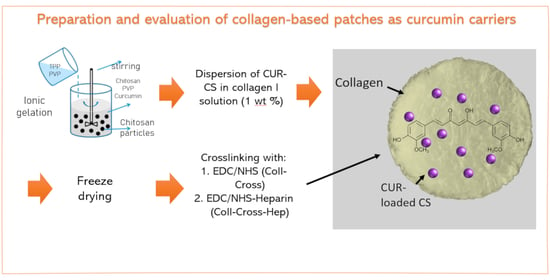
Preparation and Evaluation of Collagen-Based Patches as Curcumin Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Curcumin-Loaded Chitosan Nanoparticles
2.3. Preparation of Collagen Patches Loaded with Chitosan Nanoparticles
2.4. Isolation and Expansion of Human Keratinocytes and Fibroblasts from Skin Biopsies of Patients with Psoriasis
2.5. Characterization
2.5.1. Characterization of Curcumin-Loaded Chitosan Nanoparticles
2.5.2. Characterization of Collagen Patches
3. Results
3.1. Characterization of CS Nanoparticles
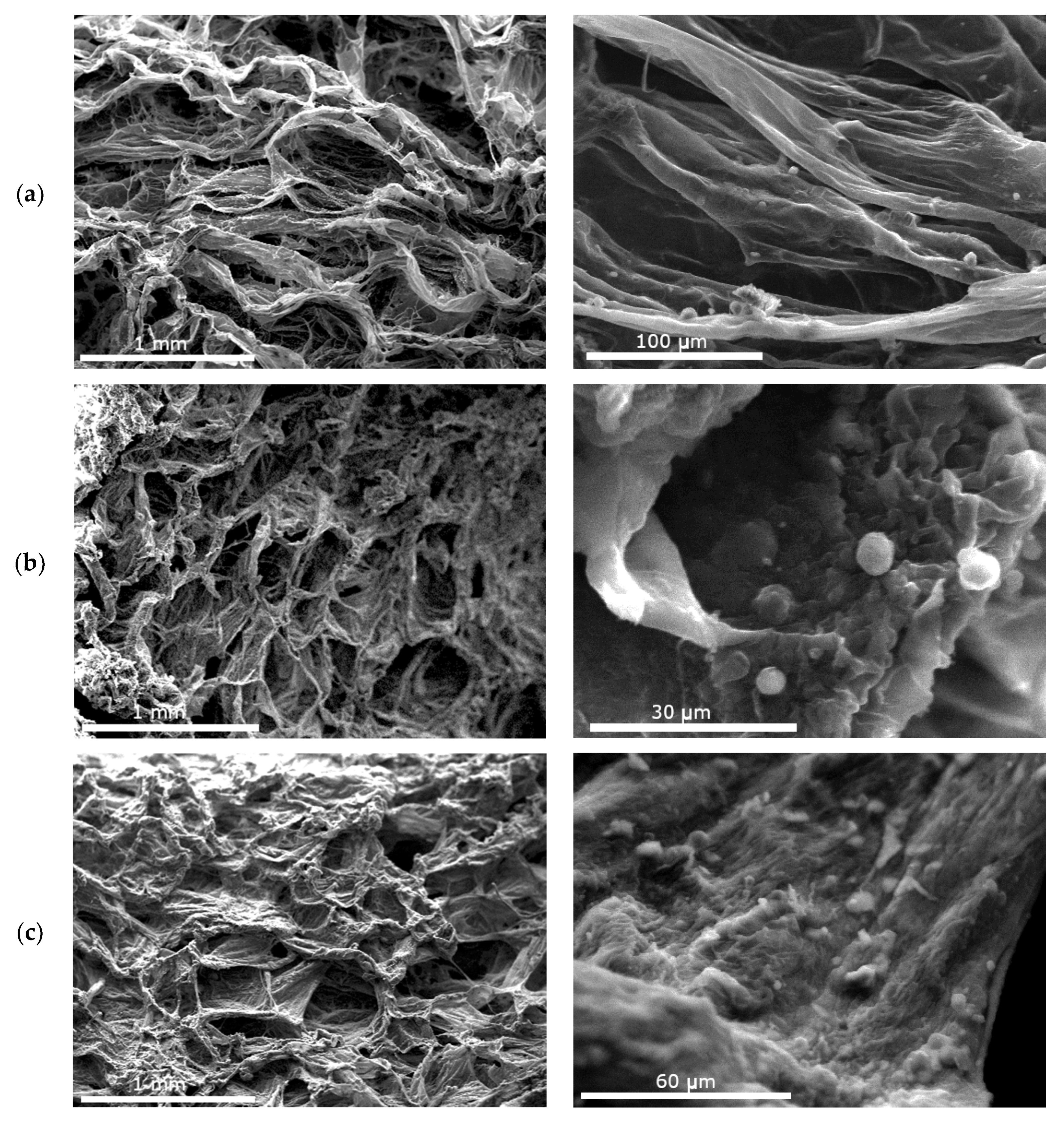
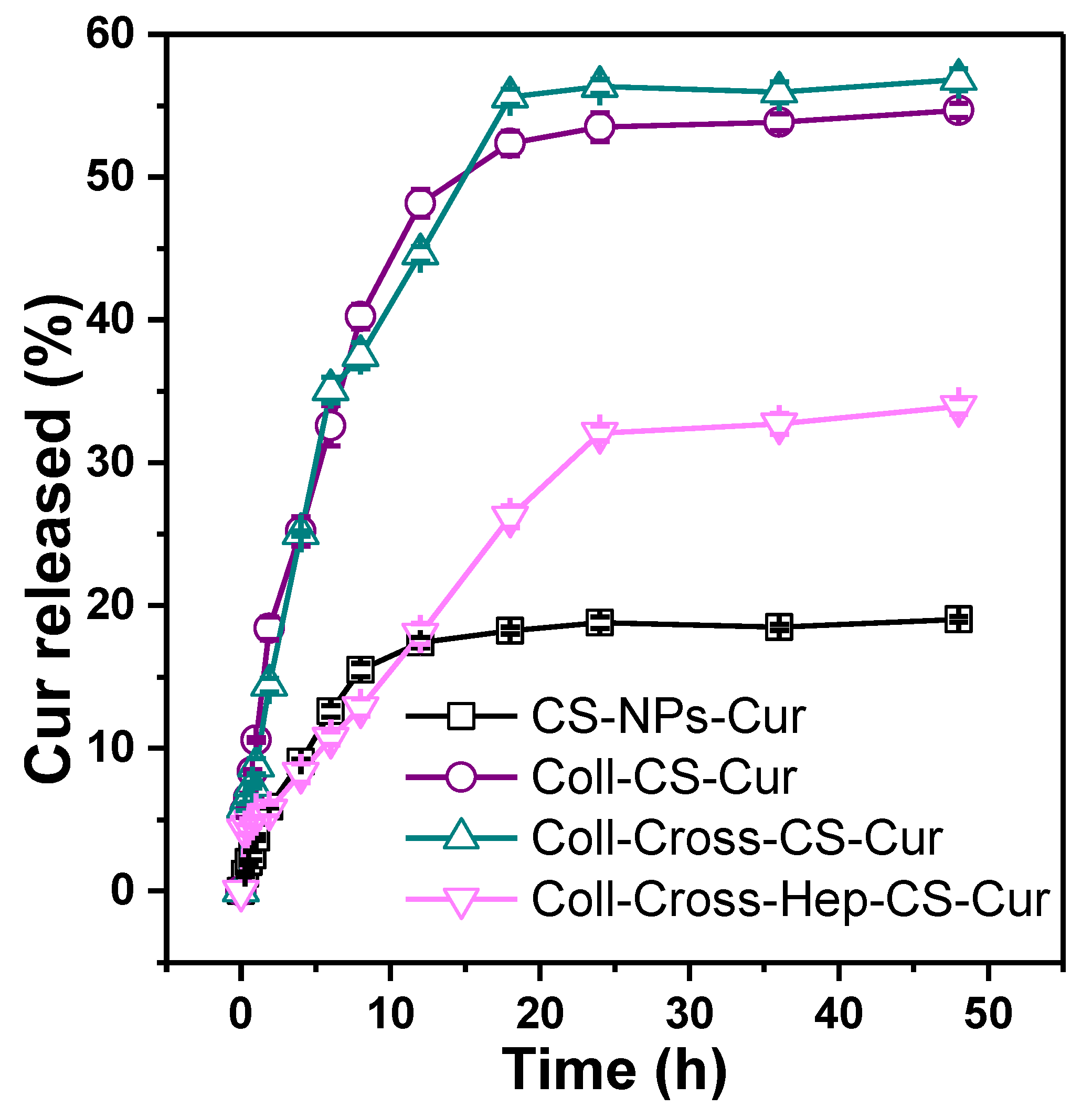
3.2. Characterization of the Patches
3.3. Cell Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santos, L.F.; Correia, I.J.; Silva, A.S.; Mano, J.F. Biomaterials for drug delivery patches. Eur. J. Pharm. Sci. 2018, 118, 49–66. [Google Scholar] [CrossRef]
- Kandavilli, S.; Nair, V.; Panchagnula, R. Polymers in transdermal drug delivery systems. Pharm. Technol. 2002, 26, 62–81. [Google Scholar]
- Shariatinia, Z.; Barzegari, A. 22—Polysaccharide hydrogel films/membranes for transdermal delivery of therapeutics. In Polysaccharide Carriers for Drug Delivery; Maiti, S., Jana, S., Eds.; Woodhead Publishing United Kingdom: Oxford, UK, 2019; pp. 639–684. ISBN 978-0-08-102553-6. [Google Scholar]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. Aaps Pharmscitech 2019, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Tracking the transdermal penetration pathways of optimized curcumin-loaded chitosan nanoparticles via confocal laser scanning microscopy. Int. J. Biol. Macromol. 2018, 108, 753–764. [Google Scholar] [CrossRef]
- Shende, P.; Gupta, H. Formulation and comparative characterization of nanoparticles of curcumin using natural, synthetic and semi-synthetic polymers for wound healing. Life Sci. 2020, 117588. [Google Scholar] [CrossRef]
- Li, F.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-loaded chitosan nanoparticles promote diabetic wound healing via attenuating inflammation in a diabetic rat model. J. Biomater. Appl. 2019, 34, 476–486. [Google Scholar] [CrossRef]
- Saheb, M.; Fereydouni, N.; Nemati, S.; Barreto, G.E.; Johnston, T.P.; Sahebkar, A. Chitosan-based delivery systems for curcumin: A review of pharmacodynamic and pharmacokinetic aspects. J. Cell. Physiol. 2019, 234, 12325–12340. [Google Scholar] [CrossRef]
