Multifunctional Hypercrosslinked Porous Organic Polymers Based on Tetraphenylethene and Triphenylamine Derivatives for High-Performance Dye Adsorption and Supercapacitor
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
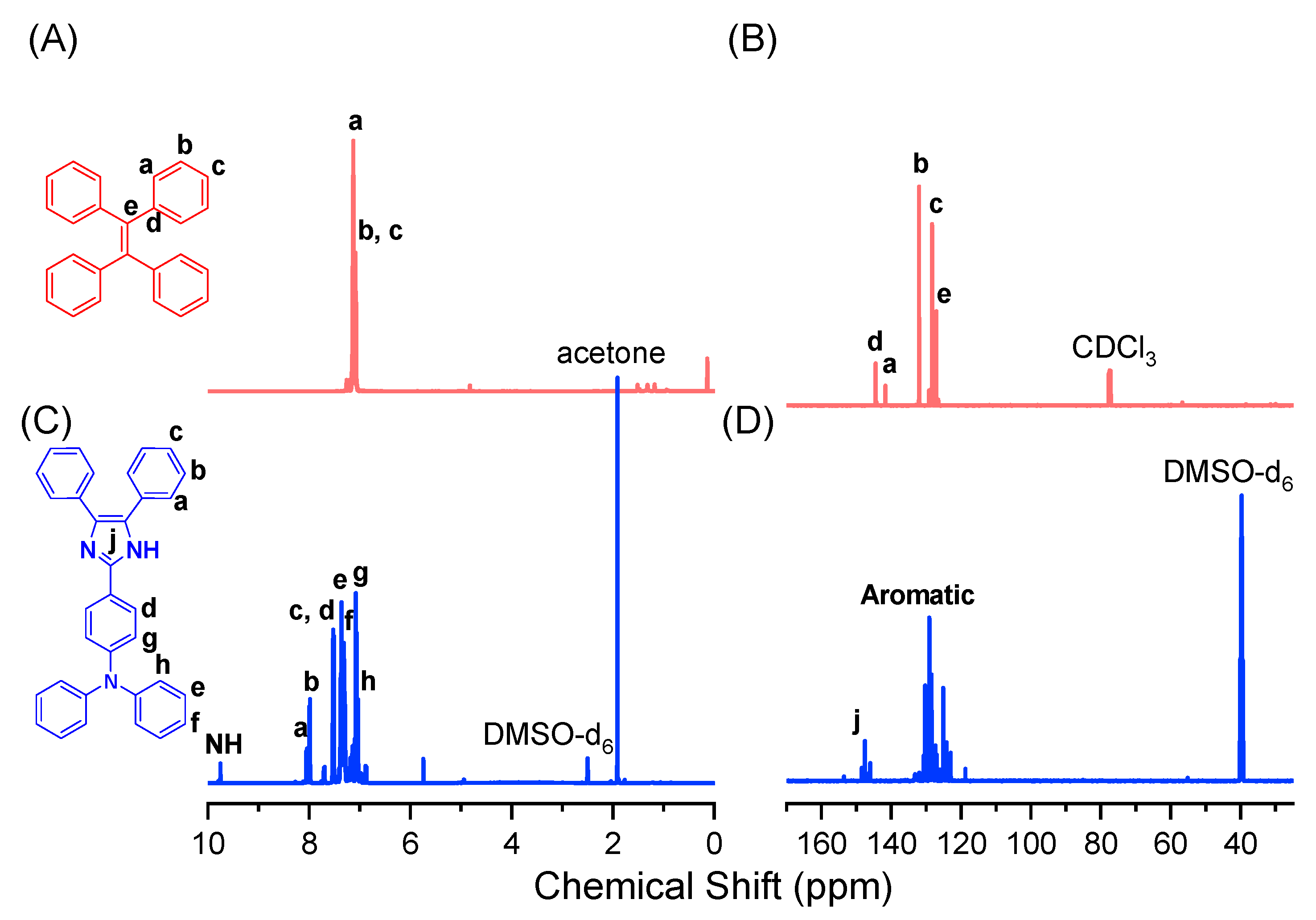
2.2. Synthesis of Tetraphenylethylene (TPE)
2.3. Synthesis of 4-(5,6-Diphenyl-1H-Benzimidazol-2-yl)-Triphenylamine (DPT)
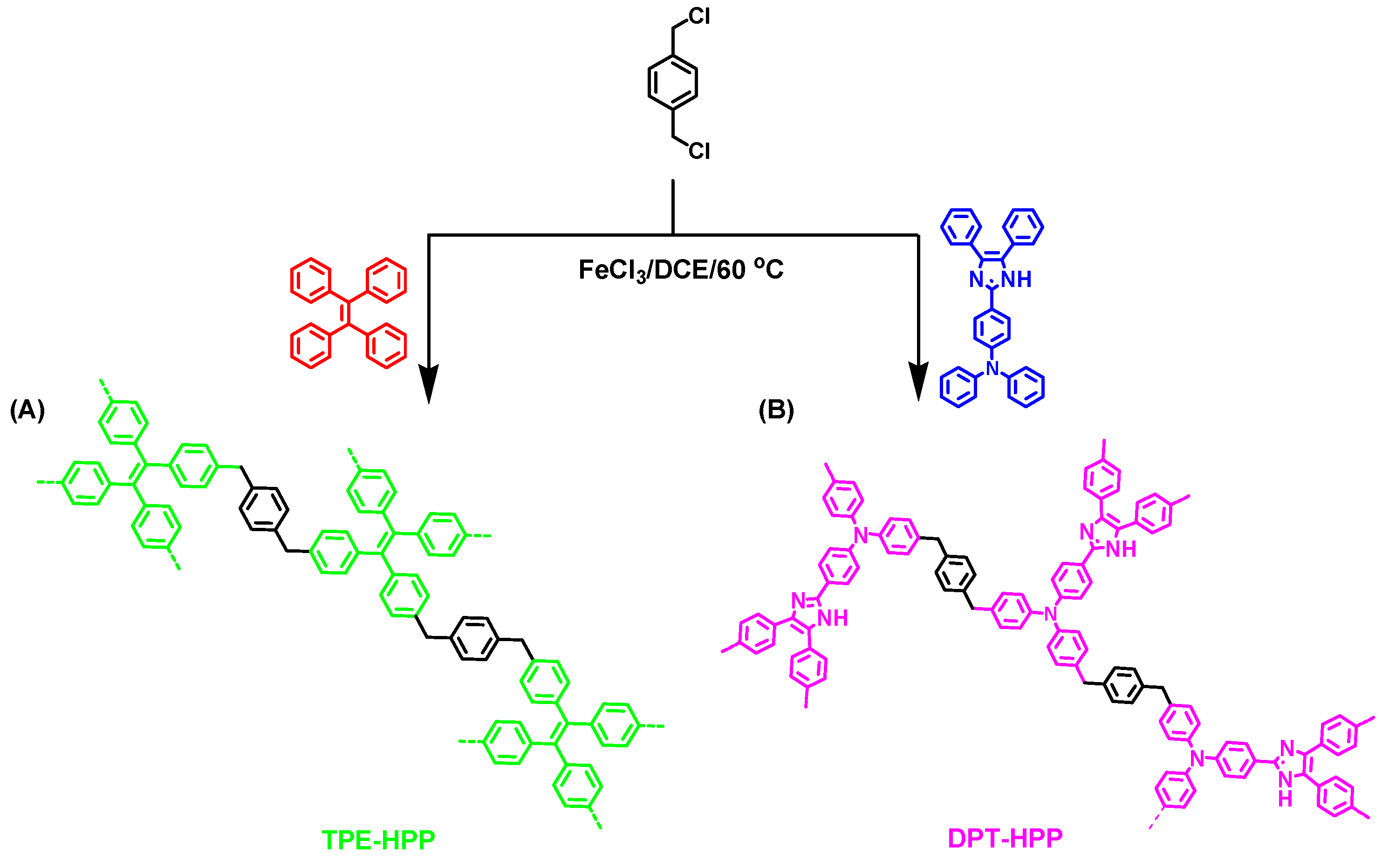
2.4. Synthesis of TPE-HPP
2.5. Synthesis of DPT-HPP
3. Results and Discussion
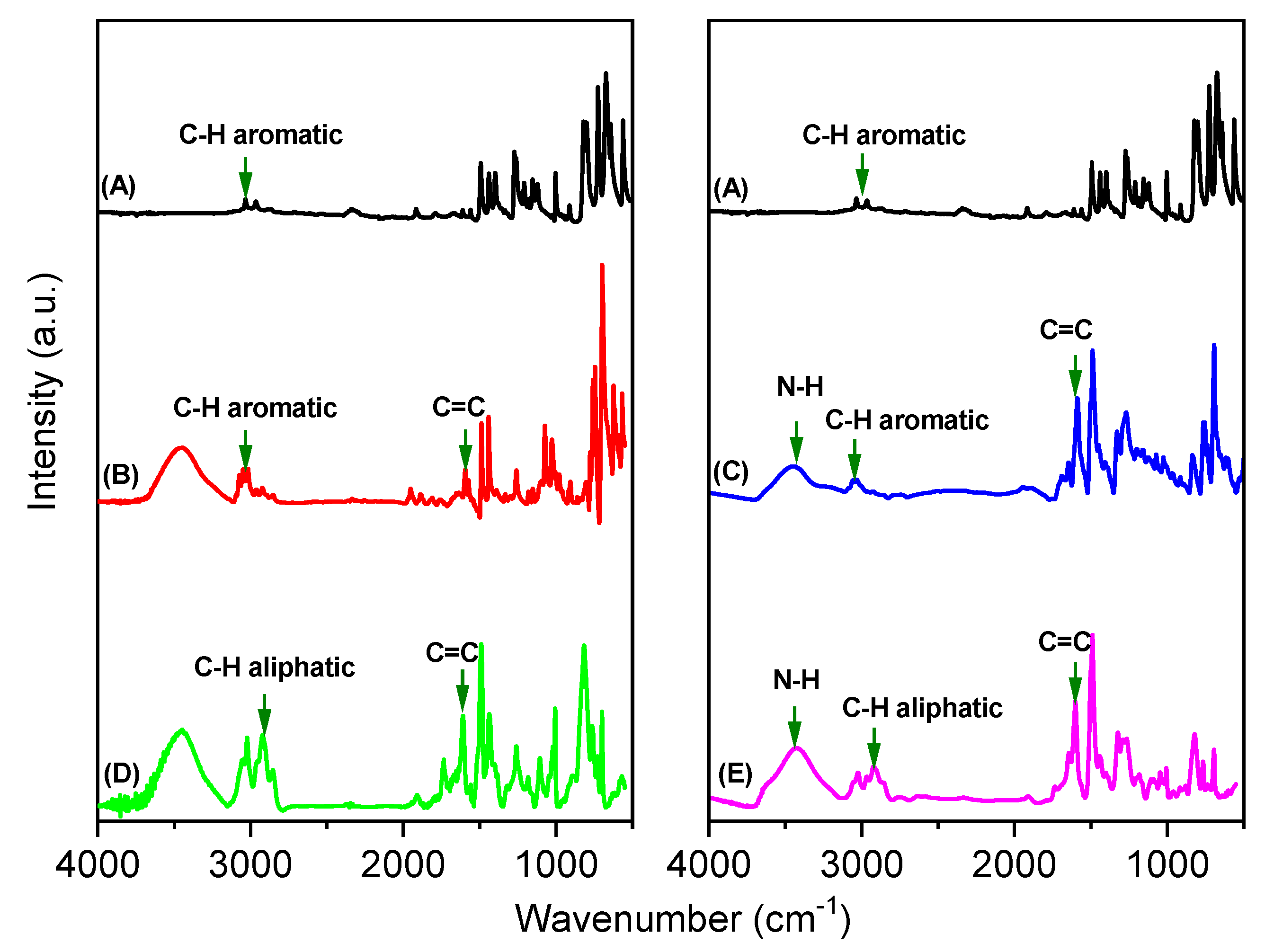
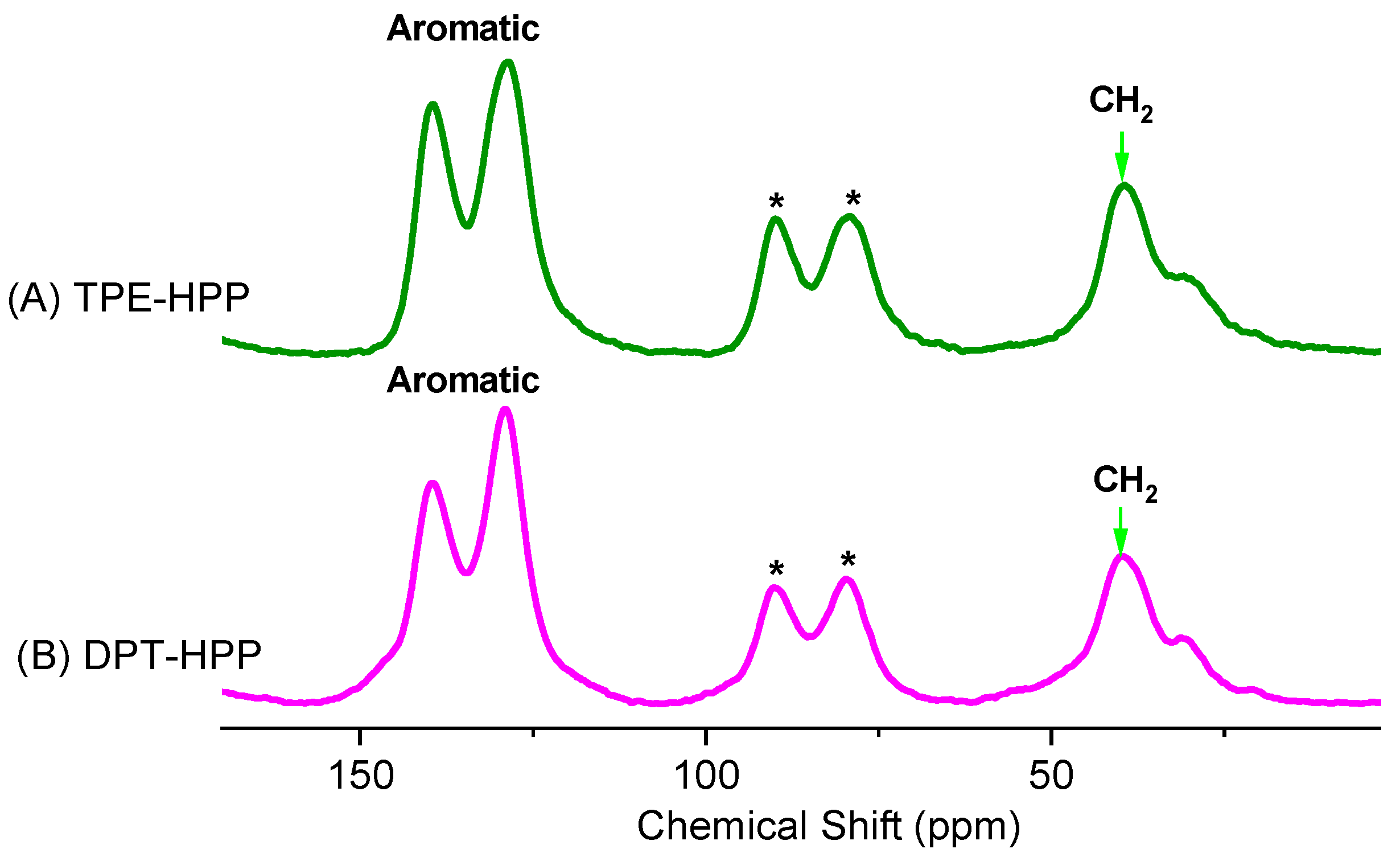
3.1. Synthesis of TPE-HPP and DPT-HPP
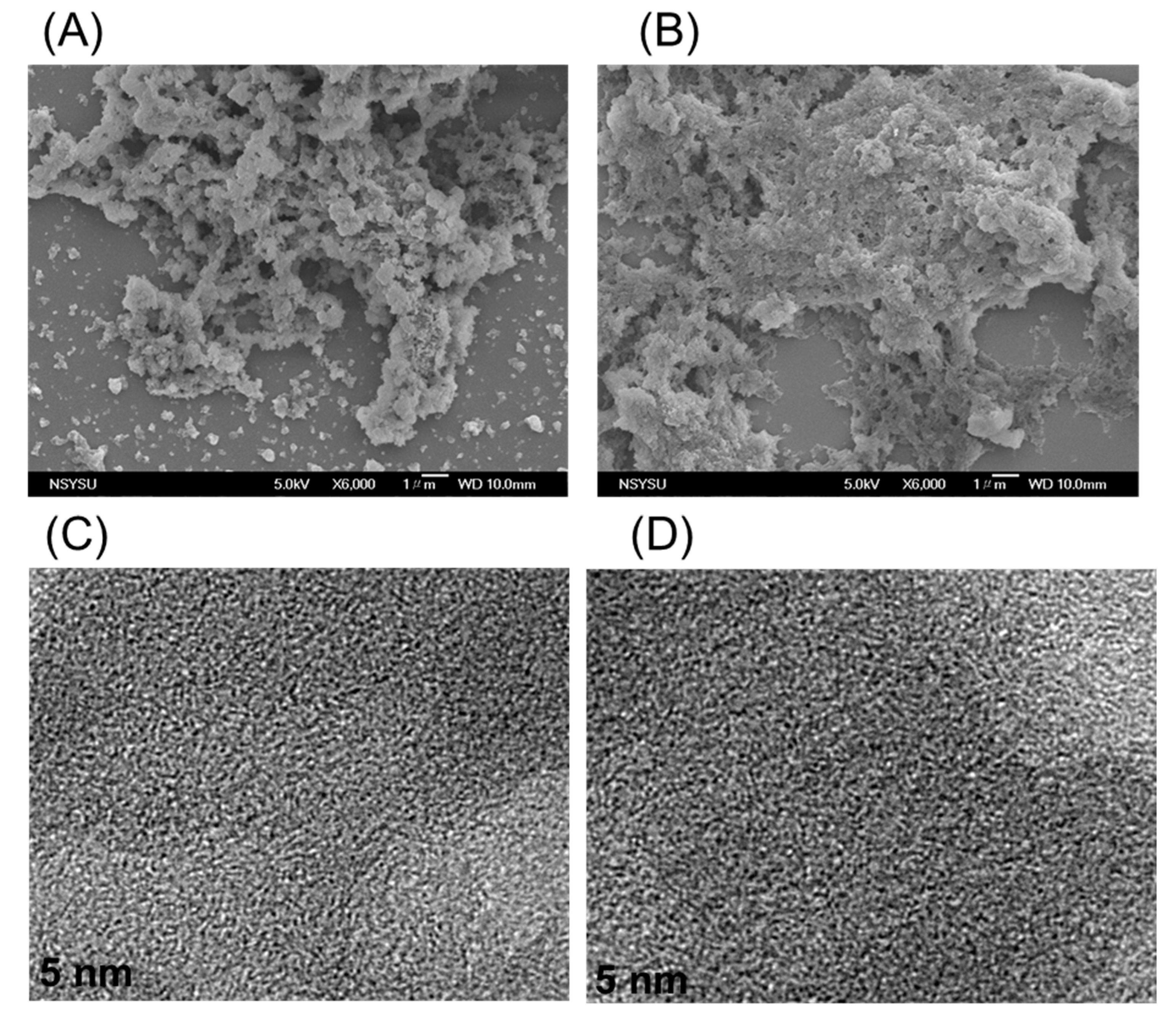
3.2. TGA, XRD, BET, SEM, and TEM Analyses for TPE-HPP and DPT-HPP
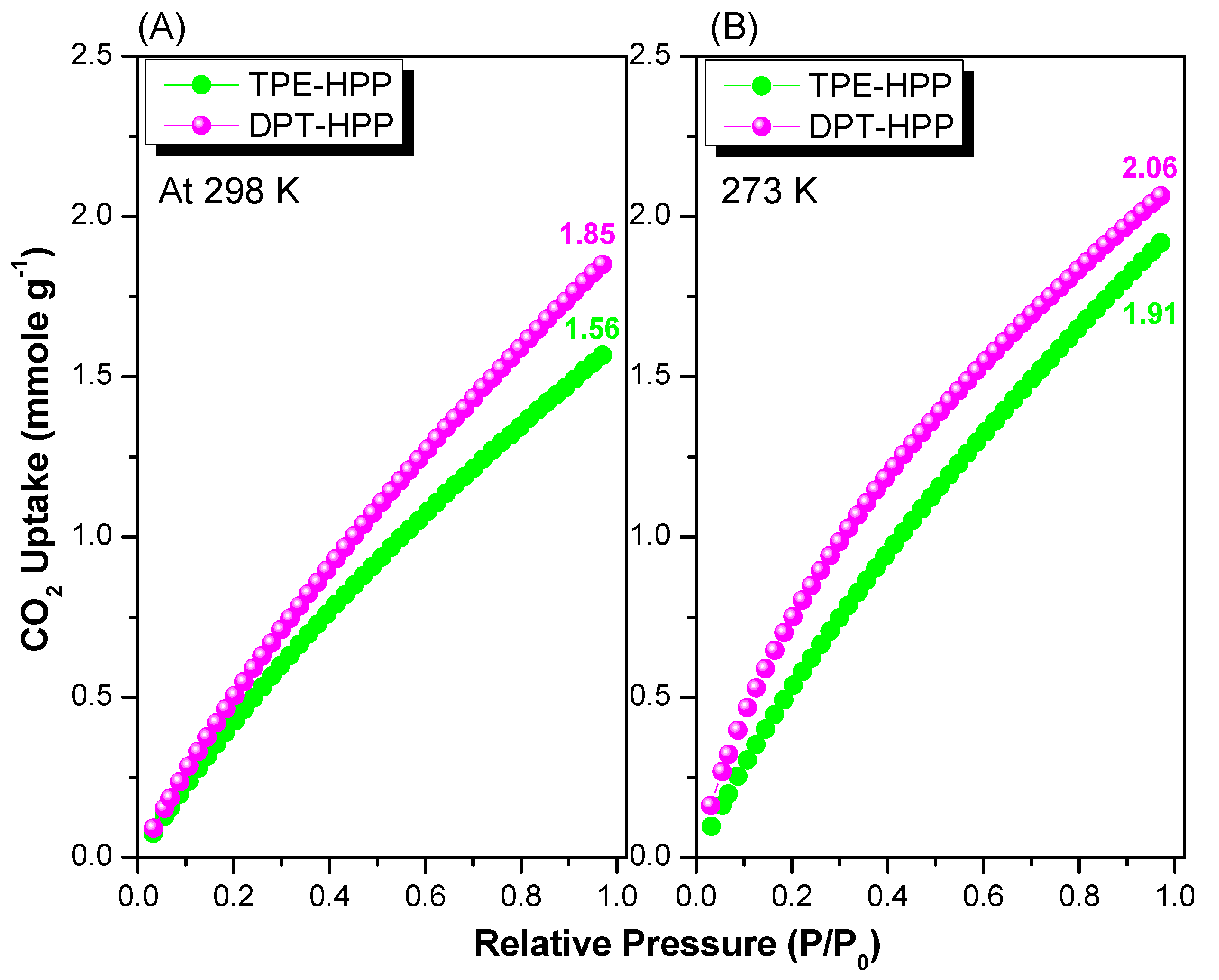
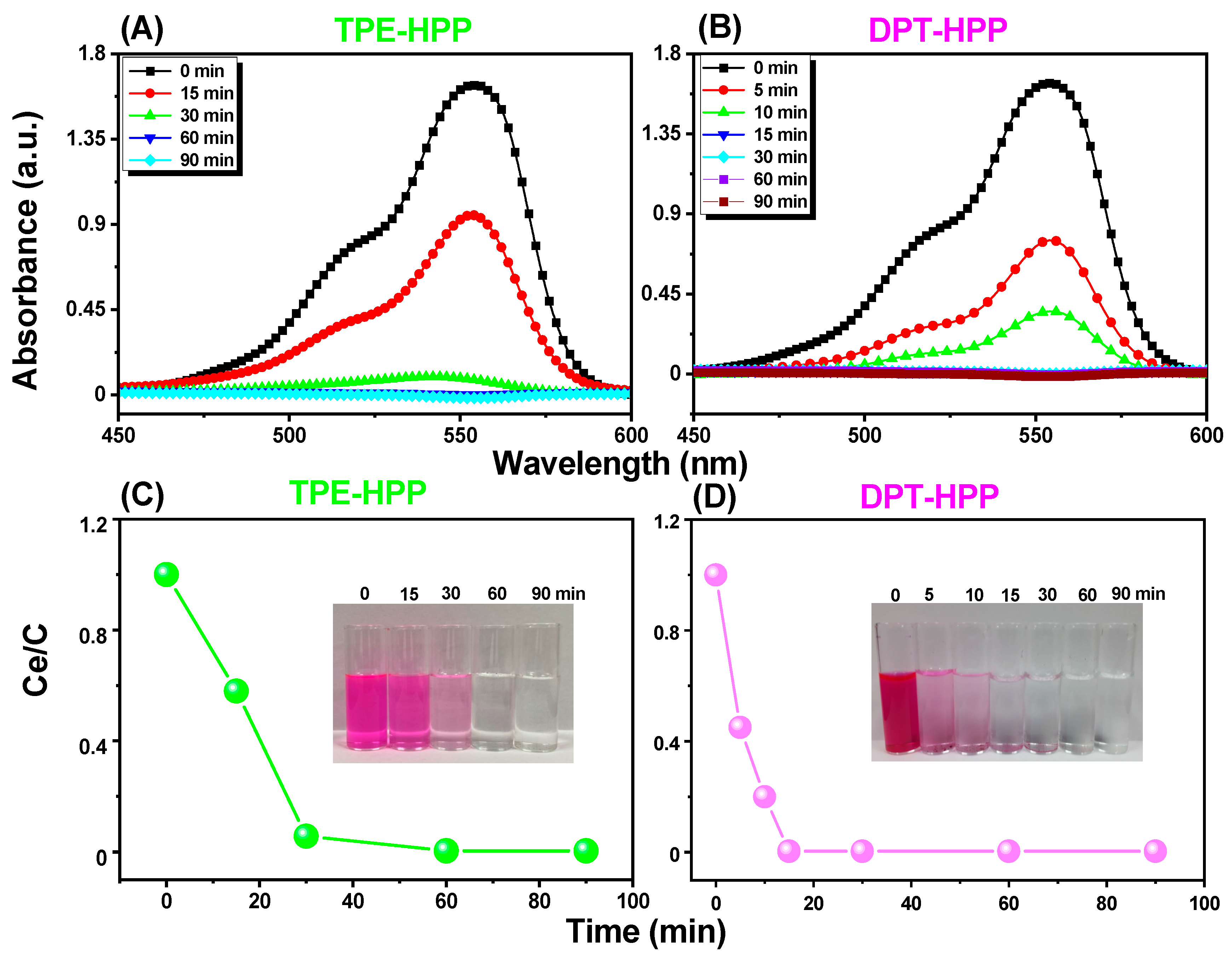
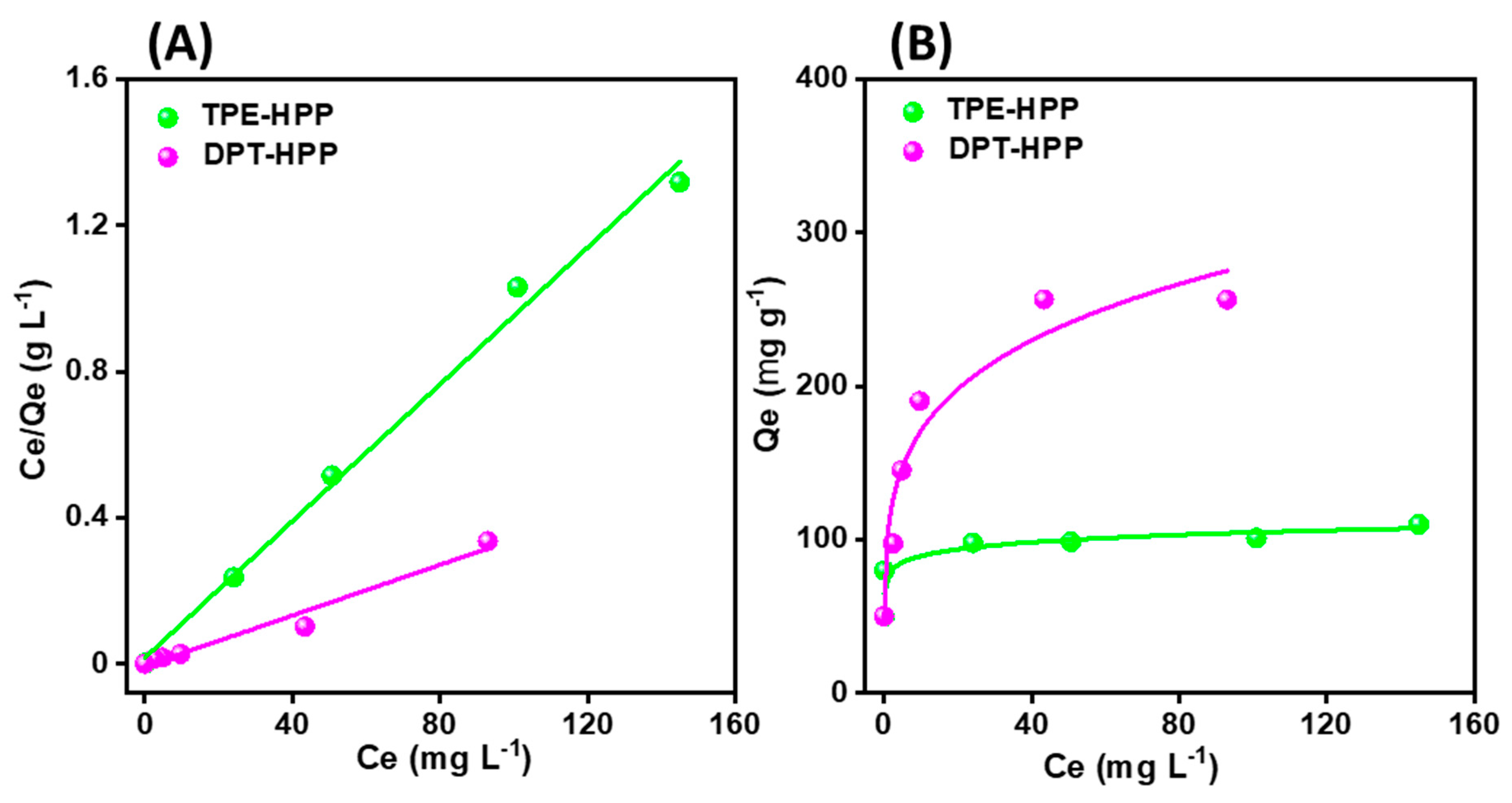
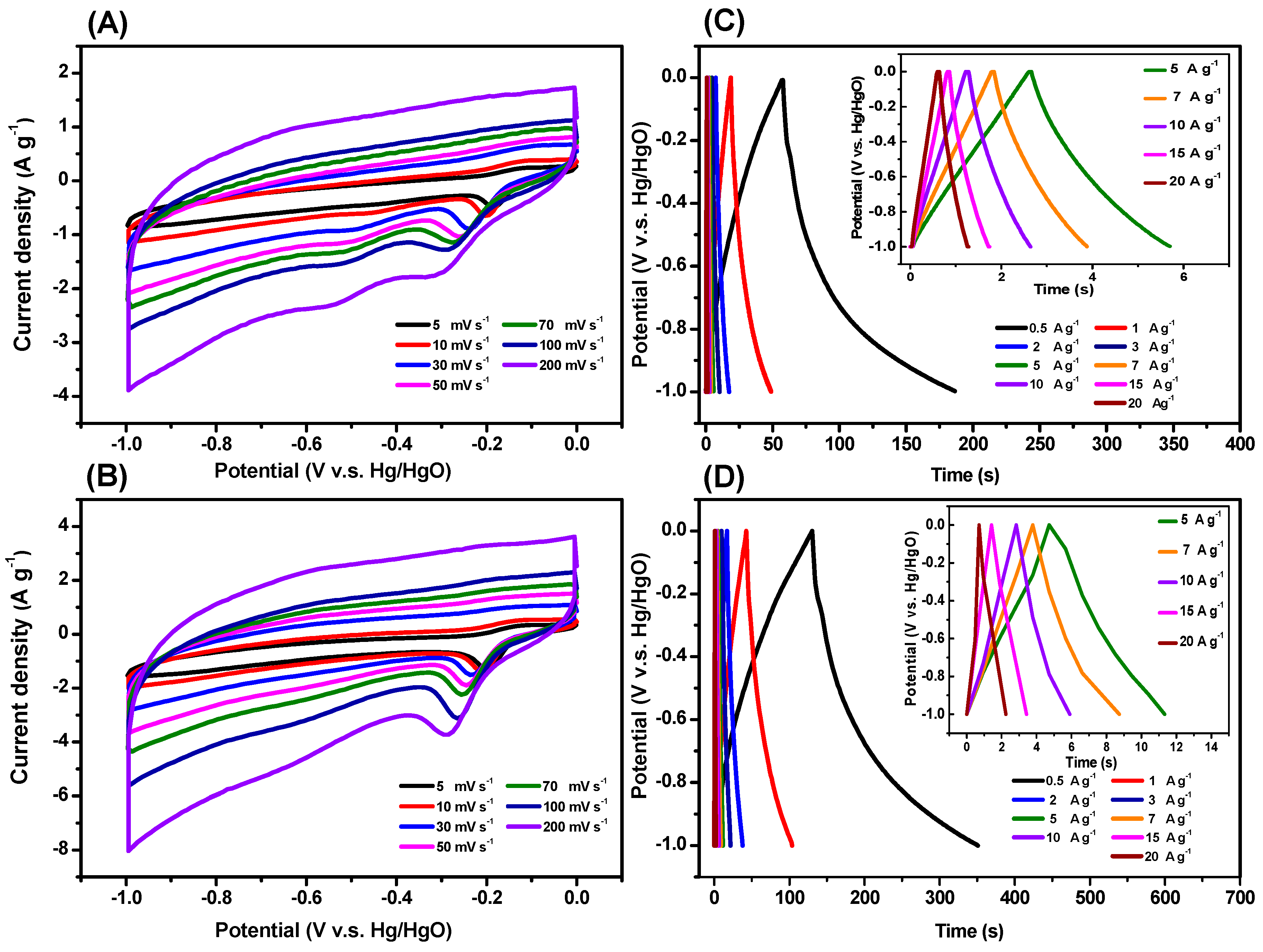
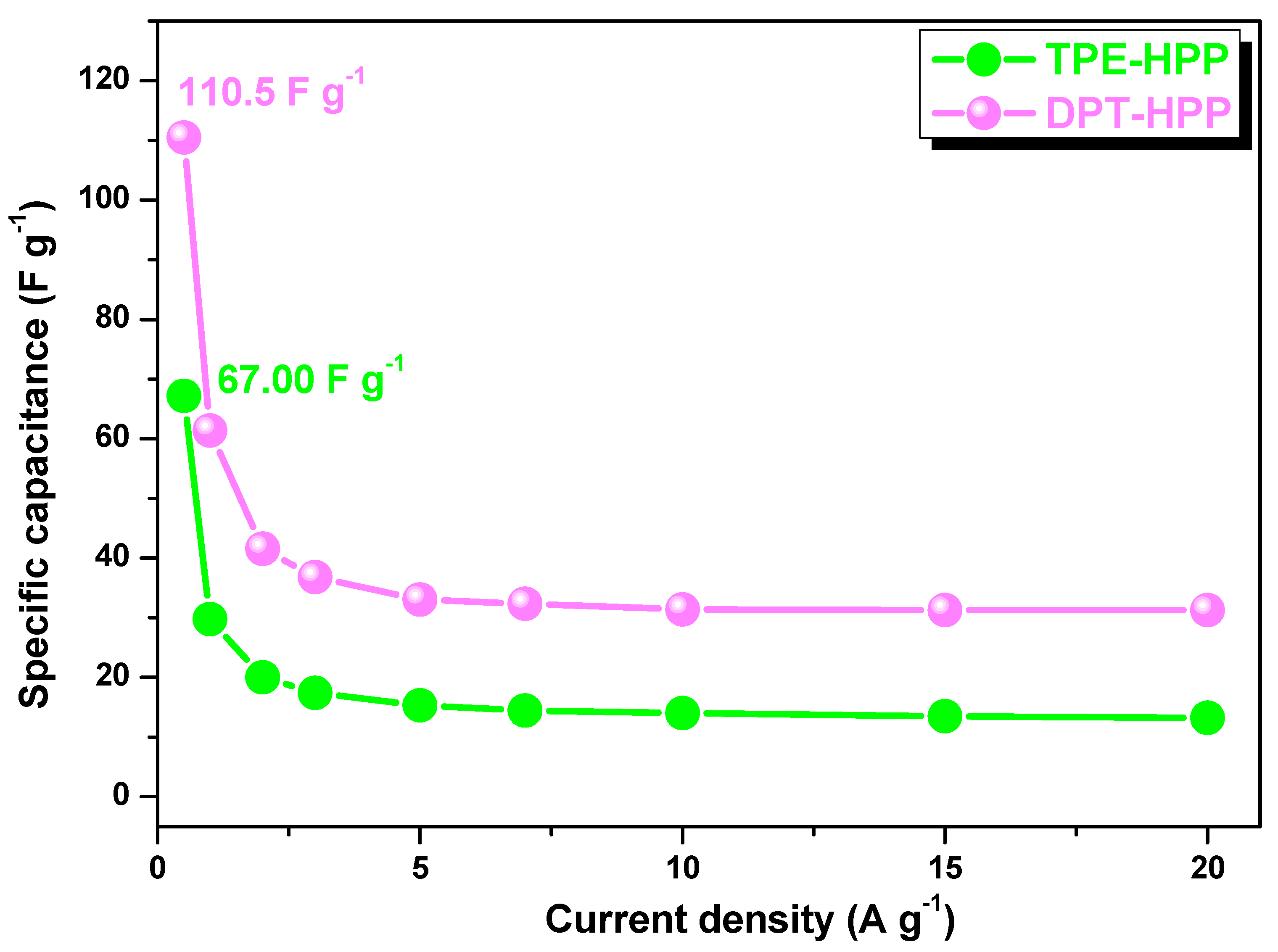
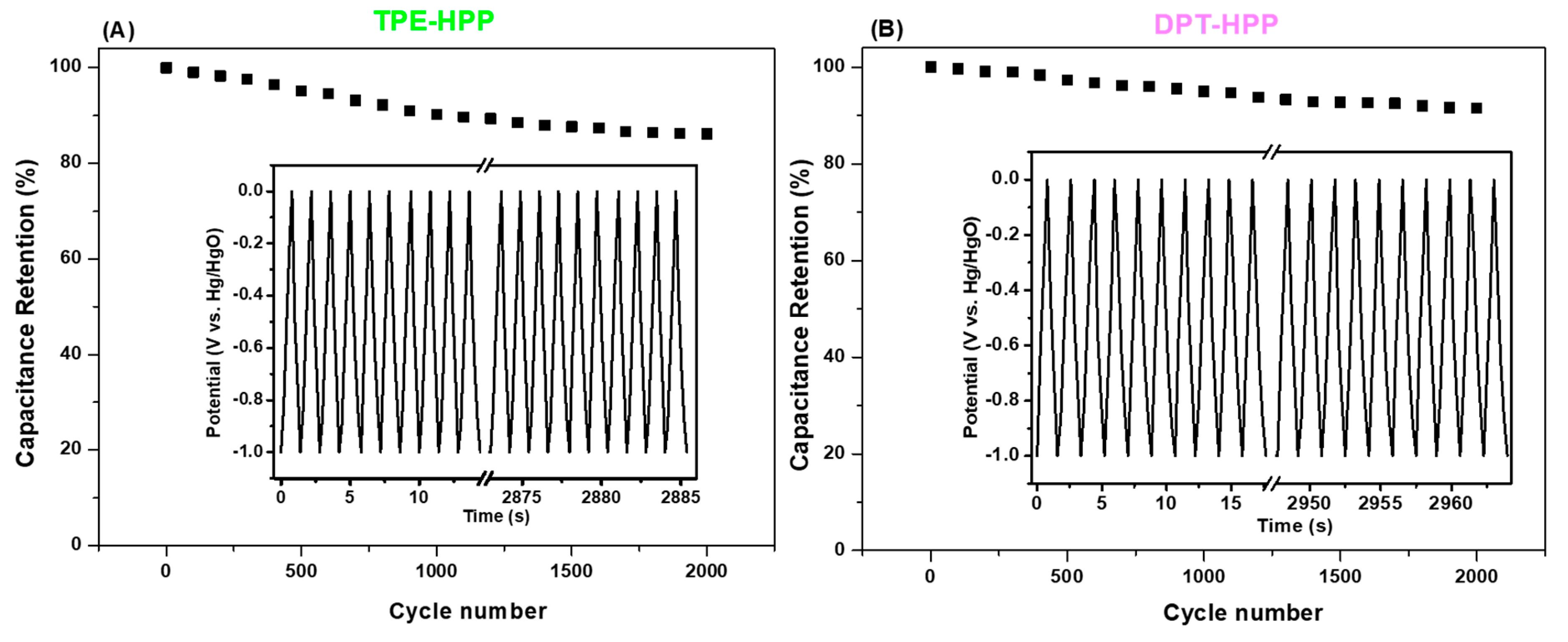
3.3. CO2 Uptake, Dye Adsorption and Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, T.X.; Liang, H.P.; Anito, D.A.; Ding, X.; Han, B.H. Emerging applications of porous organic polymers in visible-light photocatalysis. J. Mater. Chem. A 2020, 8, 7003–7034. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Chen, G.; Huang, J. Oxygen-rich porous carbons from carbonyl modified hyper-cross-linked polymers for efficient CO2 capture. J. Polym. Res. 2020, 27, 36. [Google Scholar] [CrossRef]
- Lv, H.; Wang, W.; Li, F. Porous organic polymers with built-in N-heterocyclic carbenes: Selective and efficient heterogeneous catalyst for the reductive N-formylation of amines with CO2. Chem. Eur. J. 2018, 24, 16588–16594. [Google Scholar] [CrossRef] [PubMed]
