Treatment of Palm Oil Refinery Effluent Using Tannin as a Polymeric Coagulant: Isotherm, Kinetics, and Thermodynamics Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Physicochemical Analyses of PORE
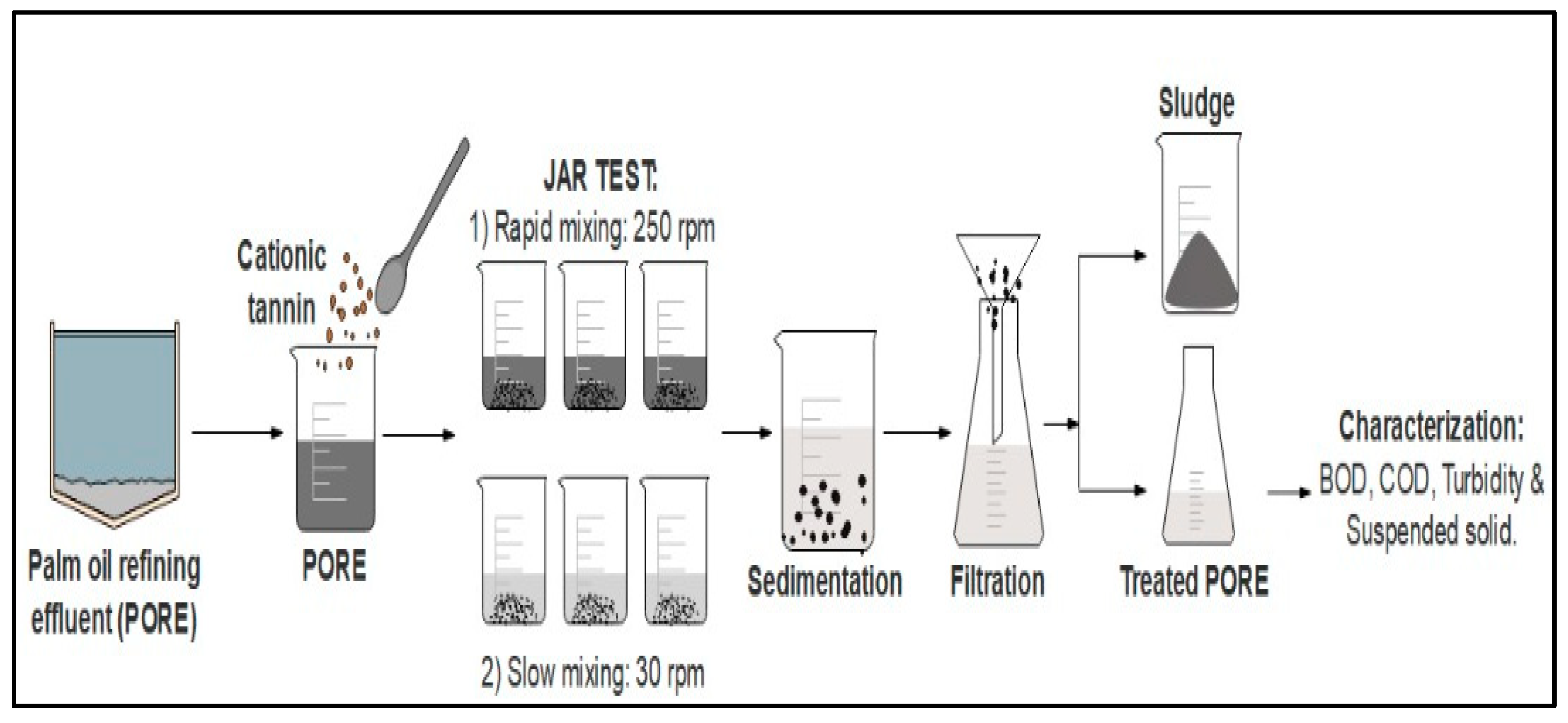
2.3. Coagulations Experiments Procedure
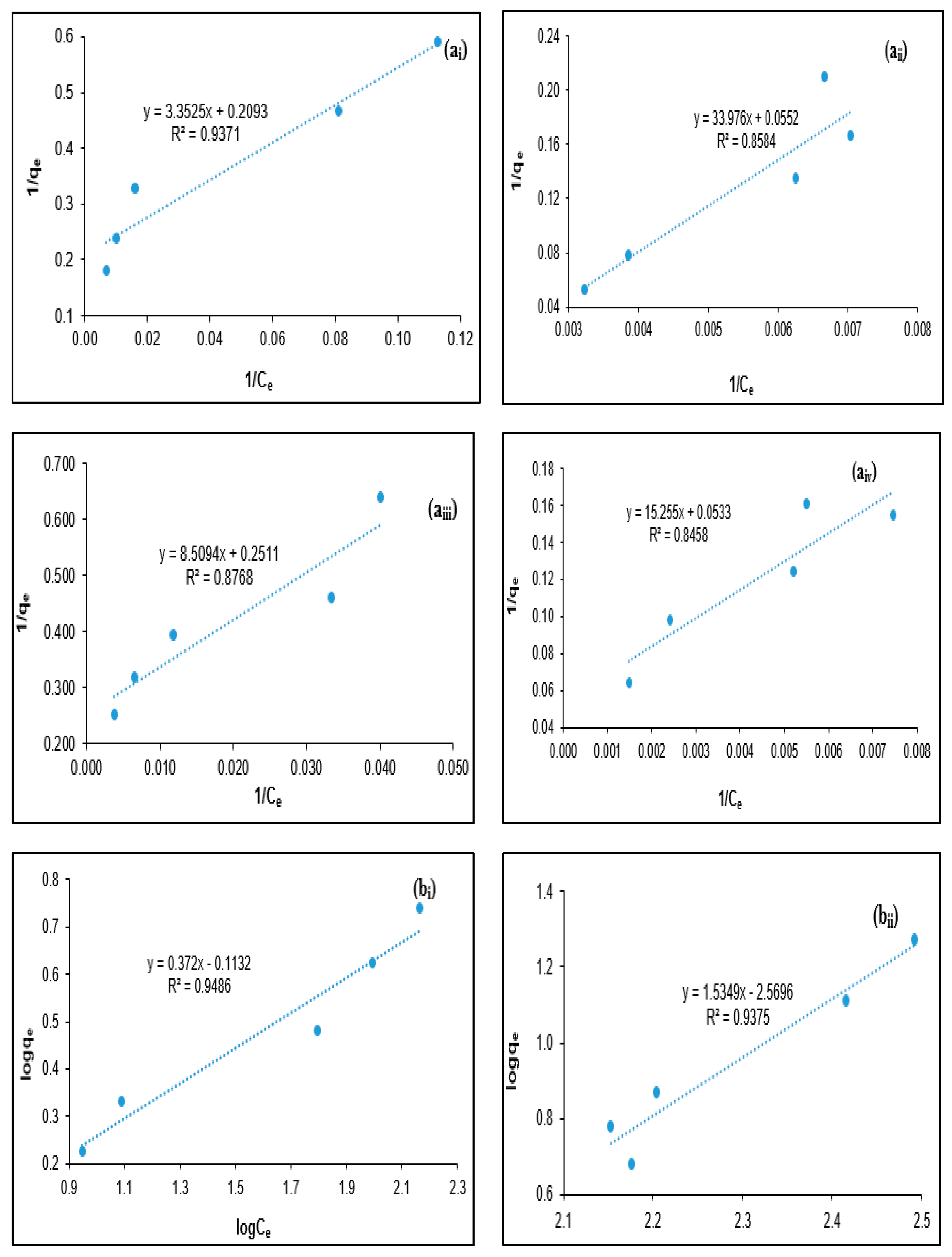
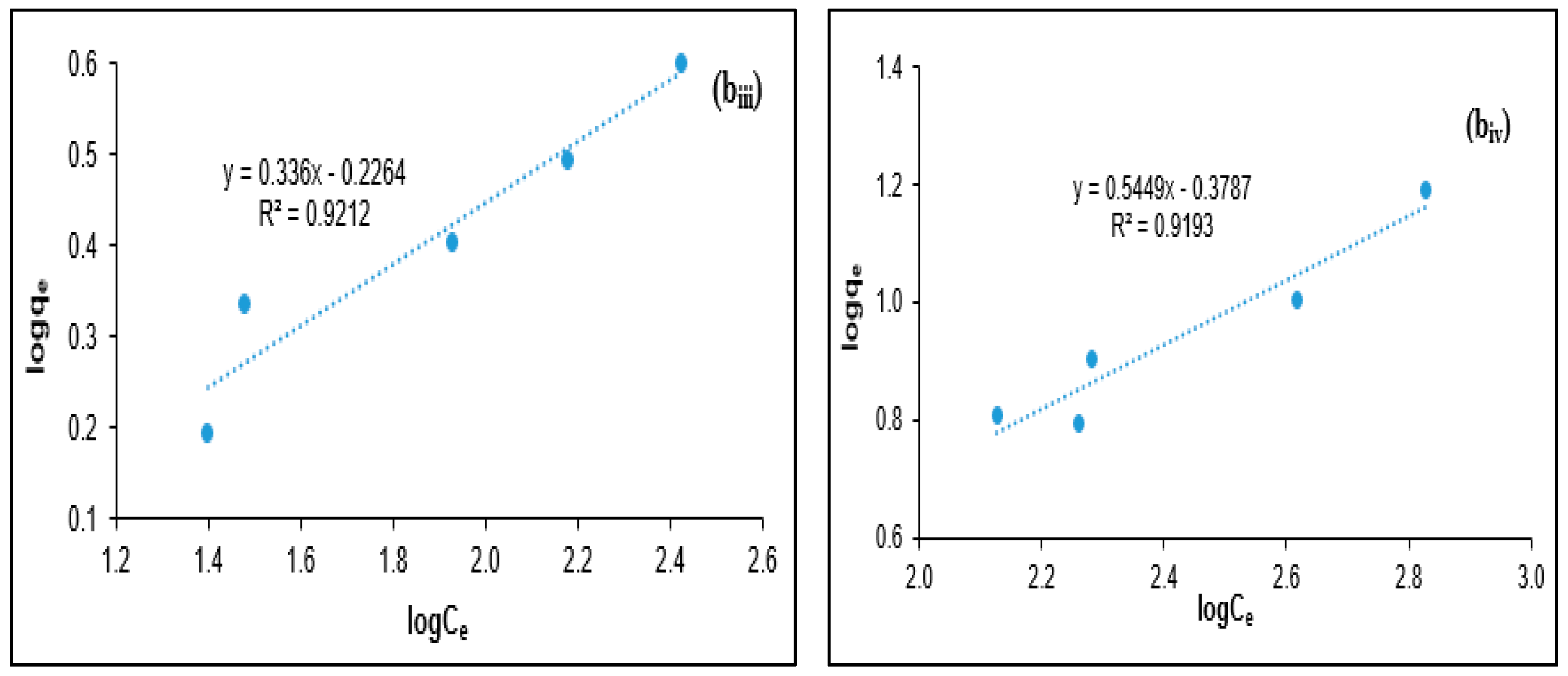
2.4. Coagulation Isotherm Modeling