- Patel, M.P.; Patel, R.R.; Patel, J.K. Chitosan mediated targeted drug delivery system: A review. J. Pharm. Pharm. Sci. 2010, 13, 536–557. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Merino, S.; Martin, C.; Kostarelos, K.; Prato, M.; Vazquez, E. Nanocomposite hydrogels: 3D polymer–nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–hydrogel composites: Concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Lecouvet, B.; Sclavons, M.; Bourbigot, S.; Bailly, C. Towards scalable production of polyamide 12/halloysite nanocomposites via water-assisted extrusion: Mechanical modeling, thermal and fire properties. Polym. Adv. Technol. 2014, 25, 137–151. [Google Scholar] [CrossRef]
- Mohanty, C.; Sahoo, S.K. Curcumin and its topical formulations for wound healing applications. Drug Discov. Today 2017, 22, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Gadekar, R.; Saurabh, M.K.; Thakur, G.S.; Saurabh, A. Study of formulation, characterisation and wound healing potential of transdermal patches of curcumin. Asian J. Pharm. Clin. Res. 2012, 5, 225–230. [Google Scholar]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef]
- Pathan, I.B.; Jaware, B.P.; Shelke, S.; Ambekar, W. Curcumin loaded ethosomes for transdermal application: Formulation, optimization, in-vitro and in-vivo study. J. Drug Deliv. Sci. Technol. 2018, 44, 49–57. [Google Scholar] [CrossRef]
- Patel, N.A.; Patel, N.J.; Patel, R.P. Design and evaluation of transdermal drug delivery system for curcumin as an anti-inflammatory drug. Drug Dev. Ind. Pharm. 2009, 35, 234–242. [Google Scholar] [CrossRef]
- Gamret, A.C.; Price, A.; Fertig, R.M.; Lev-Tov, H.; Nichols, A.J. Complementary and alternative medicine therapies for psoriasis: A systematic review. JAMA Dermatol. 2018, 154, 1330–1337. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Filippone, A.; Consoli, G.M.L.; Granata, G.; Casili, G.; Lanza, M.; Ardizzone, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Topical Delivery of Curcumin by Choline-Calix [4] arene-Based Nanohydrogel Improves Its Therapeutic Effect on a Psoriasis Mouse Model. Int. J. Mol. Sci. 2020, 21, 5053. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.-W.; Kim, M.-H.; Sohn, S.-Y.; Kim, K.-T.; Park, J.-H.; Lee, S.-Y.; Lee, J.-Y.; Kim, D.-D. Curcumin-loaded lipid-hybridized cellulose nanofiber film ameliorates imiquimod-induced psoriasis-like dermatitis in mice. Biomaterials 2018, 182, 245–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 Assists the topical anti-psoriatic efficacy of curcumin-loaded hyaluronan-modified ethosomes: A new strategy for clustering drug in inflammatory skin. Theranostics 2019, 9, 48. [Google Scholar] [CrossRef]