- Aly, K.I.; Sayed, M.M.; Mohamed, M.G.; Kuo, S.W.; Younis, O. A facile synthetic route and dual function of network luminescent porous polyester and copolyester containing porphyrin moiety for metal ions sensor and dyes adsorption. Micropor. Mesopor. Mater. 2020, 298, 110063. [Google Scholar] [CrossRef]
- Spychalska, K.; Zając, D.; Baluta, S.; Halicka, K.; Cabaj, J. Functional Polymers Structures for (Bio)Sensing Application—A Review. Polymers 2020, 12, 1154. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Je, S.H.; Talapanani, S.N.; Coskun, A. Advances in Porous Organic Polymers for Efficient Water Capture. Chem. Eur. J. 2019, 25, 10262–10283. [Google Scholar] [CrossRef] [Green Version]
- Abuzeid, H.R.; EL-Mahdy, A.F.M.; Ahmed, M.M.M.; Kuo, S.W. Triazine-functionalized covalent benzoxazine framework for direct synthesis of N-doped microporous carbon. Polym. Chem. 2019, 10, 6010–6020. [Google Scholar] [CrossRef]
- Ignacz, G.; Fei, F.; Szekely, G. Ion-Stabilized Membranes for Demanding Environments Fabricated from Polybenzimidazole and Its Blends with Polymers of Intrinsic Microporosity. ACS Appl. Nano Mater. 2018, 11, 6349–6356. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Atayde, E.C., Jr.; Matsagar, B.M.; Na, J.; Yamauchi, Y.; Wu, K.C.W.; Kuo, S.W. Construction Hierarchically Mesoporous/Microporous Materials Based on Block Copolymer and Covalent Organic Framework. J. Taiwan Inst. Chem. Eng. 2020, 122, 180–192. [Google Scholar] [CrossRef]