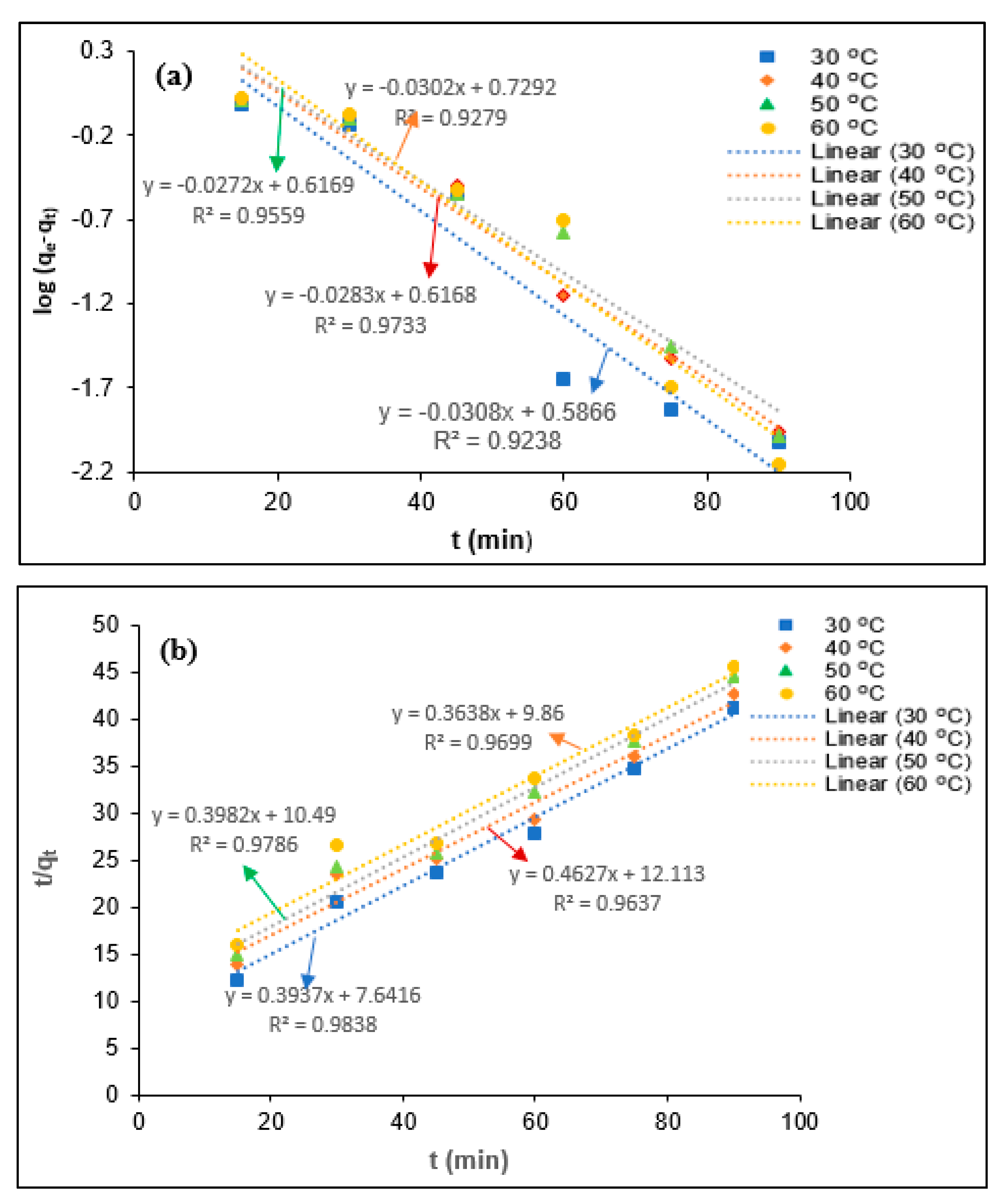
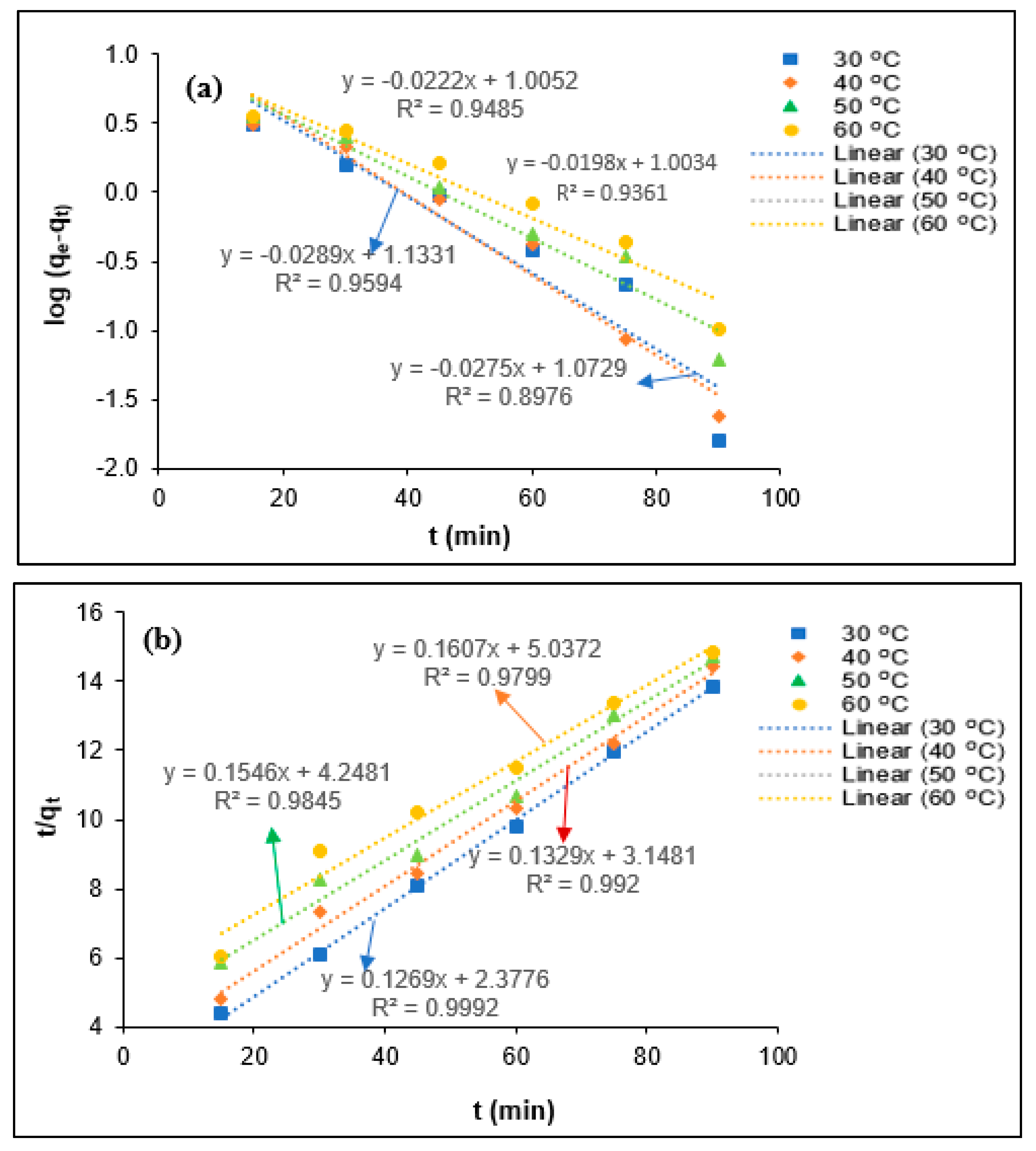
2.5. Kinetics Modeling
2.6. Thermodynamics Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of PORE
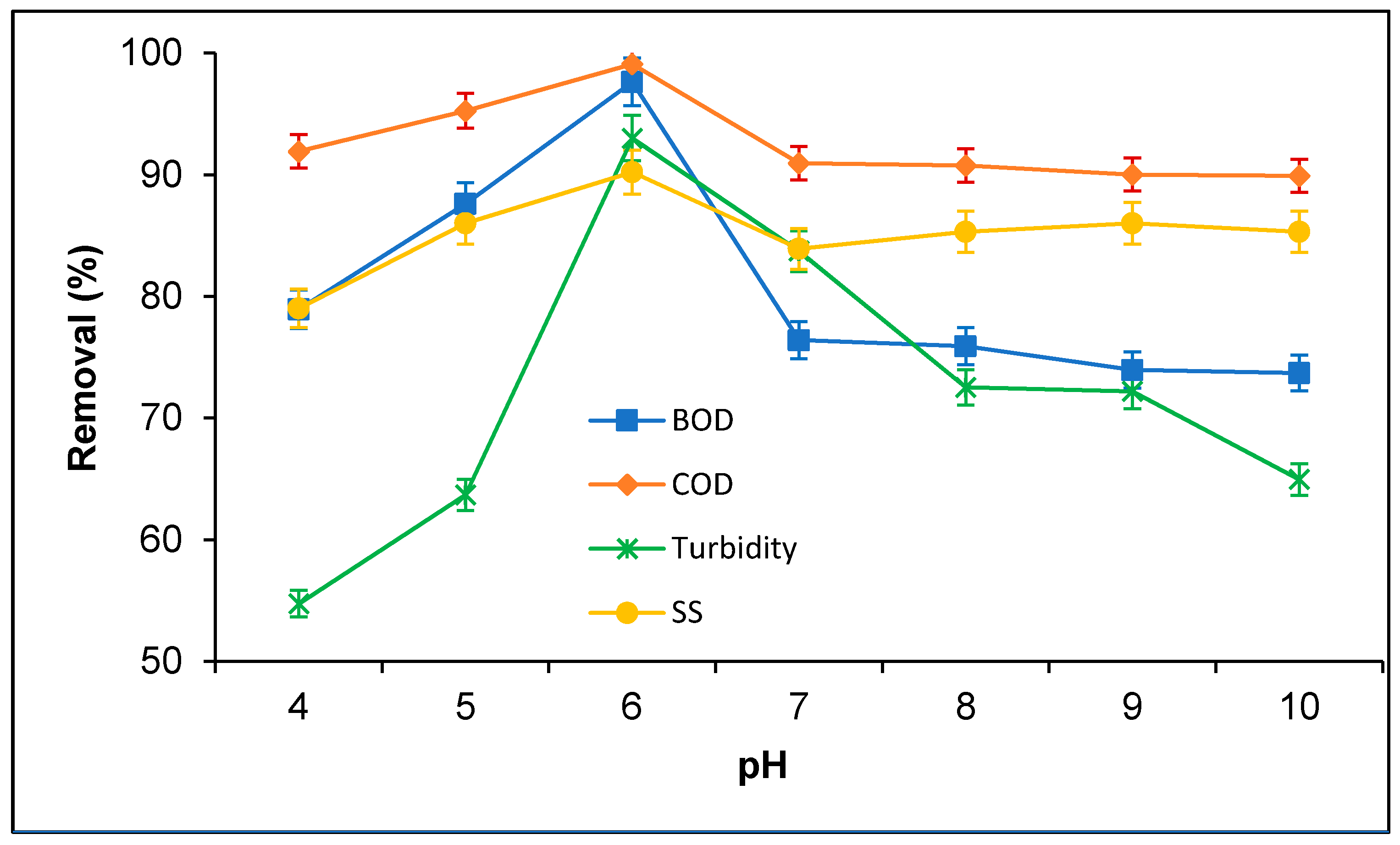
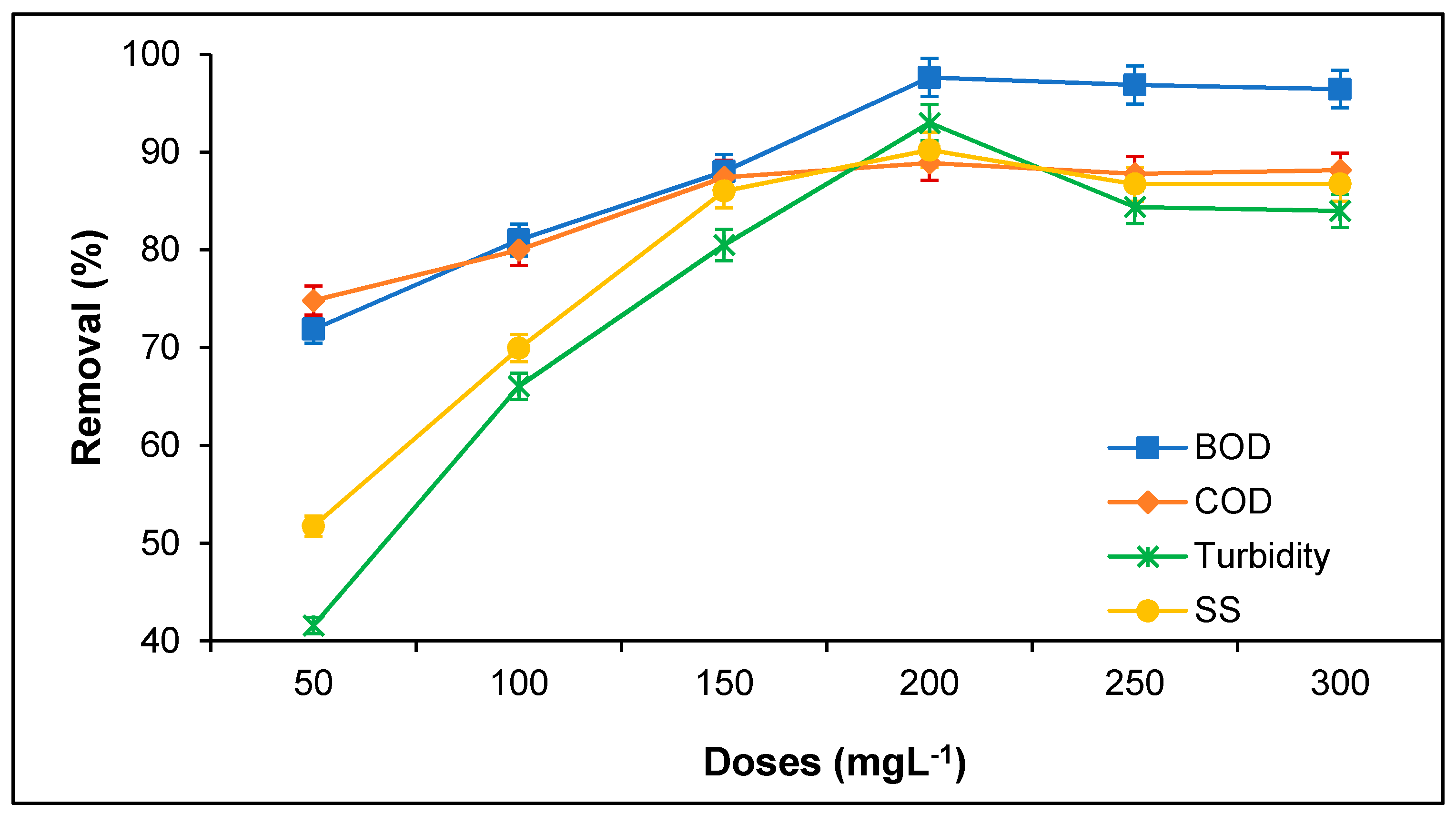
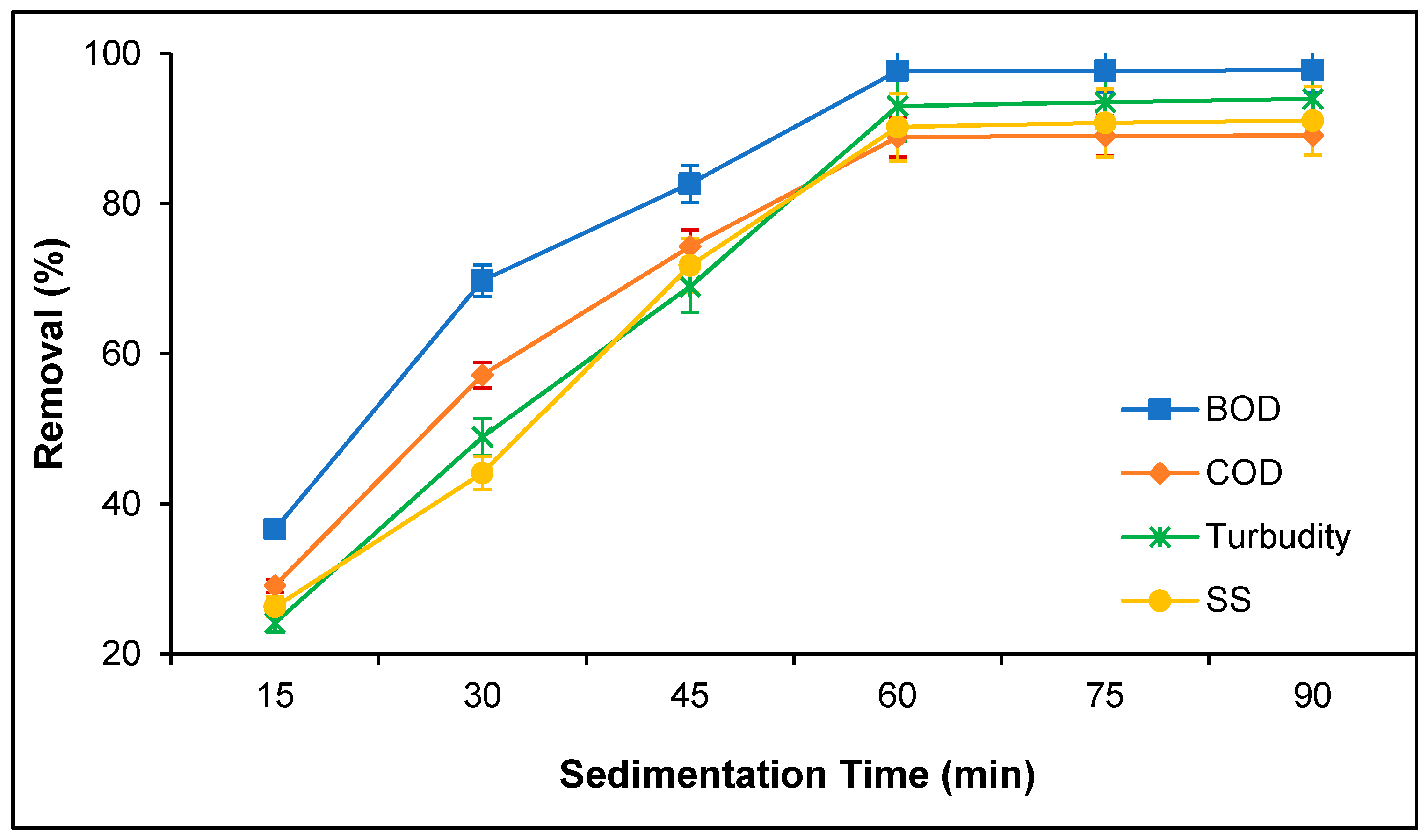
3.2. Treatment of PORE Using Tannin as a Coagulant
3.3. Isotherm Modeling for PORE Treatment Using Tannin as a Polymeric Coagulant
