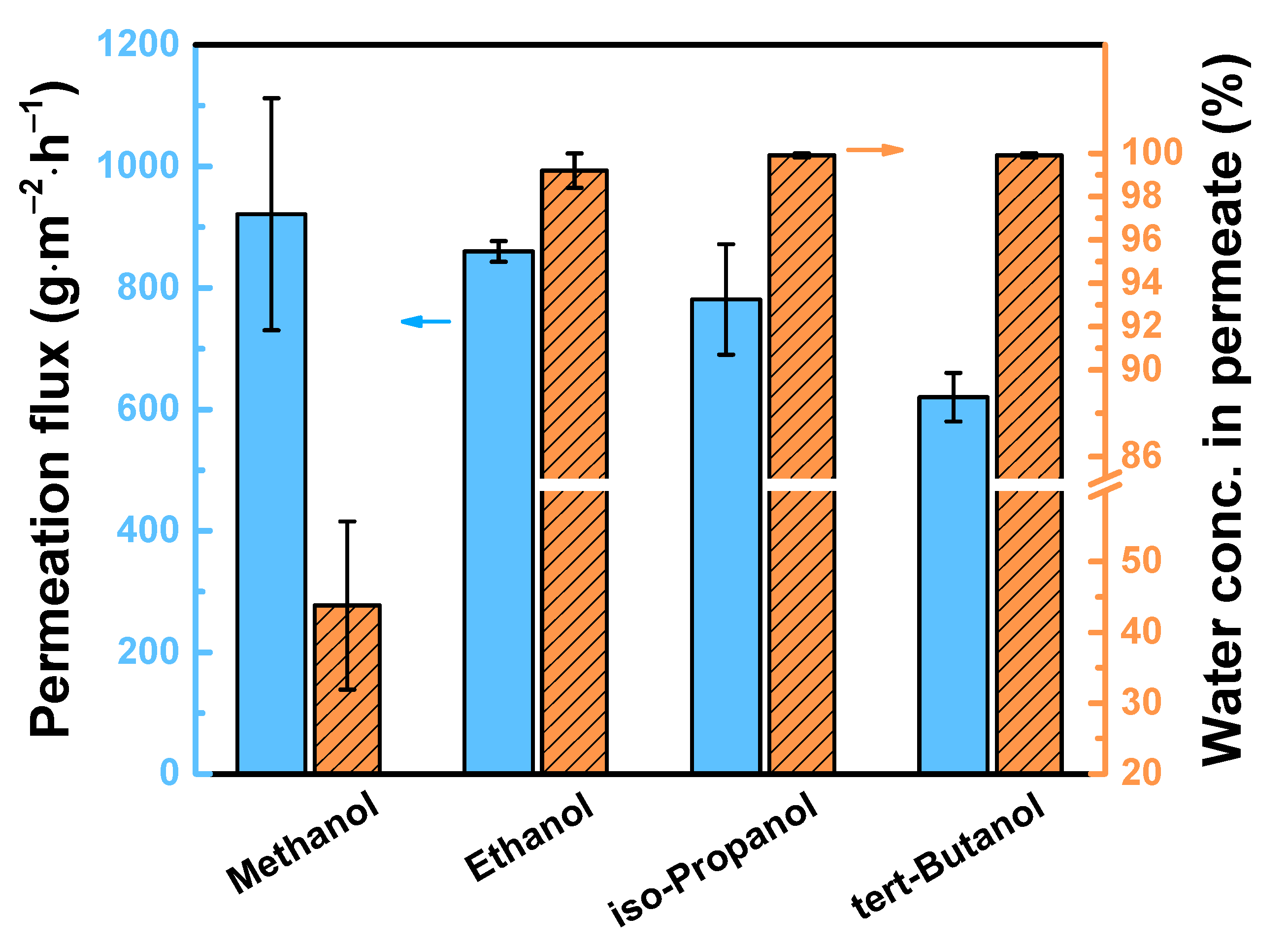
3.4. Performance of TFC Pervaporation Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Wang, Y.; Wu, L.; Wei, G. Fabrication, properties, performances, and separation application of polymeric pervaporation membranes: A review. Polymers 2020, 12, 1466. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, G.; Ye, H.; Jin, W.; Cui, Z. Two-dimensional mxene incorporated chitosan mixed-matrix membranes for efficient solvent dehydration. J. Membr. Sci. 2018, 563, 625–632. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Valdez, J.; Ahmad, M.Z. High-performance pervaporation chitosan-based membranes: New insights and perspectives. Rev. Chem. Eng. 2020, 20190051, published online ahead of print. [Google Scholar] [CrossRef]
- Du, J.R.; Hsu, L.H.; Xiao, E.S.; Guo, X.; Zhang, Y.; Feng, X. Using genipin as a “green” crosslinker to fabricate chitosan membranes for pervaporative dehydration of isopropanol. Sep. Purif. Technol. 2020, 244, 116843. [Google Scholar] [CrossRef]
- Veerapur, R.S.; Gudasi, K.B.; Patil, M.B.; Babu, V.R.; Bhat, S.D.; Sairam, M.; Aminabhavi, T.M. Sodium alginate–poly(hydroxyethylmethacrylate) interpenetrating polymeric network membranes for the pervaporation dehydration of ethanol and tetrahydrofuran. J. Appl. Polym. Sci. 2006, 101, 3324–3329. [Google Scholar] [CrossRef]
- Mokhtarzadeh, S.; Hakimpour, F.; Sarvari, R.; Agbolaghi, S.; Mansourpanah, Y. Nanocomposite membranes based on sodium alginate/poly(ε-caprolactone)/graphene oxide for methanol, ethanol and isopropanol dehydration via pervaporation. Polym. Bull. 2020, 77, 3367–3387. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; He, G.; Xing, R.; Pan, F.; Jiang, Z.; Zhang, P.; Cao, X.; Wang, B. Incorporating zwitterionic graphene oxides into sodium alginate membrane for efficient water/alcohol separation. ACS Appl. Mater. Interfaces 2016, 8, 2097–2103. [Google Scholar] [CrossRef]
- Chaudhari, S.; Kwon, Y.S.; Shon, M.Y.; Nam, S.E.; Park, Y. Surface-modified polyvinyl alcohol (PVA) membranes for pervaporation dehydration of epichlorohydrin (ECH), isopropanol (IPA), and water ternary feed mixtures. J. Ind. Eng. Chem. 2020, 81, 185–195. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Chaudhari, S.; Kim, C.E.; Son, D.H.; Park, J.H.; Moon, M.J.; Shon, M.Y.; Park, Y.; Nam, S.E. Ag-exchanged nay zeolite introduced polyvinyl alcohol/polyacrylic acid mixed matrix membrane for pervaporation separation of water/isopropanol mixture. RSC Adv. 2018, 8, 20669–20678. [Google Scholar] [CrossRef] [Green Version]
- Sajjan, A.M.; Jeevan Kumar, B.K.; Kittur, A.A.; Kariduraganavar, M.Y. Development of novel grafted hybrid pva membranes using glycidyltrimethylammonium chloride for pervaporation separation of water–isopropanol mixtures. J. Ind. Eng. Chem. 2013, 19, 427–437. [Google Scholar] [CrossRef]