- Topuz, F.; Abdulhamid, M.A.; Nunes, S.P.; Szekely, G. Hierarchically porous electrospun nanofibrous mats produced from intrinsically microporous fluorinated polyimide for the removal of oils and non-polar solvents. Environ. Sci. Nano 2020, 7, 1365–1372. [Google Scholar] [CrossRef]
- Cousins, K.; Zhang, R. Highly Porous Organic Polymers for Hydrogen Fuel Storage. Polymers 2019, 11, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.C.; Esposito, E.; Fuoco, A.; Carta, M. Microporous Organic Polymers: Synthesis, Characterization, and Applications. Polymers 2019, 11, 844. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.G.; EL-Mahdy, A.F.M.; Takashi, Y.; Kuo, S.W. Ultrastable conductive microporous covalent triazine frameworks based on pyrene moieties provide high-performance CO2 uptake and supercapacitance. New J. Chem. 2020, 44, 8241–8253. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Ebrahium, S.M.; Hammam, A.S.; Kuo, S.W.; Aly, K.I. Enhanced CO2 capture in nitrogen-enriched microporous carbons derived from Polybenzoxazines containing azobenzene and carboxylic acid units. J. Polym. Res. 2020, 27, 197. [Google Scholar] [CrossRef]
- Cui, Y.; Du, J.; Liu, Y.; Yu, Y.; Wang, S.; Pang, H.; Liang, Z.; Yu, J. Design and synthesis of multifunctional porous N-rich polymer containing s-triazine and Troöger’s base for CO2 adsorption, catalysis and sensing. Polym. Chem. 2018, 9, 2643–2649. [Google Scholar] [CrossRef]
- Jiang, W.; Yue, H.; Shuttleworth, P.S.; Xie, P.; Li, S.; Guo, J. Adamantane-Based Micro- and Ultra-Microporous Frameworks for Efficient Small Gas and Toxic Organic Vapor Adsorption. Polymers 2019, 11, 486. [Google Scholar] [CrossRef] [Green Version]