3.4. Kinetic Study
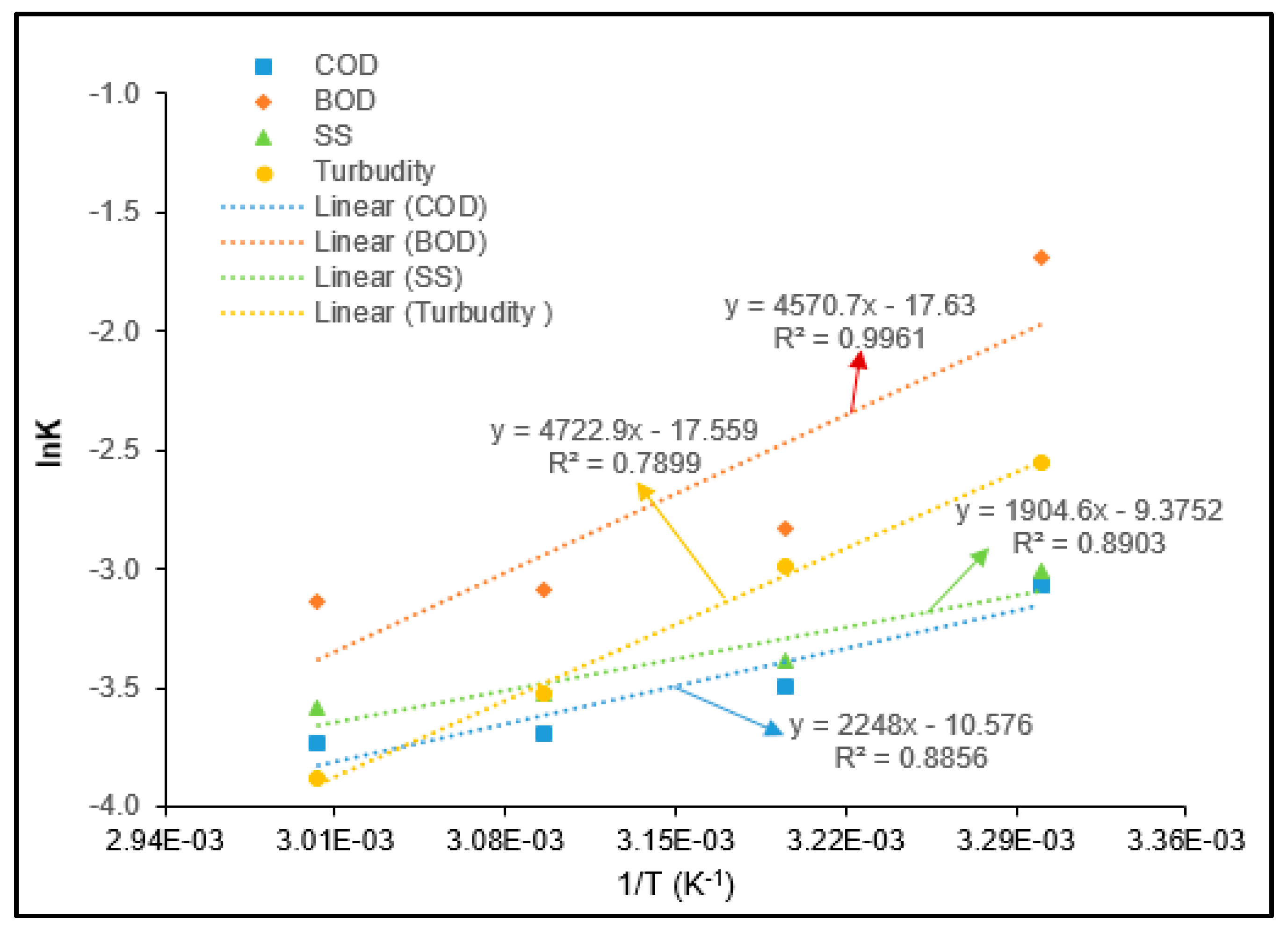
3.5. Adsorption Thermodynamics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aguiar, L.K.; Martinez, D.C.; Caleman, S.M.Q. Consumer Awareness of Palm Oil as an Ingredient in Food and Non-Food Products. J. Food Prod. Mark. 2018, 24, 297–310. [Google Scholar] [CrossRef]
- Li, L.; Yi, N.; Wang, X.; Lin, X.; Zeng, T.; Qiu, T. Novel triazolium-based