- Morgan, P.W. Condensation Polymers: By Interfacial and Solution Methods; Interscience Publishers: New York, NY, USA, 1965; Volume 10. [Google Scholar]
- Raaijmakers, M.J.; Benes, N.E. Current trends in interfacial polymerization chemistry. Prog. Polym. Sci. 2016, 63, 86–142. [Google Scholar] [CrossRef]
- An, Q.F.; Ang, M.B.M.Y.; Huang, Y.H.; Huang, S.H.; Chia, Y.H.; Lai, C.L.; Tsai, H.A.; Hun, W.S.; Hu, C.C.; Wu, Y.P.; et al. Microstructural characterization and evaluation of pervaporation performance of thin-film composite membranes fabricated through interfacial polymerization on hydrolyzed polyacrylonitrile substrate. J. Membr. Sci. 2019, 583, 31–39. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhao, Y.Y.; Wang, X.M.; Wen, X.H.; Huang, X.; Xie, Y.F. Effect of varying piperazine concentration and post-modification on prepared nanofiltration membranes in selectively rejecting organic micropollutants and salts. J. Membr. Sci. 2019, 582, 274–283. [Google Scholar] [CrossRef]
- Chiao, Y.H.; Sengupta, A.; Chen, S.T.; Huang, S.H.; Hu, C.C.; Hung, W.S.; Chang, Y.; Qian, X.; Wickramasinghe, S.R.; Lee, K.R.; et al. Zwitterion augmented polyamide membrane for improved forward osmosis performance with significant antifouling characteristics. Sep. Purif. Technol. 2019, 212, 316–325. [Google Scholar] [CrossRef]
- Marquez, J.A.D.; Ang, M.B.M.Y.; Doma, B.T.; Huang, S.H.; Tsai, H.A.; Lee, K.R.; Lai, J.Y. Application of cosolvent-assisted interfacial polymerization technique to fabricate thin-film composite polyamide pervaporation membranes with pvdf hollow fiber as support. J. Membr. Sci. 2018, 564, 722–731. [Google Scholar] [CrossRef]
- Lee, J.; Wang, R.; Bae, T.H. A comprehensive understanding of co-solvent effects on interfacial polymerization: Interaction with trimesoyl chloride. J. Membr. Sci. 2019, 583, 70–80. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Z.; Zhao, S.; Wang, J.; Zhang, P.; Cao, X. Combining co-solvent-optimized interfacial polymerization and protective coating-controlled chlorination for highly permeable reverse osmosis membranes with high rejection. J. Membr. Sci. 2019, 572, 61–72. [Google Scholar] [CrossRef]
- Kim, I.C.; Jegal, J.; Lee, K.H. Effect of aqueous and organic solutions on the performance of polyamide thin-film-composite nanofiltration membranes. J. Polym. Sci. Pt. B-Polym. Phys. 2002, 40, 2151–2163. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Jeong, B.H.; Huang, X.; Hoek, E.M.V. Impacts of reaction and curing conditions on polyamide composite reverse osmosis membrane properties. J. Membr. Sci. 2008, 311, 34–45. [Google Scholar] [CrossRef]
- Park, S.J.; Kwon, S.J.; Kwon, H.E.; Shin, M.G.; Park, S.H.; Park, H.; Park, Y.I.; Nam, S.E.; Lee, J.H. Aromatic solvent-assisted interfacial polymerization to prepare high performance thin film composite reverse osmosis membranes based on hydrophilic supports. Polymer 2018, 144, 159–167. [Google Scholar] [CrossRef]
