Extracellular Vesicle Isolation Yields Increased by Low-Temperature Gaseous Plasma Treatment of Polypropylene Tubes
Abstract
:1. Introduction
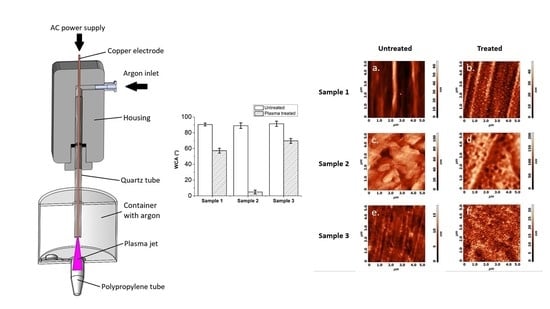
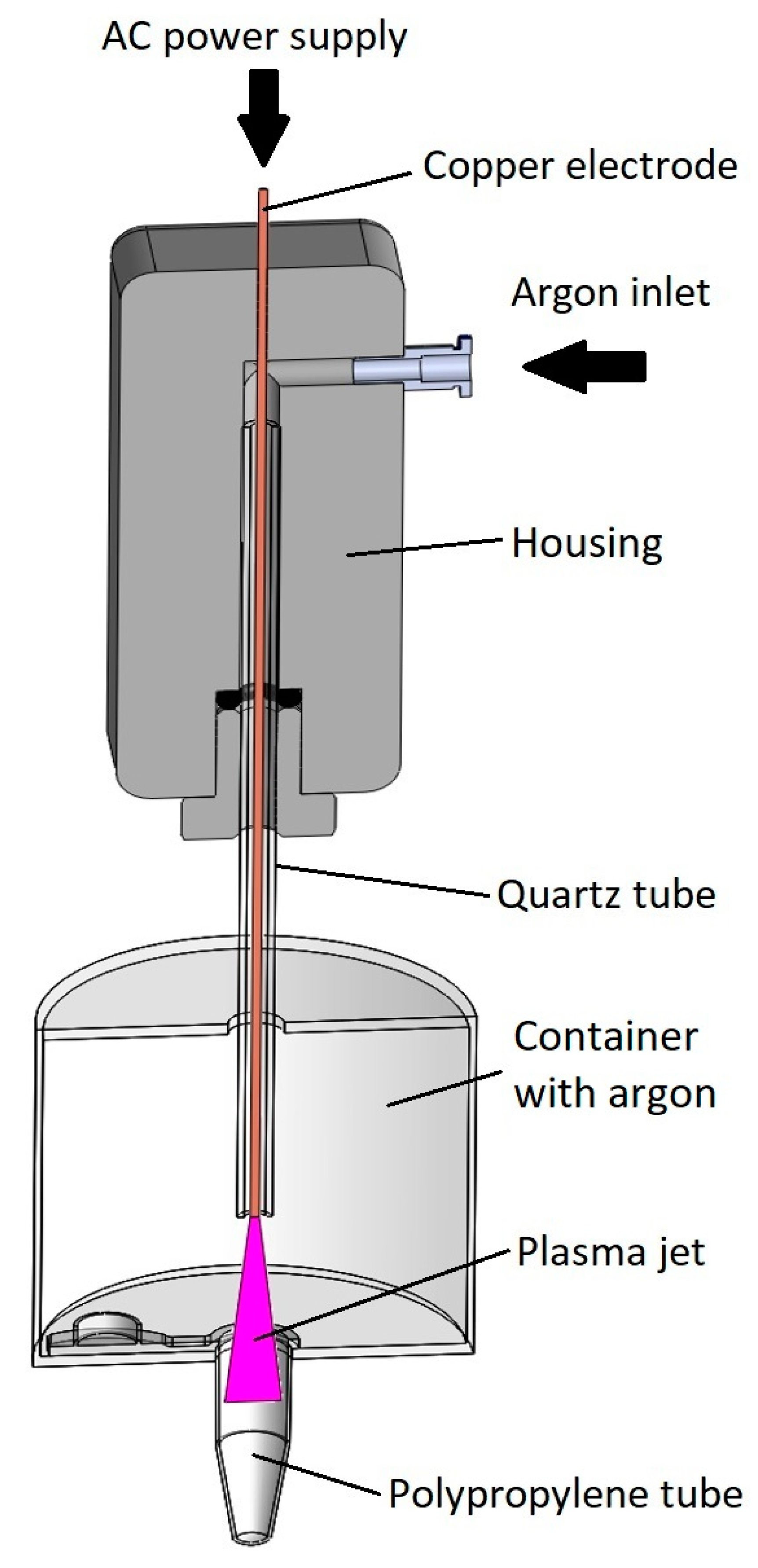
- The vertical position of the gaseous plasma jet ensures that the ground is coaxially positioned with the axis of symmetry. In the case of horizontal setup lower part of the tube was more effectively treated by gaseous plasma compared to the upper part due to the jet being attracted to the ground (Figure 1).