ionic liquids as effective catalysts for transesterification of palm oil to biodiesel. J. Mol. Liq. 2018, 249, 732–738. [Google Scholar] [CrossRef]
- Palm Oil Trade Statistics from January to December, 2019; Malaysian Palm Oil Council (MPOC): Selangor, Malaysia, 2019.
- Omar, A.K.M.; Tengku Norsalwani, T.L.; Asmah, M.S.; Badrulhisham, Z.Y.; Easa, A.M.; Omar, F.M.; Hossain, M.S.; Zuknik, M.H.; Nik Norulaini, N.A. Implementation of the supercritical carbon dioxide technology in oil palm fresh fruits bunch sterilization: A review. J. CO2 Util. 2018, 25, 205–215. [Google Scholar] [CrossRef]
- Dkhissi, O.; Hakmaoui, A.E.; Souabi, S.; Chatoui, M.; Jada, A.; Akssira, M. Treatment of vegetable oil refinery wastewater by coagulation-flocculation process using the cactus as a bio-flocculant. J. Mater. Environ. Sci. 2018, 9, 18–25. [Google Scholar]
- Tan, Y.D.; Lim, J.S. Feasibility of palm oil mill effluent elimination towards sustainable Malaysian palm oil industry. Renew. Sustain. Energy Rev. 2019, 111, 507–522. [Google Scholar] [CrossRef]
- Environmental Quality Act 1974, Environmental Quality (Industrial Effluent) Regulations 2009; Department of Environment, Ministry of Science, Technology and the Environment: Kuala Lumpur, Malaysia, 2009.