- McKeown, N.B.; Budd, P.M.; Book, D. Microporous polymers as potential hydrogen storage materials. Macromol. Rapid Commun. 2007, 28, 995–1002. [Google Scholar] [CrossRef]
- Li, J.G.; Lee, P.Y.; Ahmed, M.M.M.; Mohamed, M.G.; Kuo, S.W. Varying the Hydrogen Bonding Strength in Phenolic/PEO-b-PLA Blends Provides Mesoporous Carbons Having Large Accessible Pores Suitable for Energy Storage. Macromol. Chem. Phys. 2020, 221, 2000040. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Hung, W.S.; EL-Mahdy, A.F.M.; Ahmed, M.M.M.; Dai, L.; Chen, T.; Kuo, S.W. High-Molecular-Weight PLA-b-PEO-b-PLA Triblock Copolymer Templated Large Mesoporous Carbons for Supercapacitors and CO2 Capture. Polymers 2020, 12, 1193. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Ye, Y.; Meng, X.; Du, J.; Song, X.; Liang, Z. Ultrahigh volatile iodine capture by conjugated microporous polymer based on N,N,N’,N’-tetraphenyl-1,4-phenylenediamine. Polym. Chem. 2019, 10, 2608–2615. [Google Scholar]
- Li, W.T.; Zhuang, Y.T.; Wang, J.Y.; Yang, T.; Yu, Y.L.; Chen, M.L.; Wang, J.H. A three-dimensional porous organic framework for highly selective capture of mercury and copper ions. ACS Appl. Polym. Mater. 2019, 1, 2797–2806. [Google Scholar]
- Kumar, R.; Shunmugam, R. Unique design of porous organic framework Showing efficiency toward removal of toxicants. ACS Omega 2017, 2, 4100–4107. [Google Scholar] [PubMed] [Green Version]
- Tan, Z.; Su, H.; Guo, Y.; Liu, H.; Liao, B.; Amin, A.M.; Liu, Q. Ferrocene-Based Conjugated Microporous Polymers Derived from Yamamoto Coupling for Gas Storage and Dye Removal. Polymers 2020, 12, 719. [Google Scholar]