- Gaseous plasma treatment of the tube was conducted inside an enclosed container to ensure argon atmosphere with less quenching, as in this setting plasma jet is wider and less focused, streamers are more branched. Gas flow is initiated before the plasma jet is turned on. Argon is heavier than air and displaces some of the air from the bottom of the container.
- Treatment is not stationary, the plasma jet is moving in and out of the tube during treatment. The movement is enabled by a linear stage to ensure equal speeds and acceleration/deceleration and consequently even and repeatable gaseous plasma treatment.
- Gas flow is optimized in a manner that the plasma jet is stable.
2. Materials and Methods
2.1. Preparation of Samples
2.2. X-ray Photoelectron Spectroscopy
2.3. Atomic Force Microscopy
2.4. Differential Scanning Calorimetry
2.5. Water Contact Angle
2.6. Blood Sampling
2.7. Isolation of EVs
2.8. Flow Cytometry
3. Results
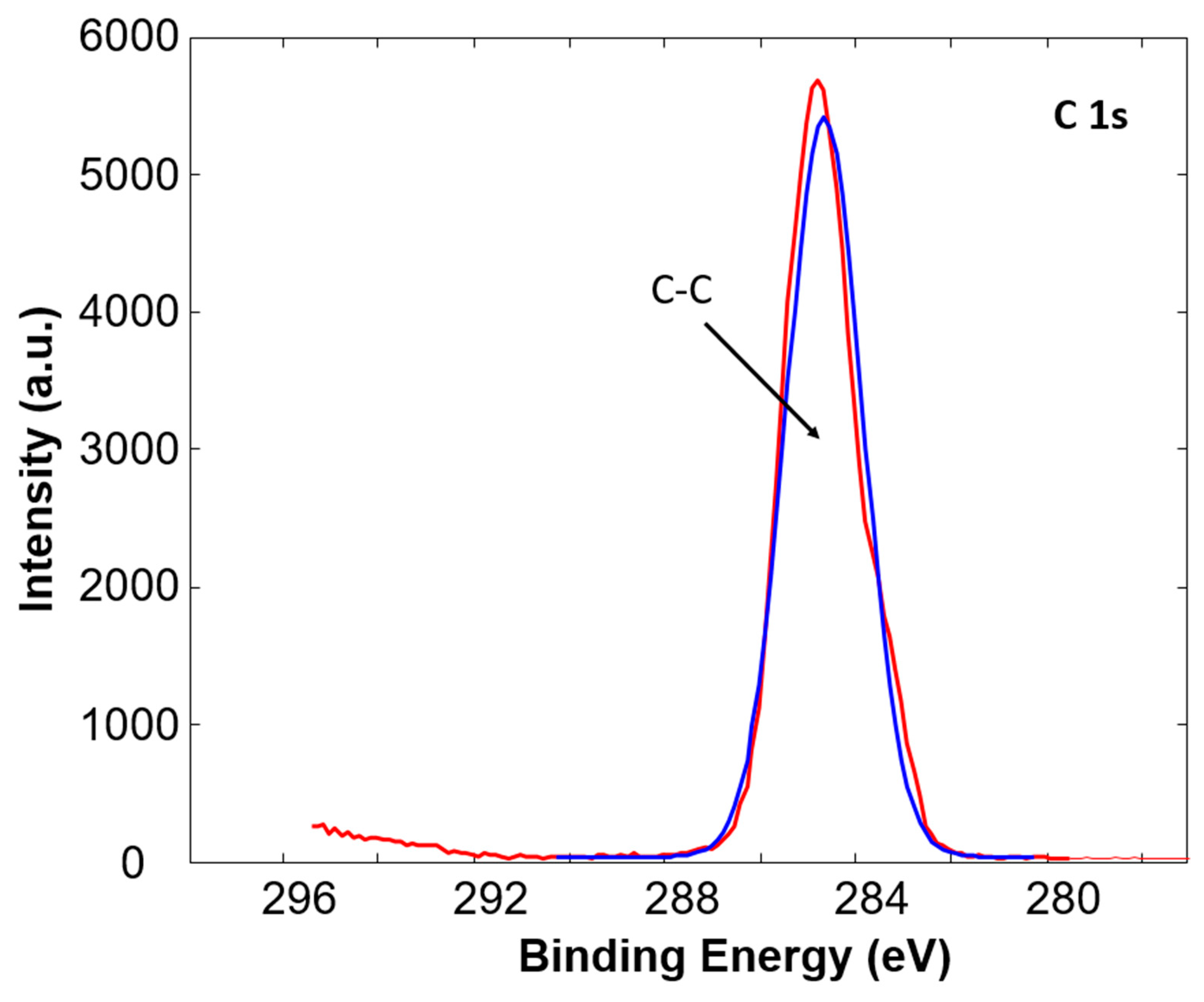
3.1. X-ray Photoelectron Spectroscopy
3.2. Atomic Force Microscopy
3.3. Differential Scanning Calorimetry
3.4. Water Contact Angle
3.5. Flow Cytometry
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Trends in aging--United States and worldwide. MMWR Morb. Mortal. Wkly. Rep. 2003, 52, 101. [Google Scholar]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; D’Asti, E.; Magnus, N.; Al-Nedawi, K.; Meehan, B.; Rak, J. Microvesicles as mediators of intercellular communication in cancer—The emerging science of cellular ‘debris’. Semin. Immunopathol. 2011, 33, 455–467. [Google Scholar] [CrossRef] [Green Version]
- Schara, K.; Janša, V.; Šuštar, V.; Dolinar, D.; Pavlič, J.I.; Lokar, M.; Kralj-Iglič, V.; Veranič, P.; Iglič, A. Mechanisms for the formation of membranous nanostructures in cell-to-cell communication. Cell. Mol. Biol. Lett. 2009, 14, 636–656. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of extracellular communication during cancer progression. J. Cell Sci. 2010, 123, 1603–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aurelian, S.M.; Cheta, D.M.; Onicescu, D. Microvesicles-potential biomarkers for the interrelations atherosclerosis/type 2 diabetes mellitus. Rom. J. Morphol. Embryol. 2014, 55, 1035–1039. [Google Scholar]
- Sluijter, J.P.; Verhage, V.; Deddens, J.C.; van den Akker, F.; Doevendans, P.A. Microvesicles and exosomes for intracardiac communication. Cardiovasc. Res. 2014, 102, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S. Micro RNA Expression in Circulating Microvesicles Predicts Cardiovascular Events in Patients With Coronary Artery Disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef] [Green Version]