- Gomez, C.; Muangnoi, C.; Sorasitthiyanukarn, F.N.; Wongpiyabovorn, J.; Rojsitthisak, P.; Rojsitthisak, P. Synergistic Effects of Photo-Irradiation and Curcumin-Chitosan/Alginate Nanoparticles on Tumor Necrosis Factor-Alpha-Induced Psoriasis-Like Proliferation of Keratinocytes. Molecules 2019, 24, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Ng, S.-F.; Khan, S.; Katas, H. Nanoencapsulation, an efficient and promising approach to maximize wound healing efficacy of curcumin: A review of new trends and state-of-the-art. Colloids Surf. B Biointerfaces 2017, 150, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Tackling the various classes of nano-therapeutics employed in topical therapy of psoriasis. Drug Deliv. 2020, 27, 662–680. [Google Scholar] [CrossRef]
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, M.K.; Ahmad, F.J.; Iqbal, Z. Development of Curcumin loaded chitosan polymer based nanoemulsion gel: In vitro, ex vivo evaluation and in vivo wound healing studies. Int. J. Biol. Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Liu, Z.; Wang, L.; Cun, D.; Tong, H.H.Y.Y.; Yan, R.; Chen, X.; Wang, R.; Zheng, Y. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J. Control. Release 2017, 254, 44–54. [Google Scholar] [CrossRef]
- Mao, K.-L.; Fan, Z.-L.; Yuan, J.-D.; Chen, P.-P.; Yang, J.-J.; Xu, J.; ZhuGe, D.-L.; Jin, B.-H.; Zhu, Q.-Y.; Shen, B.-X. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf. B Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Kaul, V.; Waghule, T.; Gorantla, S.; Sharma, S.; Roy, A.; Dubey, S.K.; Singhvi, G. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: Optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur. J. Pharm. Sci. 2020, 152, 105438. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.A.; Kazi, M.; Hadi Albgomi, M.; Ahad, A.; Raish, M. Development and optimization of self-nanoemulsifying drug delivery systems (SNEDDS) for curcumin transdermal delivery: An anti-inflammatory exposure. Drug Dev. Ind. Pharm. 2019, 45, 1073–1078. [Google Scholar] [CrossRef]