- Xie, Y.; Wang, T.T.; Liu, X.H.; Zou, K.; Deng, W.Q. Capture and conversion of CO2 at ambient conditions by a conjugated microporous polymer. Nat. Commun. 2013, 4, 1960. [Google Scholar] [PubMed] [Green Version]
- Jiang, J.X.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z.; et al. Conjugated Microporous Poly(aryleneethynylene) Networks. Angew. Chem. Int. Ed. 2007, 46, 8574–8578. [Google Scholar]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [PubMed] [Green Version]
- Mohamed, M.G.; Lee, C.C.; EL-Mahdy, A.F.M.; Luder, J.; Yu, M.H.; Li, Z.; Zhu, Z.; Chueh, C.C.; Kuo, S.W. Exploitation of Two-Dimensional Conjugated Covalent Organic Frameworks Based on Tetraphenylethylene with Bicarbazole and Pyrene Units and Applications in Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 11448–11459. [Google Scholar]
- EL-Mahdy, A.F.M.; Mohamed, M.G.; Mansoure, T.H.; Yu, H.H.; Chen, T.; Kuo, S.W. Ultrastable tetraphenyl-p-phenylenediamine-based covalent organic frameworks as platforms for high-performance electrochemical supercapacitors. Chem. Commun. 2019, 55, 14890–14893. [Google Scholar]
- EL-Mahdy, A.F.M.; Kuo, C.H.; Alshehri, A.; Young, C.; Yamauchi, Y.; Kim, J.; Kuo, S.W. Strategic design of triphenylamine- and triphenyltriazine-based two-dimensional covalent organic frameworks for CO2 uptake and energy storage. J. Mater. Chem. A 2018, 6, 19532–19541. [Google Scholar]
- Vopička, O.; Friess, K.; Hynek, V.; Sysel, P.; Zgazar, M.; Sipek, M.; Pilnacek, M.K.; Lanc, M.; Jansen, J.C.; Mason, C.R.; et al. Equilibrium and transient sorption of vapours and gases in the polymer of intrinsic microporosity PIM-1. J. Membr. Sci. 2013, 434, 148–160. [Google Scholar] [CrossRef]
