The Degradation and Repolymerization Analysis on Solvolysis Liquefaction of Corn Stalk
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Liquefaction Process
2.3. Characterization of the Corn Stalk and Liquefaction Residues
2.4. Characterization of the Liquefied Product
3. Results and Discussion
3.1. The Investigation on the Liquefaction Conditions of Corn Stalk
3.2. The Analysis of Chemical Functional Groups
3.3. Thermal Behaviors of the Corn Stalk and Liquefaction Residues
3.4. Elemental Analysis of Corn Stalk and Liquefaction Residues
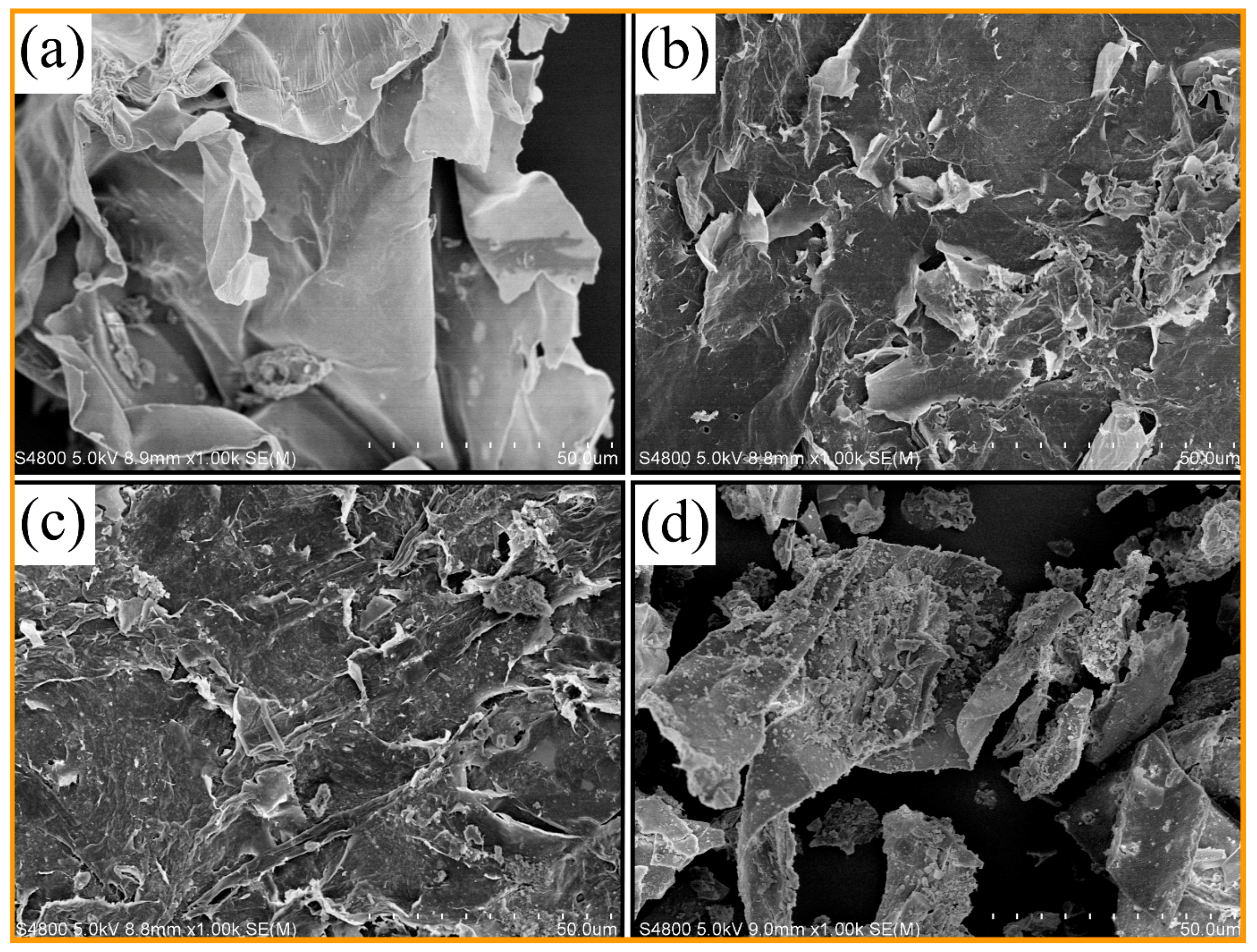
3.5. Morphology of the Corn Stalk and Liquefaction Residues
3.6. Depolymerization and Repolymerization Mechanism of Corn Stalk in the Solvolysis Liquefaction Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yue, D.; Oribayo, O.; Rempel, G.L.; Pan, Q. Liquefaction of waste pine wood and its application in the synthesis of a flame retardant polyurethane foam. RSC Adv. 2017, 7, 30334–30344. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Zhang, C.; Hao, S.; Luo, G.; Zhang, S.; Chen, J. Effect of glycerol as co-solvent on yields of bio-oil from rice straw through hydrothermal liquefaction. Bioresour. Technol. 2016, 220, 471–478. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.; Camargo, R.; Yan, N. Biomass Liquefaction and Alkoxylation: A Review of Structural Characterization Methods for Bio-based Polyols. Polym. Rev. 2017, 57, 668–694. [Google Scholar] [CrossRef]
- Kastner, J.R.; Miller, J.; Geller, D.P.; Locklin, J.; Keith, L.H.; Johnson, T. Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catal. Today 2012, 190, 122–132. [Google Scholar] [CrossRef]
- Tenhaeff, W.E.; Rios, O.; More, K.; McGuire, M.A. Highly Robust Lithium Ion Battery Anodes from Lignin: An Abundant, Renewable, and Low-Cost Material. Adv. Funct. Mater. 2014, 24, 86–94. [Google Scholar] [CrossRef]
- Xu, J.; Xie, X.; Wang, J.; Jiang, J. Directional liquefaction coupling fractionation of lignocellulosic biomass for platform chemicals. Green Chem. 2016, 18, 3124–3138. [Google Scholar] [CrossRef]
- D’Souza, J.; Camargo, R.; Yan, N. Polyurethane foams made from liquefied bark-based polyols. J. Appl. Polym. Sci. 2014, 131, 40599. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Yu, D.; Chen, G. Hydrothermal liquefaction of barley straw to bio-crude oil: Effects of reaction temperature and aqueous phase recirculation. Appl. Energy 2015, 137, 183–192. [Google Scholar] [CrossRef]
- Jasiūnas, L.; Pedersen, T.H.; Toor, S.S.; Rosendahl, L.A. Biocrude production via supercritical hydrothermal co-liquefaction of spent mushroom compost and aspen wood sawdust. Renew. Energy 2017, 111, 392–398. [Google Scholar] [CrossRef]
- Isa, K.M.; Abdullah, T.A.T.; Ali, U.F.M. Hydrogen donor solvents in liquefaction of biomass: A review. Renew. Sustain. Energy Rev. 2018, 81, 1259–1268. [Google Scholar] [CrossRef]