- Jantarat, C.; Sirathanarun, P.; Boonmee, S.; Meechoosin, W.; Wangpittaya, H. Effect of Piperine on Skin Permeation of Curcumin from a Bacterially Derived Cellulose-Composite Double-Layer Membrane for Transdermal Curcumin Delivery. Sci. Pharm. 2018, 86, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpagavalli, L.; Mahaeswaran, A.; Praveena, P.; Sharmila, S.; Meena, B. Formulation and evaluation of transdermal patches of curcumin. Int. J. Nov. Trends Pharm. Sci. 2017, 7, 22–26. [Google Scholar]
- Kaur, R.; Sharma, A.; Puri, V.; Singh, I. Preparation and characterization of biocomposite films of carrageenan/locust bean gum/montmorrillonite for transdermal delivery of curcumin. Bioimpacts BI 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravikumar, R.; Ganesh, M.; Senthil, V.; Ramesh, Y.V.; Jakki, S.L.; Choi, E.Y. Tetrahydro curcumin loaded PCL-PEG electrospun transdermal nanofiber patch: Preparation, characterization, and in vitro diffusion evaluations. J. Drug Deliv. Sci. Technol. 2018, 44, 342–348. [Google Scholar] [CrossRef]
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S. Understanding the prospective of nano-formulations towards the treatment of psoriasis. Biomed. Pharmacother. 2018, 107, 447–463. [Google Scholar] [CrossRef]
- Liu, H.; Danthi, S.J.; Enyeart, J.J. Curcumin potently blocks Kv1. 4 potassium channels. Biochem. Biophys. Res. Commun. 2006, 344, 1161–1165. [Google Scholar] [CrossRef] [Green Version]
- Iriventi, P.; Gupta, N.V.; Osmani, R.A.M.; Balamuralidhara, V. Design & development of nanosponge loaded topical gel of curcumin and caffeine mixture for augmented treatment of psoriasis. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2020. [Google Scholar]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef]
- Niranjan, R.; Kaushik, M.; Prakash, J.; Venkataprasanna, K.S.; Arpana, C.; Balashanmugam, P.; Venkatasubbu, G.D. Enhanced wound healing by PVA/Chitosan/Curcumin patches: In vitro and in vivo study. Colloids Surf. B Biointerfaces 2019, 182, 110339. [Google Scholar]
- Liu, X.; You, L.; Tarafder, S.; Zou, L.; Fang, Z.; Chen, J.; Lee, C.H.; Zhang, Q. Curcumin-releasing chitosan/aloe membrane for skin regeneration. Chem. Eng. J. 2019, 359, 1111–1119. [Google Scholar] [CrossRef]
- Rezaii, M.; Oryan, S.; Javeri, A. Curcumin nanoparticles incorporated collagen-chitosan scaffold promotes cutaneous wound healing through regulation of TGF-β1/Smad7 gene expression. Mater. Sci. Eng. C 2019, 98, 347–357. [Google Scholar] [CrossRef]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Mannemala, S.S.; Kollipara, R.; Wadhwani, A.D.; Mulukutla, S.; Raju, K.R.S.; Malayandi, R. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar] [CrossRef]