- Esfandian, F.; Peyravi, M.; Ghoreyshi, A.A.; Jahanshahi, M.; Rad, A.S. Fabrication of tfc nanofiltration membranes via co-solvent assisted interfacial polymerization for lactose recovery. Arab. J. Chem. 2019, 12, 5325–5338. [Google Scholar] [CrossRef]
- Huang, S.H.; Hung, W.S.; Liaw, D.J.; Tsai, H.A.; Jiang, G.J.; Lee, K.R.; Lai, J.Y. Positron annihilation study on thin-film composite pervaporation membranes: Correlation between polyamide fine structure and different interfacial polymerization conditions. Polymer 2010, 51, 1370–1376. [Google Scholar] [CrossRef]
- Tsai, H.A.; Chen, Y.L.; Huang, S.H.; Hu, C.C.; Hung, W.S.; Lee, K.R.; Lai, J.Y. Preparation of polyamide/polyacrylonitrile composite hollow fiber membrane by synchronous procedure of spinning and interfacial polymerization. J. Membr. Sci. 2018, 551, 261–272. [Google Scholar] [CrossRef]
- Chiao, Y.H.; Chen, S.T.; Patra, T.; Hsu, C.H.; Sengupta, A.; Hung, W.S.; Huang, S.H.; Qian, X.; Wickramasinghe, R.; Chang, Y. Zwitterionic forward osmosis membrane modified by fast second interfacial polymerization with enhanced antifouling and antimicrobial properties for produced water pretreatment. Desalination 2019, 469, 114090. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Lau, V., Jr.; Ji, Y.L.; Huang, S.H.; An, Q.F.; Caparanga, A.R.; Tsai, H.A.; Hung, W.S.; Hu, C.C.; Lee, K.R.; et al. Correlating PSf support physicochemical properties with the formation of piperazine-based polyamide and evaluating the resultant nanofiltration membrane performance. Polymers 2017, 9, 505. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Impact of support layer pore size on performance of thin film composite membranes for forward osmosis. J. Membr. Sci. 2015, 483, 25–33. [Google Scholar] [CrossRef]
- Yakavalangi, M.E.; Rimaz, S.; Vatanpour, V. Effect of surface properties of polysulfone support on the performance of thin film composite polyamide reverse osmosis membranes. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hoek, E.M.V. Impacts of support membrane structure and chemistry on polyamide–polysulfone interfacial composite membranes. J. Membr. Sci. 2009, 336, 140–148. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Ji, Y.L.; Huang, S.H.; Lee, K.R.; Lai, J.Y. A facile and versatile strategy for fabricating thin-film nanocomposite membranes with polydopamine-piperazine nanoparticles generated in situ. J. Membr. Sci. 2019, 579, 79–89. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Tang, C.L.; De Guzman, M.R.; Maganto, H.L.C.; Caparanga, A.R.; Huang, S.H.; Tsai, H.A.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Improved performance of thin-film nanofiltration membranes fabricated with the intervention of surfactants having different structures for water treatment. Desalination 2020, 481, 114352. [Google Scholar] [CrossRef]