- Mohamed, M.G.; EL-Mahdy, A.F.M.; Ahmed, M.M.M.; Kuo, S.W. Direct Synthesis of Microporous Bicarbazole-Based Covalent Triazine Frameworks for High-Performance Energy Storage and Carbon Dioxide Uptake. ChemPlusChem 2019, 84, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, L.; Jin, S.; Tan, B. Covalent triazine frameworks: Synthesis and applications. J. Mater. Chem. A 2019, 7, 5153–5172. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Hung, Y.H.; Mansoure, T.H.; Yu, H.H.; Hsu, Y.S.; Wu, K.C.W.; Kuo, S.W. Synthesis of [3+3] β-ketoenamine-tethered covalent organic frameworks (COFs) for high-performance supercapacitance and CO2 storage. J. Taiwan Inst. Chem. Eng. 2019, 103, 199–208. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wang, D.; Suwansoontorn, A.; Du, G.; Liu, Z.; Hasan, M.D.; Nagao, Y. A simple and cost-effective synthesis of ionic porous organic polymers with excellent porosity for high iodine capture. Polymer 2020, 204, 122796. [Google Scholar]
- Penchah, H.R.; Ghaemi, A.; Gilani, H.G. Benzene-Based Hyper-Cross-Linked Polymer with Enhanced Adsorption Capacity for CO2 Capture. Energy Fuels 2019, 33, 12578–12586. [Google Scholar]
- Li, B.; Gong, R.; Wang, W.; Huang, X.; Zhangm, W.; Li, H.; Hu, C.; Tan, B. A New Strategy to Microporous Polymers: Knitting Rigid Aromatic Building Blocks by External Cross-Linker. Macromolecules 2011, 4, 2410–2414. [Google Scholar] [CrossRef]