- Laghezza Masci, V.; Taddei, A.-R.; Courant, T.; Tezgel, O.; Navarro, F.; Giorgi, F.; Mariolle, D.; Fausto, A.-M.; Texier, I. Characterization of Collagen/Lipid Nanoparticle–Curcumin Cryostructurates for Wound Healing Applications. Macromol. Biosci. 2019, 19, 1800446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Song, Z.; Wen, Y.; Xu, H.; Zhu, L.; Feng, R. Transdermal delivery of curcumin-loaded supramolecular hydrogels for dermatitis treatment. J. Mater. Sci. Mater. Med. 2019, 30, 11. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Lan, Y.; Xue, W.; Cheng, B.; Zhang, Y.; Wang, C.; Ramakrishna, S. Collagen-cellulose nanocrystal scaffolds containing curcumin-loaded microspheres on infected full-thickness burns repair. J. Tissue Eng. Regen. Med. 2017, 11, 3544–3555. [Google Scholar] [CrossRef]
- Mitra, T.; Manna, P.J.; Raja, S.T.K.; Gnanamani, A.; Kundu, P.P. Curcumin loaded nano graphene oxide reinforced fish scale collagen–a 3D scaffold biomaterial for wound healing applications. RSC Adv. 2015, 5, 98653–98665. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cyclodextrin nanosponge based hydrogel for the transdermal co-delivery of curcumin and resveratrol: Development, optimization, in vitro and ex vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 55–64. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciuc, A.-M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Saleem, S.; Iqubal, M.K.; Garg, S.; Ali, J.; Baboota, S. Trends in nanotechnology-based delivery systems for dermal targeting of drugs: An enticing approach to offset psoriasis. Expert Opin. Drug Deliv. 2020, 17, 817–838. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Wissink, M.J.B.; Beernink, R.; Pieper, J.S.; Poot, A.A.; Engbers, G.H.M.; Beugeling, T.; Van Aken, W.G.; Feijen, J. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials 2001, 22, 151–163. [Google Scholar] [CrossRef]
- Lee, K.-S.; Chang, Y.-W. Thermal, mechanical, and rheological properties of poly(ε-caprolactone)/halloysite nanotube nanocomposites. J. Appl. Polym. Sci. 2013, 128, 2807–2816. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- United States Pharmacopeia. USP29-Transdermal Delivery Systems-General Drug Release Standards; United States Pharmacopeia: Rockville, MD, USA, 2009. [Google Scholar]