- Kim, I.C.; Jeong, B.R.; Kim, S.J.; Lee, K.H. Preparation of high flux thin film composite polyamide membrane: The effect of alkyl phosphate additives during interfacial polymerization. Desalination 2013, 308, 111–114. [Google Scholar] [CrossRef]
- Yung, L.; Ma, H.; Wang, X.; Yoon, K.; Wang, R.; Hsiao, B.S.; Chu, B. Fabrication of thin-film nanofibrous composite membranes by interfacial polymerization using ionic liquids as additives. J. Membr. Sci. 2010, 365, 52–58. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Huang, S.H.; Li, Y.C.; Cahatol, A.T.C.; Tayo, L.L.; Hung, W.S.; Tsai, H.A.; Hu, C.C.; Lee, K.R.; Lai, J.Y. High-performance thin-film composite polyetheramide membranes for the dehydration of tetrahydrofuran. J. Membr. Sci. 2020, 611, 118373. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, G.; Zhang, H.; Yang, F. Exploration of permeability and antifouling performance on modified cellulose acetate ultrafiltration membrane with cellulose nanocrystals. Carbohydr. Polym. 2017, 174, 190–199. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Huang, C.H.; Liu, Y.L. Self-healing polymeric materials for membrane separation: An example of a polybenzimidazole-based membrane for pervaporation dehydration on isopropanol aqueous solution. RSC Adv. 2017, 7, 38360–38366. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.H.; Huang, S.H.; Chao, W.C.; Li, C.L.; Hsieh, Y.Y.; Hung, W.-S.; Liaw, D.J.; Hu, C.C.; Lee, K.R.; Lai, J.Y. A study on the characteristics and pervaporation performance of polyamide thin-film composite membranes with modified polyacrylonitrile as substrate for bioethanol dehydration. Polym. Int. 2014, 63, 1478–1486. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Huang, S.H.; Chang, M.W.; Lai, C.L.; Tsai, H.A.; Hung, W.S.; Hu, C.C.; Lee, K.R. Ultraviolet-initiated graft polymerization of acrylic acid onto thin-film polyamide surface for improved ethanol dehydration performance of pervaporation membranes. Sep. Purif. Technol. 2020, 235, 116155. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A.; Fotouhi, M. Preparation and characterization of thin film nanocomposite membrane for pervaporative dehydration of aqueous alcohol solutions. Desalination 2013, 314, 20–27. [Google Scholar] [CrossRef]
- Xie, H.R.; Ji, C.H.; Xue, S.M.; Xu, Z.L.; Yang, H.; Ma, X.H. Enhanced pervaporation performance of sa-pfsa/ceramic hybrid membranes for ethanol dehydration. Sep. Purif. Technol. 2018, 206, 218–225. [Google Scholar] [CrossRef]
- Zuo, J.; Lai, J.Y.; Chung, T.S. In-situ synthesis and cross-linking of polyamide thin film composite (tfc) membranes for bioethanol applications. J. Membr. Sci. 2014, 458, 47–57. [Google Scholar] [CrossRef]
- Xia, L.L.; Li, C.L.; Wang, Y. In-situ crosslinked PVA/organosilica hybrid membranes for pervaporation separations. J. Membr. Sci. 2016, 498, 263–275. [Google Scholar] [CrossRef]