- Pan, L.; Chen, Q.; Zhu, J.H.; Yu, J.G.; He, Y.J.; Han, B.H. Hypercrosslinked porous polycarbazoles via one-step oxidative coupling reaction and Friedel–Crafts alkylation. Polym. Chem. 2015, 6, 2478–2487. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Zhang, X.; Mansoure, T.H.; EL-Mahdy, A.F.M.; Huang, C.F.; Danko, M.; Xin, Z.; Kuo, S.W. Hypercrosslinked porous organic polymers based on tetraphenylanthraquinone for CO2 uptake and high-performance supercapacitor. Polymer 2020, 205, 122857. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, X.; Matsuo, Y.; Song, J.; Zhao, R.; Faheem, M.; Chen, M.; Zhang, Y.; Tian, Y.; Zhu, G. Fluorescein-based fluorescent porous aromatic framework for Fe3+ detection with high sensitivity. J. Mater. Chem. C 2019, 7, 2327–2332. [Google Scholar] [CrossRef]
- Wang, S.; Tu, M.; Peng, T.; Zhang, C.; Li, T.; Hussain, I.; Wang, J.; Tan, B. Porous hypercrosslinked polymer-TiO2-graphene composite photocatalysts for visible-light-driven CO2 conversion. Nat. Commun. 2019, 10, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Fontanals, N.; Marce, R.M.; Borrull, F.; Cormack, P.A.G. Hypercrosslinked materials: Preparation, characterisation, and applications. Polym. Chem. 2015, 6, 7231–7244. [Google Scholar] [CrossRef] [Green Version]
- Tsyurupa, M.P.; Davankov, V.A. Porous structure of hypercrosslinked polystyrene: State-of-the-art mini review. React. Funct. Polym. 2006, 66, 768–779. [Google Scholar] [CrossRef]
- Chen, D.; Fu, Y.; Yu, W.; Yu, G.; Pan, C. Versatile Adamantane-based porous polymers with enhanced microporosity for efficient CO2 capture and iodine removal. Chem. Eng. J. 2018, 334, 900–906. [Google Scholar] [CrossRef]