- Commission, E.P. 2.9. 4 Dissolution Test for Transdermal Patches; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2008. [Google Scholar]
- Nguyen, M.-H.; Lee, S.E.; Tran, T.-T.; Bui, C.-B.; Nguyen, T.-H.-N.; Vu, N.-B.-D.; Tran, T.-T.; Nguyen, T.-H.-P.; Nguyen, T.-T.; Hadinoto, K. A simple strategy to enhance the in vivo wound-healing activity of curcumin in the form of self-assembled nanoparticle complex of curcumin and oligochitosan. Mater. Sci. Eng. C 2019, 98, 54–64. [Google Scholar] [CrossRef]
- Akhtar, F.; Rizvi, M.M.A.; Kar, S.K. Oral delivery of curcumin bound to chitosan nanoparticles cured Plasmodium yoelii infected mice. Biotechnol. Adv. 2012, 30, 310–320. [Google Scholar] [CrossRef]
- Chuah, L.H.; Roberts, C.J.; Billa, N.; Abdullah, S.; Rosli, R. Cellular uptake and anticancer effects of mucoadhesive curcumin-containing chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 116, 228–236. [Google Scholar] [CrossRef]
- Khan, M.A.; Zafaryab, M.; Mehdi, S.H.; Ahmad, I.; Rizvi, M.M.A. Characterization and anti-proliferative activity of curcumin loaded chitosan nanoparticles in cervical cancer. Int. J. Biol. Macromol. 2016, 93, 242–253. [Google Scholar] [CrossRef]
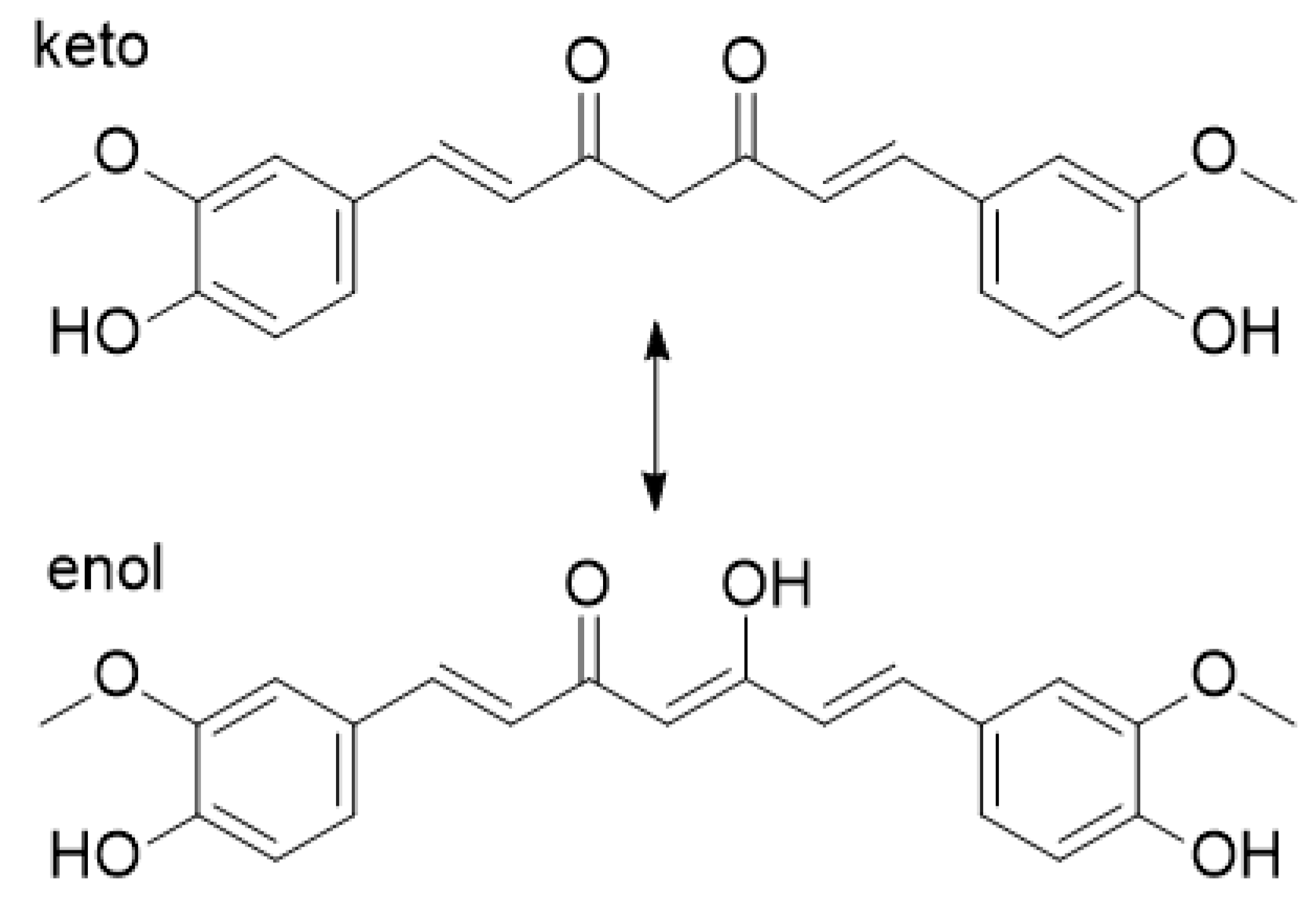
- Kawano, S.; Inohana, Y.; Hashi, Y.; Lin, J.-M. Analysis of keto-enol tautomers of curcumin by liquid chromatography/mass spectrometry. Chin. Chem. Lett. 2013, 24, 685–687. [Google Scholar] [CrossRef]
- Mohan, P.R.K.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water soluble complexes of curcumin with cyclodextrins: Characterization by FT-Raman spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Abureesh, M.A.; Oladipo, A.A.; Gazi, M. Facile synthesis of glucose-sensitive chitosan–poly (vinyl alcohol) hydrogel: Drug release optimization and swelling properties. Int. J. Biol. Macromol. 2016, 90, 75–80. [Google Scholar] [CrossRef]
- Khan, M.A.; Ahmad, S.; Ahmad, I.; Rizvi, M.M.A. Anti-Proliferative Activity of Curcumin Loaded PLGA Nanoparticles for Prostate Cancer. In Nanotechnology Applied To Pharmaceutical Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 267–278. [Google Scholar]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Wickramathilaka, M.P.; Tao, B.Y. Characterization of covalent crosslinking strategies for synthesizing DNA-based bioconjugates. J. Biol. Eng. 2019, 13, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michailidou, G.; Ainali, N.M.; Xanthopoulou, E.; Nanaki, S.; Kostoglou, M.; Koukaras, E.N.; Bikiaris, D.N. Effect of Poly (vinyl alcohol) on Nanoencapsulation of Budesonide in Chitosan Nanoparticles via Ionic Gelation and Its Improved Bioavailability. Polym. 2020, 12, 1101. [Google Scholar] [CrossRef]