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef]
- Meckes, D.G.; Raab-Traub, N. Microvesicles and viral infection. J. Virol. 2011, 85, 12844–12854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verderio, C.; Muzio, L.; Turola, E.; Bergami, A.; Novellino, L.; Ruffini, F.; Riganti, L.; Corradini, I.; Francolini, M.; Garzetti, L. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 2012, 72, 610–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Mantel, P.-Y.; Halleck, A.E.; Trachtenberg, A.J.; Soria, C.E.; Oquin, S.; Bonebreak, C.M.; Saracoglu, E. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Weiß, N.; Wente, W.; Müller, P. Eppendorf LoBind®: Evaluation of Protein Recovery in Eppendorf Protein LoBind Tubes and Plates; Technical report Eppendorf Application Note 180; Eppendorf Instrumente GmbH: Hamburg, Germany, 2010. [Google Scholar]
- Penkov, O.V.; Khadem, M.; Lim, W.-S.; Kim, D.-E. A review of recent applications of atmospheric pressure plasma jets for materials processing. J. Coat. Technol. Res. 2015, 12, 225–235. [Google Scholar] [CrossRef]
- Flamm, D.L.; Auciello, O. Plasma Deposition, Treatment, and Etching of Polymers: The Treatment and Etching of Polymers; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Polak, M.; Masur, K.; von Woedtke, T.; Winter, J.; Reuter, S. Plasma processes and plasma sources in medicine. Contrib. Plasma Phys. 2012, 52, 644–654. [Google Scholar] [CrossRef]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.-U.; Morfill, G.E.; Klämpfl, T.; Steffes, B.; Thomas, H. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin. Plasma Med. 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Schlegel, J.; Köritzer, J.; Boxhammer, V. Plasma in cancer treatment. Clin. Plasma Med. 2013, 1, 2–7. [Google Scholar] [CrossRef]
- Iseki, S.; Nakamura, K.; Hayashi, M.; Tanaka, H.; Kondo, H.; Kajiyama, H.; Kano, H.; Kikkawa, F.; Hori, M. Selective killing of ovarian cancer cells through induction of apoptosis by nonequilibrium atmospheric pressure plasma. Appl. Phys. Lett. 2012, 100, 113702. [Google Scholar] [CrossRef]
- De Valence, S.; Tille, J.-C.; Chaabane, C.; Gurny, R.; Bochaton-Piallat, M.-L.; Walpoth, B.H.; Möller, M. Plasma treatment for improving cell biocompatibility of a biodegradable polymer scaffold for vascular graft applications. Eur. J. Pharm. Biopharm. 2013, 85, 78–86. [Google Scholar] [CrossRef]
- Junkar, I.; Vesel, A.; Cvelbar, U.; Mozetič, M.; Strnad, S. Influence of oxygen and nitrogen plasma treatment on polyethylene terephthalate (PET) polymers. Vacuum 2009, 84, 83–85. [Google Scholar] [CrossRef]