- Dutta, P.K.; Rabaey, K.; Yuan, Z.; Rozendal, R.A.; Keller, J. Electrochemical sulfide removal and recovery from paper mill anaerobic treatment effluent. Water Res. 2010, 44, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Dissolved air flotation combined to biosurfactants: A clean and efficient alternative to treat industrial oily water. Rev. Environ. Sci. Bio/Technol. 2018, 17, 591–602. [Google Scholar] [CrossRef]
- Mazhar, S.; Ditta, A.; Bulgariu, L.; Ahmad, I.; Ahmed, M.; Nadiri, A.A. Sequential treatment of paper and pulp industrial wastewater: Prediction of water quality parameters by Mamdani Fuzzy Logic model and phytotoxicity assessment. Chemosphere 2019, 227, 256–268. [Google Scholar] [CrossRef]
- Hossain, M.S.; Omar, F.; Asis, A.J.; Bachmann, R.T.; Islam Sarker, M.Z.; Ab Kadir, M.O. Effective treatment of palm oil mill effluent using FeSO4.7H2O waste from titanium oxide industry: Coagulation adsorption isotherm and kinetics studies. J. Clean. Prod. 2019, 219, 86–98. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Ngteni, R.; Hossain, M.S.; Ab Kadir, M.O.; Asis, A.J.; Tajudin, Z. Kinetics and Isotherm Modeling for the Treatment of Rubber Processing Effluent Using Iron (II) Sulphate Waste as a Coagulant. Water 2020, 12, 1747. [Google Scholar] [CrossRef]
- Bacelo, H.A.M.; Santos, S.C.R.; Botelho, C.M.S. Tannin-based biosorbents for environmental applications—A review. Chem. Eng. J. 2016, 303, 575–587. [Google Scholar] [CrossRef]
- Ibrahim, A.; Yaser, A.Z. Colour removal from biologically treated landfill leachate with tannin-based coagulant. J. Environ. Chem. Eng. 2019, 7, 103483. [Google Scholar] [CrossRef]
- Lopes, E.C.; Santos, S.C.R.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Evaluation of a tannin-based coagulant on the decolorization of synthetic effluents. J. Environ. Chem. Eng. 2019, 7, 103125. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association, American Water Works Association, and Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Iftekhar, S.; Ramasamy, D.L.; Srivastava, V.; Asif, M.B.; Sillanpää, M. Understanding the factors affecting the adsorption of Lanthanum using different adsorbents: A critical review. Chemosphere 2018, 204, 413–430. [Google Scholar] [CrossRef]
- Hassan, A.; Abdullah, M.; Khalik, W.M.A.W.M. Removal of Residual Oil from Palm Oil Refinery Factory Effluent by Chitosan and Its Derivatives. Asian J. Chem. 2018, 30, 76–80. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Solera-Hernández, C. Removal of sodium dodecyl benzene sulfonate from water by means of a new tannin-based coagulant: Optimisation studies through design of experiments. Chem. Eng. J. 2009, 153, 56–61. [Google Scholar] [CrossRef]
- Dela Justina, M.; Rodrigues Bagnolin Muniz, B.; Mattge Bröring, M.; Costa, V.J.; Skoronski, E. Using vegetable tannin and polyaluminium chloride as coagulants for dairy wastewater treatment: A comparative study. J. Water Process Eng. 2018, 25, 173–181. [Google Scholar] [CrossRef]
- Graham, N.; Gang, F.; Fowler, G.; Watts, M. Characterisation and coagulation performance of a tannin-based cationic polymer: A preliminary assessment. Colloids Surf. A Physicochem. Eng. Asp. 2008, 327, 9–16. [Google Scholar] [CrossRef]
- Tangarfa, M.; Semlali Aouragh Hassani, N.; Alaoui, A. Behavior and Mechanism of Tannic Acid Adsorption on the Calcite Surface: Isothermal, Kinetic, and Thermodynamic Studies. ACS Omega 2019, 4, 19647–19654. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Frutos-Blanco, G. Schinopsis balansae tannin-based flocculant in removing sodium dodecyl benzene sulfonate. Sep. Purif. Technol. 2009, 67, 295–303. [Google Scholar] [CrossRef]
- Özacar, M.; Şengil, İ.A. Evaluation of tannin biopolymer as a coagulant aid for coagulation of colloidal particles. Colloids Surf. A Physicochem. Eng. Asp. 2003, 229, 85–96. [Google Scholar] [CrossRef]
- Zahrim, A.Y.; Dexter, Z.D.; Joseph, C.G.; Hilal, N. Effective coagulation-flocculation treatment of highly polluted palm oil mill biogas plant wastewater using dual coagulants: Decolourisation, kinetics and phytotoxicity studies. J. Water Process Eng. 2017, 16, 258–269. [Google Scholar] [CrossRef] [Green Version]
- Kamaraj, R.; Pandiarajan, A.; Jayakiruba, S.; Naushad, M.; Vasudevan, S. Kinetics, thermodynamics and isotherm modeling for removal of nitrate from liquids by facile one-pot electrosynthesized nano zinc hydroxide. J. Mol. Liq. 2016, 215, 204–211. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Badawi, M.A.; Negm, N.A.; Abou Kana, M.T.H.; Hefni, H.H.; Abdel Moneem, M.M. Adsorption of aluminum and lead from wastewater by chitosan-tannic acid modified biopolymers: Isotherms, kinetics, thermodynamics and process mechanism. Int. J. Biol. Macromol. 2017, 99, 465–476. [Google Scholar] [CrossRef]