- Koumentakou, I.; Terzopoulou, Z.; Michopoulou, A.; Kalafatakis, I.; Theodorakis, K.; Tzetzis, D.; Bikiaris, D. Chitosan dressings containing inorganic additives and levofloxacin as potential wound care products with enhanced hemostatic properties. Int. J. Biol. Macromol. 2020, 162, 693–703. [Google Scholar] [CrossRef]
- Suchý, T.; Šupová, M.; Sauerová, P.; Verdánová, M.; Sucharda, Z.; Rýglová, Š.; Žaloudková, M.; Sedláček, R.; Kalbáčová, M.H. The effects of different cross-linking conditions on collagen-based nanocomposite scaffolds—An in vitro evaluation using mesenchymal stem cells. Biomed. Mater. 2015, 10, 65008. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skopinska-Wisniewska, J.; Gawron, M.; Kozlowska, J.; Planecka, A. Chemical and thermal cross-linking of collagen and elastin hydrolysates. Int. J. Biol. Macromol. 2010, 47, 570–577. [Google Scholar] [CrossRef]
- Bak, S.Y.; Lee, S.W.; Choi, C.H.; Kim, H.W. Assessment of the Influence of Acetic Acid Residue on Type I Collagen during Isolation and Characterization. Materials 2018, 11, 2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Z.; Zheng, X.; Tang, K.; Liu, J.; Ma, Z.; Zhao, Q. Dissolution and regeneration of collagen fibers using ionic liquid. Int. J. Biol. Macromol. 2012, 51, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Huh, C.H.; Seo, K.I.; Suh, D.H.; Youn, J.I. Evaluation of skin surface hydration in Korean psoriasis patients: A possible factor influencing psoriasis. Clin. Exp. Dermatol. 2002, 27, 147–152. [Google Scholar] [CrossRef]
- Rim, J.H.; Jo, S.J.; Park, J.Y.; Park, B.D.; Youn, J.I. Electrical measurement of moisturizing effect on skin hydration and barrier function in psoriasis patients. Clin. Exp. Dermatol. 2005, 30, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Charulatha, V.; Rajaram, A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 2003, 24, 759–767. [Google Scholar] [CrossRef]