- Brand, S.; Susanti, R.F.; Kim, S.K.; Lee, H.; Kim, J.; Sang, B.-I. Supercritical ethanol as an enhanced medium for lignocellulosic biomass liquefaction: Influence of physical process parameters. Energy 2013, 59, 173–182. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Wang, J.; Zhao, T.; Yang, H.; Jiang, J.; Jiang, X. Kinetic and product composition study on the cellulose liquefaction in polyhydric alcohols. Bioresour. Technol. 2016, 214, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Rachel-Tang, D.Y.; Islam, A.; Taufiq-Yap, Y.H. Bio-oil production via catalytic solvolysis of biomass. RSC Adv. 2017, 7, 7820–7830. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, E.Y. Biobutanediol-mediated liquefaction of empty fruit bunch saccharification residues to prepare lignin biopolyols. Bioresour. Technol. 2016, 208, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Wang, Y.; Ruan, R.; Tayier, M.; Dai, L.; Zhao, Y.; Zhou, Y.; Liu, Y. Comparative study on various alcohols solvolysis of organosolv lignin using microwave energy: Physicochemical and morphological properties. Chem. Eng. Process. Process Intensif. 2018, 126, 38–44. [Google Scholar] [CrossRef]
- Aysu, T.; Küçük, M.M. Liquefaction of giant fennel (Ferula orientalis L.) in supercritical organic solvents: Effects of liquefaction parameters on product yields and character. J. Supercrit. Fluids 2013, 83, 104–123. [Google Scholar] [CrossRef]
- Kosmela, P.; Hejna, A.; Formela, K.; Haponiuk, J.T.; Piszczyk, Ł. Biopolyols obtained via crude glycerol-based liquefaction of cellulose: Their structural, rheological and thermal characterization. Cellulose 2016, 23, 2929–2942. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Jo, Y.J.; Lee, C.G.; Lee, E. Solvothermal liquefaction of microalgal Tetraselmis sp. biomass to prepare biopolyols by using PEG#400-blended glycerol. Algal Res. 2015, 12, 539–544. [Google Scholar]
- Hassan, E.M.; Shukry, N. Polyhydric alcohol liquefaction of some lignocellulosic agricultural residues. Ind. Crops Prod. 2008, 27, 33–38. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, S.; Lan, Z.; Zhang, X.; Yin, Y.; Tu, S.; Yang, S.; Ye, L. Fast liquefaction of bamboo shoot shell with liquid-phase microplasma assisted technology. Bioresour. Technol. 2016, 218, 1275–1278. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, Z.; Fan, L.; Zhang, H.; Liao, Y.; Zheng, D.; Wang, S. Rapid and solvent-saving liquefaction of woody biomass using microwave-ultrasonic assisted technology. Bioresour. Technol. 2016, 199, 423–426. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Shu, R.; Xu, Y.; Ma, L.; Wang, T. Catalytic depolymerization of the hydrolyzed lignin over mesoporous catalysts. Bioresour. Technol. 2017, 226, 125–131. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Qu, G.; Lin, X.; Han, D.; Yan, X.; Zhang, H. Liquefaction of Peanut Shells with Cation Exchange Resin and Sulfuric Acid as Dual Catalyst for the Subsequent Synthesis of Rigid Polyurethane Foam. Polymers 2019, 11, 993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Fan, L.; Wu, Z.; Zhang, H.; Liao, Y.; Zheng, D.; Wang, S. Efficient liquefaction of woody biomass in polyhydric alcohol with acidic ionic liquid as a green catalyst. Biomass Bioenergy 2015, 81, 154–161. [Google Scholar] [CrossRef]