- Aljerf, L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study. J. Environ. Manag. 2018, 225, 120–132. [Google Scholar] [CrossRef]
- Yoro, K.O.; Amosa, M.K.; Sekoai, P.T.; Mulopo, J.; Daramola, M.O. Diffusion mechanism and effect of mass transfer limitation during the adsorption of CO2 by polyaspartamide in a packed-bed unit. Int. J. Sustain. Eng. 2020, 13, 54–67. [Google Scholar] [CrossRef]
- Húmpola, P.D.; OdettI, H.S.; Fertitta, A.E.; Vicente, J.L. Thermodynamic analysis of adsorption models of phenol in liquid phase on different activated carbons. J. Chil. Chem. Soc. 2013, 58, 1541–1544. [Google Scholar] [CrossRef] [Green Version]



| Parameters | Unit | Concentration | * Discharge Limits [6] | |
|---|---|---|---|---|
| Standard A | Standard B | |||
| pH | - | 5.86 ± 0.22 | 6.0–9.0 | 5.5–9.0 |
| Temperature | °C | 28 ± 2 | ≤40 | ≤40 |
| BOD | mg/L | 518 ± 5 | 20 | 40 |
| COD | mg/L | 1350 ± 25 | 80 | 200 |
| SS | mg/L | 1430 ± 12 | 50 | 100 |
| ** Turbidity | mg/L | 464 ± 8 | - | - |
| Parameters | Unit | Concentration | Residual Concentration |
|---|---|---|---|
| BOD | mg/L | 518 ± 5 | 12 ± 5 |
| COD | mg/L | 1350 ± 25 | 150 ± 5 |
| SS | mg/L | 1430 ± 12 | 140 ± 5 |
| Turbidity | mg/L | 464 ± 8 | 32 ± 5 |
| Adsorbate | Freundlich Model | Langmuir Model | ||||
|---|---|---|---|---|---|---|
| R2 | Kf (L/mg) | n | R2 | a (L/mg) | b (mg/mg) | |
| BOD | 0.9486 | 0.7705 | 2.6882 | 0.9371 | 0.0624 | 4.7778 |
| COD | 0.9375 | 0.0027 | 0.6515 | 0.8584 | 0.0016 | 18.116 |
| Turbidity | 0.9212 | 0.5937 | 2.9762 | 0.8768 | 0.0295 | 3.9825 |
| SS | 0.9193 | 0.4181 | 1.8352 | 0.8458 | 0.0035 | 18.7617 |
| Parameters | T (°C) | qe (exp) (mg/mg) | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | ||||
|---|---|---|---|---|---|---|---|---|
| qe (mg/mg) | k1 (min−1) | R2 | qe (mg/mg min) | K2 (mg/mg min) | R2 | |||
| BOD | 30 | 2.54 | 3.06 | 0.0792 | 0.9548 | 2.88 | 0.0340 | 0.9951 |
| 40 | 2.34 | 2.85 | 0.0654 | 0.994 | 2.76 | 0.0263 | 0.9954 | |
| 50 | 2.23 | 4.16 | 0.0707 | 0.9741 | 2.58 | 0.0236 | 0.9964 | |
| 60 | 2.21 | 5.95 | 0.0719 | 0.8729 | 2.24 | 0.0246 | 0.9991 | |
| COD | 30 | 6.09 | 8.33 | 0.0772 | 0.9423 | 6.89 | 0.0140 | 0.9981 |
| 40 | 5.79 | 6.04 | 0.0548 | 0.9498 | 5.95 | 0.0125 | 0.9973 | |
| 50 | 5.62 | 14.88 | 0.0730 | 0.9289 | 5.83 | 0.0093 | 0.9941 | |
| 60 | 5.58 | 8.78 | 0.0534 | 0.9695 | 5.34 | 0.0095 | 0.9891 | |
| SS | 30 | 6.49 | 11.83 | 0.0633 | 0.8976 | 7.88 | 0.0068 | 0.9992 |
| 40 | 6.23 | 13.59 | 0.0666 | 0.9594 | 7.52 | 0.0056 | 0.9921 | |
| 50 | 6.11 | 10.12 | 0.0511 | 0.9485 | 6.47 | 0.0056 | 0.9845 | |
| 60 | 6.05 | 10.08 | 0.0456 | 0.9361 | 6.22 | 0.0051 | 0.9799 | |
| Turbidity | 30 | 2.18 | 3.86 | 0.0709 | 0.9238 | 2.75 | 0.0134 | 0.9699 |
| 40 | 2.02 | 4.14 | 0.0652 | 0.9733 | 2.54 | 0.0203 | 0.9838 | |
| 50 | 2.11 | 4.14 | 0.0638 | 0.9559 | 2.51 | 0.0151 | 0.9637 | |
| 60 | 1.97 | 5.36 | 0.0696 | 0.9279 | 2.16 | 0.0177 | 0.9786 | |
| T (K) | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (kJ/mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD | BOD | SS | Turbidity | COD | BOD | SS | Turbidity | COD | BOD | SS | Turbidity | |
| 303.15 | −45.332 | −83.505 | −39.472 | −82.544 | −18.690 | −39.267 | −15.838 | −38.003 | 0.088 | 0.146 | 0.078 | 0.147 |
| 313.15 | −46.212 | −84.965 | −40.252 | −84.014 | ||||||||
| 333.15 | −47.091 | −86.425 | −41.032 | −85.484 | ||||||||
| 353.15 | −47.970 | −87.885 | −41.812 | −86.954 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mat Yasin, N.M.F.; Hossain, M.S.; H.P.S., A.K.; Zulkifli, M.; Al-Gheethi, A.; Asis, A.J.; Yahaya, A.N.A. Treatment of Palm Oil Refinery Effluent Using Tannin as a Polymeric Coagulant: Isotherm, Kinetics, and Thermodynamics Analyses. Polymers 2020, 12, 2353. https://doi.org/10.3390/polym12102353
Mat Yasin NMF, Hossain MS, H.P.S. AK, Zulkifli M, Al-Gheethi A, Asis AJ, Yahaya ANA. Treatment of Palm Oil Refinery Effluent Using Tannin as a Polymeric Coagulant: Isotherm, Kinetics, and Thermodynamics Analyses. Polymers. 2020; 12(10):2353. https://doi.org/10.3390/polym12102353
Chicago/Turabian StyleMat Yasin, Nik Mohd Farid, Md. Sohrab Hossain, Abdul Khalil H.P.S., Muzafar Zulkifli, Adel Al-Gheethi, Ahmad Jaril Asis, and Ahmad Naim Ahmad Yahaya. 2020. "Treatment of Palm Oil Refinery Effluent Using Tannin as a Polymeric Coagulant: Isotherm, Kinetics, and Thermodynamics Analyses" Polymers 12, no. 10: 2353. https://doi.org/10.3390/polym12102353