- Machala, Z.; Hensel, K.; Akishev, Y. Plasma for Bio-Decontamination, Medicine and Food Security; Springer Science & Business Media: Cham, Switzerland, 2012. [Google Scholar]
- Sichina, W. DSC as Problem Solving Tool: Measurement of Percent Crystallinity of Thermoplastics. Perkin Elmer Instrum. PETech 2000, 40. Available online: https://www.perkinelmer.com/content/applicationnotes/app_thermalcrystallinitythermoplastics.pdf (accessed on 14 October 2020).
- Šuštar, V.; Bedina-Zavec, A.; Štukelj, R.; Frank, M.; Bobojević, G.; Janša, R.; Ogorevc, E.; Kruljc, P.; Mam, K.; Šimunič, B. Nanoparticles isolated from blood: A reflection of vesiculability of blood cells during the isolation process. Int. J. Nanomed. 2011, 6, 2737. [Google Scholar]
- Maurer-Spurej, E.; Pfeiler, G.; Maurer, N.; Lindner, H.; Glatter, O.; Devine, D.V. Room temperature activates human blood platelets. Lab. Investig. 2001, 81, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.-J.; Tang, S.; Myung, S.-W.; Lu, N.; Choi, H.-S. Surface characteristics of polypropylene film treated by an atmospheric pressure plasma. Surf. Coat. Technol. 2005, 192, 1–10. [Google Scholar] [CrossRef]
- Cheng, C.; Liye, Z.; Zhan, R.-J. Surface modification of polymer fibre by the new atmospheric pressure cold plasma jet. Surf. Coat. Technol. 2006, 200, 6659–6665. [Google Scholar] [CrossRef]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.d.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Sarani, A.; Nikiforov, A.Y.; De Geyter, N.; Morent, R.; Leys, C. Surface modification of polypropylene with an atmospheric pressure plasma jet sustained in argon and an argon/water vapour mixture. Appl. Surf. Sci. 2011, 257, 8737–8741. [Google Scholar] [CrossRef]
- Kwon, O.-J.; Myung, S.-W.; Lee, C.-S.; Choi, H.-S. Comparison of the surface characteristics of polypropylene films treated by Ar and mixed gas (Ar/O2) atmospheric pressure plasma. J. Colloid Interface Sci. 2006, 295, 409–416. [Google Scholar] [CrossRef]
- Lu, X.; Wu, S. On the active species concentrations of atmospheric pressure nonequilibrium plasma jets. IEEE Trans. Plasma Sci. 2013, 41, 2313–2326. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High resolution monochromated X-ray photoelectron spectroscopy of organic polymers: A comparison between solid state data for organic polymers and gas phase data for small molecules. Mol. Phys. 1992, 76, 919–936. [Google Scholar] [CrossRef]
- Matthews, S.R.; Hwang, Y.J.; McCord, M.G.; Bourham, M.A. Investigation into etching mechanism of polyethylene terephthalate (PET) films treated in helium and oxygenated-helium atmospheric plasmas. J. Appl. Polym. Sci. 2004, 94, 2383–2389. [Google Scholar] [CrossRef]