- Lv, G.; Wu, S.; Lou, R. Kinetic study for the thermal decomposition of hemicellulose isolated from corn stalk. BioResources 2010, 5, 1281–1291. [Google Scholar]
- Lv, G.; Wu, S. Analytical pyrolysis studies of corn stalk and its three main components by TG-MS and Py-GC/MS. J. Anal. Appl. Pyrolysis 2012, 97, 11–18. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008; Volume 1617, pp. 1–16.
- Chen, Y.; Wu, Y.L.; Zhang, P.L.; Hua, D.R.; Yang, M.D.; Li, C.; Chen, Z.; Liu, J. Direct liquefaction of Dunaliella tertiolecta for bio-oil in sub/supercritical ethanol-water. Bioresour. Technol. 2012, 124, 190–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Liu, H.; Hui, L.; Wang, H.; Liu, H. Characterization of liquefied products from corn stalk and its biomass components by polyhydric alcohols with phosphoric acid. Carbohydr. Polym. 2019, 215, 170–178. [Google Scholar] [CrossRef]
- Long, J.; Zhang, Q.; Wang, T.; Zhang, X.; Xu, Y.; Ma, L. An efficient and economical process for lignin depolymerization in biomass-derived solvent tetrahydrofuran. Bioresour. Technol. 2014, 154, 10–17. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, J.; Zhao, J.; Tu, S. Liquefaction of bamboo shoot shell for the production of polyols. Bioresour. Technol. 2014, 153, 147–153. [Google Scholar] [CrossRef]
- Amran, U.A.; Zakaria, S.; Chia, C.H.; Fang, Z.; Masli, M.Z. Production of Liquefied Oil Palm Empty Fruit Bunch Based Polyols via Microwave Heating. Energy Fuels 2017, 31, 10975–10982. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Pasa, V.M.D. Production and characterization of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur. Polym. J. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- Long, J.; Li, X.; Guo, B.; Wang, F.; Yu, Y.; Wang, L. Simultaneous delignification and selective catalytic transformation of agricultural lignocellulose in cooperative ionic liquid pairs. Green Chem. 2012, 14, 1935–1941. [Google Scholar] [CrossRef]
- Tan, S.S.Y.; MacFarlane, D.R.; Upfal, J.; Edye, L.A.; Doherty, W.O.S.; Patti, A.F.; Pringle, J.M.; Scott, J.L. Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzenesulfonate ionic liquid. Green Chem. 2009, 11, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Tejado, A.; Pena, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Kunov-Kruse, A.J.; Riisager, A.; Saravanamurugan, S.; Berg, R.W.; Kristensen, S.B.; Fehrmann, R. Revisiting the Brønsted acid catalysed hydrolysis kinetics of polymeric carbohydrates in ionic liquids by in situ ATR-FTIR spectroscopy. Green Chem. 2013, 15, 2843–2848. [Google Scholar] [CrossRef] [Green Version]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Kaleem, I.; Chun, L.; Tong, J. Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy Environ. 2010, 35, 5406–5411. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Li, F.; Xie, J.-L.; Cornelis, F.; Hse, C.-Y.; Qi, J.-Q.; Xiao, H. Microwave-assisted Liquefaction of Rape Straw for the Production of Bio-oils. BioResources 2017, 12, 1968–1981. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Hse, C.-Y.; Shupe, T.F.; Hu, T. Influence of solvent type on microwave-assisted liquefaction of bamboo. Eur. J. Wood Wood Prod. 2016, 74, 249–254. [Google Scholar] [CrossRef]
- Amran, U.A.; Zakaria, S.; Chia, C.H.; Roslan, R.; Jaafar, S.N.S.; Salleh, K.M. Polyols and rigid polyurethane foams derived from liquefied lignocellulosic and cellulosic biomass. Cellulose 2019, 26, 3231–3246. [Google Scholar] [CrossRef]
- Phan Thi, A.; Lin, J.; Cao, J.-Z. Fabrication and characterization of isolated lignin as adhesive for three-ply plywood. Polym. Compos. 2018, 39, 484–490. [Google Scholar] [CrossRef]
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, X.; Yu, W.; Zhang, Y. Kinetic analysis of cellulose extraction from banana pseudo-stem by liquefaction in polyhydric alcohols. Ind. Crop. Prod. 2019, 137, 377–385. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Y.; Liu, D.; Petrus, L. Qualitative analysis of products formed during the acid catalyzed liquefaction of bagasse in ethylene glycol. Bioresour. Technol. 2007, 98, 1454–1459. [Google Scholar] [CrossRef]





| Samples | TMax1 (°C) | TMax2 (°C) | TMax3 (°C) |
|---|---|---|---|
| CS | 210 ± 1 | 320 ± 1 | – |
| LR-130 | 265 ± 1 | 348 ± 1 | – |
| LR-150 | 270 ± 1 | 376 ± 1 | 546 ± 1 |
| LR-170 | 283 ± 1 | 392 ± 1 | 552 ± 1 |
| Samples | C (wt %) | H (wt %) | N (wt %) | O a (wt %) | H/C | O/C | HHV (MJ kg−1) |
|---|---|---|---|---|---|---|---|
| CS | 45.74 ± 0.21 | 6.22 ± 0.08 | 1.39 ± 0.11 | 46.65 | 0.1359 | 1.02 | 16.04 |
| LR-130 | 43.12 ± 0.15 | 6.16 ± 0.12 | 0.66 ± 0.03 | 50.06 | 0.1428 | 1.16 | 14.44 |
| LR-150 | 37.83 ± 0.30 | 5.10 ± 0.15 | 0.92 ± 0.08 | 56.15 | 0.1348 | 1.48 | 10.03 |
| LR-170 | 39.15 ± 0.19 | 4.57 ± 0.10 | 0.98 ± 0.05 | 55.30 | 0.1167 | 1.41 | 9.87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, Q.; Lin, X.; Jiang, K.; Han, D. The Degradation and Repolymerization Analysis on Solvolysis Liquefaction of Corn Stalk. Polymers 2020, 12, 2337. https://doi.org/10.3390/polym12102337
Chen W, Zhang Q, Lin X, Jiang K, Han D. The Degradation and Repolymerization Analysis on Solvolysis Liquefaction of Corn Stalk. Polymers. 2020; 12(10):2337. https://doi.org/10.3390/polym12102337
Chicago/Turabian StyleChen, Weisheng, Qinqin Zhang, Xiaoqi Lin, Kaisen Jiang, and Dezhi Han. 2020. "The Degradation and Repolymerization Analysis on Solvolysis Liquefaction of Corn Stalk" Polymers 12, no. 10: 2337. https://doi.org/10.3390/polym12102337