- Yang, C. Enhanced physicochemical properties of collagen by using EDC/NHS-crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Parsa, P.; Paydayesh, A.; Davachi, S.M. Investigating the effect of tetracycline addition on nanocomposite hydrogels based on polyvinyl alcohol and chitosan nanoparticles for specific medical applications. Int. J. Biol. Macromol. 2019, 121, 1061–1069. [Google Scholar] [CrossRef]
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly (ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr. Polym. 2012, 88, 84–90. [Google Scholar] [CrossRef]
- Afshar, M.; Dini, G.; Vaezifar, S.; Mehdikhani, M.; Movahedi, B. Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Coburn, J.M.; Lozano-Pérez, A.A.; Cenis, J.L.; Víllora, G.; Kaplan, D.L. Production of curcumin-loaded silk fibroin nanoparticles for cancer therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Guo, B.; Ma, P.X. Injectable alginate microsphere/PLGA–PEG–PLGA composite hydrogels for sustained drug release. RSC Adv. 2014, 4, 17736–17742. [Google Scholar] [CrossRef]
- Rezaei, M.; Oryan, S.; Nourani, M.R.; Mofid, M.; Mozafari, M. Curcumin nanoparticle-incorporated collagen/chitosan scaffolds for enhanced wound healing. Bioinspiredbiomim Nanobiomater. 2018, 7, 159–166. [Google Scholar] [CrossRef]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef] [PubMed]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef]






| Sample | Diameter (nm) | Z-Potential (mV) | Nanoparticle Yield (%) | Encapsulation Efficiency (%) | Loading Capacity (%) |
|---|---|---|---|---|---|
| CS-NPs | 160 ± 40 | 38 | 43.0 | - | - |
| CS-Cur-NPs | 164 ± 52 | 38 | 46.8 | 34% | 3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzopoulou, Z.; Michopoulou, A.; Palamidi, A.; Koliakou, E.; Bikiaris, D. Preparation and Evaluation of Collagen-Based Patches as Curcumin Carriers. Polymers 2020, 12, 2393. https://doi.org/10.3390/polym12102393
Terzopoulou Z, Michopoulou A, Palamidi A, Koliakou E, Bikiaris D. Preparation and Evaluation of Collagen-Based Patches as Curcumin Carriers. Polymers. 2020; 12(10):2393. https://doi.org/10.3390/polym12102393
Chicago/Turabian StyleTerzopoulou, Zoi, Anna Michopoulou, Artemis Palamidi, Elena Koliakou, and Dimitrios Bikiaris. 2020. "Preparation and Evaluation of Collagen-Based Patches as Curcumin Carriers" Polymers 12, no. 10: 2393. https://doi.org/10.3390/polym12102393
APA StyleTerzopoulou, Z., Michopoulou, A., Palamidi, A., Koliakou, E., & Bikiaris, D. (2020). Preparation and Evaluation of Collagen-Based Patches as Curcumin Carriers. Polymers, 12(10), 2393. https://doi.org/10.3390/polym12102393