- Junkar, I.; Cvelbar, U.; Vesel, A.; Hauptman, N.; Mozetič, M. The role of crystallinity on polymer interaction with oxygen plasma. Plasma Process. Polym. 2009, 6, 667–675. [Google Scholar] [CrossRef]
- Wang, C.; He, X. Polypropylene surface modification model in atmospheric pressure dielectric barrier discharge. Surf. Coat. Technol. 2006, 201, 3377–3384. [Google Scholar] [CrossRef]
- Akishev, Y.S.; Grushin, M.; Monich, A.; Napartovich, A.; Trushkin, N. One-atmosphere argon dielectric-barrier corona discharge as an effective source of cold plasma for the treatment of polymer films and fabrics. High Energy Chem. 2003, 37, 286–291. [Google Scholar] [CrossRef]



| C1s (at%) | O1s (at%) | O/C | ||
|---|---|---|---|---|
| Sample 1 | Untreated | 91.5 | 8.5 | 0.09 |
| Treated | 84.8 | 15.2 | 0.18 | |
| Sample 2 | Untreated | 90.2 | 9.8 | 0.11 |
| Treated | 81.0 | 19.0 | 0.24 | |
| Sample 3 | Untreated | 99.4 | 0.6 | 0.01 |
| Treated | 85.1 | 14.9 | 0.18 |
| C-C (%) | C-O (%) | C=O (%) | O=C-O (%) | ||
|---|---|---|---|---|---|
| Sample 1 | Untreated | 96.3 | 2.4 | 0 | 1.2 |
| Treated | 82.1 | 10.2 | 3.7 | 4.0 | |
| Sample 2 | Untreated | 90.4 | 7.4 | 0 | 2.2 |
| Treated | 75.0 | 16.5 | 3.2 | 5.3 | |
| Sample 3 | Untreated | 100 | 0 | 0 | 0 |
| Treated | 81.8 | 9.1 | 4.8 | 4.3 | |
| Untreated (nm) | Treated (nm) | |
|---|---|---|
| Sample 1 | 2.48 ± 0.51 | 3.90 ± 1.32 |
| Sample 2 | 2.40 ± 0.34 | 2.64 ± 0.52 |
| Sample 3 | 0.74 ± 0.21 | 2.58 ± 0.05 |
| Tm (°C) | Tc (°C) | Crystallinity (%) | |
|---|---|---|---|
| Sample 1 | 161.3 | 119.1 | 53.6 |
| Sample 2 | 149.3 | 120.6 | 41.8 |
| Sample 3 | 166.0 | 133.4 | 58.0 |
| Improvement (%) | |
|---|---|
| Sample 1 | 42 ± 23 |
| Sample 2 | 17 ± 31 |
| Sample 3 | 26 ± 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resnik, M.; Kovač, J.; Štukelj, R.; Kralj-Iglič, V.; Humpolíček, P.; Junkar, I. Extracellular Vesicle Isolation Yields Increased by Low-Temperature Gaseous Plasma Treatment of Polypropylene Tubes. Polymers 2020, 12, 2363. https://doi.org/10.3390/polym12102363
Resnik M, Kovač J, Štukelj R, Kralj-Iglič V, Humpolíček P, Junkar I. Extracellular Vesicle Isolation Yields Increased by Low-Temperature Gaseous Plasma Treatment of Polypropylene Tubes. Polymers. 2020; 12(10):2363. https://doi.org/10.3390/polym12102363
Chicago/Turabian StyleResnik, Matic, Janez Kovač, Roman Štukelj, Veronika Kralj-Iglič, Petr Humpolíček, and Ita Junkar. 2020. "Extracellular Vesicle Isolation Yields Increased by Low-Temperature Gaseous Plasma Treatment of Polypropylene Tubes" Polymers 12, no. 10: 2363. https://doi.org/10.3390/polym12102363