| Membrane | C (%) | O (%) | N (%) | N/O |
|---|---|---|---|---|
| CA | 54.93 | 45.07 | − | − |
| TFCtetralin | 68.42 | 21.06 | 10.52 | 0.4995 |
| TFCtoluene | 67.50 | 23.80 | 8.70 | 0.3655 |
| TFChexane | 59.20 | 32.21 | 8.60 | 0.2670 |
| TFCisopentane | 55.19 | 40.65 | 4.17 | 0.1026 |
| Compound | Viscosity (cp) | δd (MPa1/2) | δp (MPa1/2) | δh (MPa1/2) | δt (MPa1/2) | Rasolvent-water c | Rasolvent-DETA c |
|---|---|---|---|---|---|---|---|
| Water | 0.895 a | 15.50 | 16.00 | 42.30 | 47.81 | − | − |
| Tetralin | 2.023 a | 19.60 | 2.00 | 2.90 | 19.91 | 42.61 | 17.07 |
| Toluene | 0.560 a | 18.00 | 1.40 | 2.00 | 18.16 | 43.15 | 17.31 |
| Hexane | 0.326 b | 14.90 | 0.00 | 0.00 | 14.90 | 45.24 | 19.86 |
| Isopentane | 0.214 b | 13.70 | 0.00 | 0.00 | 13.70 | 45.37 | 20.43 |
| DETA | 7.14 b | 16.70 | 13.30 | 14.30 | 25.70 | − | − |
| Membrane | Contact Angle (°) a |
|---|---|
| CA | 45.40 ± 2.04 |
| TFCtetralin | 45.43 ± 1.58 |
| TFCtoluene | 39.46 ± 1.75 |
| TFCn-hexane | 42.09 ± 1.51 |
| TFCiso-pentane | 40.66 ± 2.36 |
| Membrane | Feed Ethanol Conc. (wt%) | Operating Temperature (°C) | Permeation Flux (g∙m−2∙h−1) | Water Conc. in Permeate (wt%) | Ref. |
|---|---|---|---|---|---|
| DETA/TMC | 90 | 25 | 860 | 99.2 | This study |
| DAPL-SCC/mPAN | 90 | 25 | 600 | 96.7 | [13] |
| m-tolidine-H-TMC/mPAN TFC | 90 | 25 | 2191 | 99.5 | [39] |
| PAA-PA/PAN | 90 | 25 | 830 | 99.5 | [40] |
| PA+nano-NaX zeolite/mPAN | 90 | 25 | 4500 | 77 | [41] |
| SA/PFSA/ceramic hybrid membrane | 85 | 75 | 821 | 99.9 | [42] |
| TDI cross-linked PA | 85 | 50 | 2000 | 95.9 | [43] |
| PVA-P4-80 hybrid | 85 | 40 | 145 | 99.5 | [44] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ang, M.B.M.Y.; Huang, S.-H.; Wei, S.-W.; Chiao, Y.-H.; Aquino, R.R.; Hung, W.-S.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. Surface Properties, Free Volume, and Performance for Thin-Film Composite Pervaporation Membranes Fabricated through Interfacial Polymerization Involving Different Organic Solvents. Polymers 2020, 12, 2326. https://doi.org/10.3390/polym12102326
Ang MBMY, Huang S-H, Wei S-W, Chiao Y-H, Aquino RR, Hung W-S, Tsai H-A, Lee K-R, Lai J-Y. Surface Properties, Free Volume, and Performance for Thin-Film Composite Pervaporation Membranes Fabricated through Interfacial Polymerization Involving Different Organic Solvents. Polymers. 2020; 12(10):2326. https://doi.org/10.3390/polym12102326
Chicago/Turabian StyleAng, Micah Belle Marie Yap, Shu-Hsien Huang, Shi-Wei Wei, Yu-Hsuan Chiao, Ruth R. Aquino, Wei-Song Hung, Hui-An Tsai, Kueir-Rarn Lee, and Juin-Yih Lai. 2020. "Surface Properties, Free Volume, and Performance for Thin-Film Composite Pervaporation Membranes Fabricated through Interfacial Polymerization Involving Different Organic Solvents" Polymers 12, no. 10: 2326. https://doi.org/10.3390/polym12102326