- Wang, X.; Mu, P.; Zhang, C.; Chen, Y.; Zeng, J.; Wang, F.; Jiang, J.X. Control Synthesis of Tubular Hyper-Cross- Linked Polymers for Highly Porous Carbon Nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 20779–20786. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, J.; Deng, S.; He, Q.; Deng, S.; Hu, Y.; Wang, D. Hypercrosslinked polymers enabled micropore-dominant N, S Co-Doped porous carbon for ultrafast electron/ion transport supercapacitors. Nano Energy 2019, 65, 103993. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Liu, J.; Fu, X.; Luo, Y.; Lyu, Y. Hypercrosslinked conjugated microporous polymers for carbon capture and energy storage. New J. Chem. 2017, 41, 3915–3919. [Google Scholar] [CrossRef]
- Pola, S.; Kuo, C.H.; Peng, W.T.; Islam, M.M.; Chao, I.; Tao, Y.T. Contorted Tetrabenzocoronene Derivatives for Single Crystal Field Effect Transistors: Correlation between Packing and Mobility. Chem. Mater. 2012, 24, 2566–2571. [Google Scholar] [CrossRef]
- Tian, M.; Wang, C.; Wang, L.; Luo, K.; Zhao, A.; Guo, C. Study on the synthesis and structure–effect relationship of multi-aryl imidazoles with their fluorescence properties. Luminescence 2014, 29, 540–548. [Google Scholar] [CrossRef]
- Li, Y.H.; Chen, Y.C. Triphenylamine-hexaarylbiimidazole derivatives as hydrogen acceptor photoinitiators for free radical photopolymerization under UV and LED light. Polym. Chem. 2020, 11, 1504–1513. [Google Scholar] [CrossRef]
- Lin, W.; Yuan, L.; Tan, W.; Feng, J.; Long, L. Construction of fluorescent probes via protection/deprotection of functional groups: A ratiometric fluorescent probe for Cu2+. Chem. Eur. J. 2008, 15, 1030–1035. [Google Scholar] [CrossRef]
- Bu, L.; Sawada, T.; Kuwahara, Y.; Shosenji, H.; Yoshida, K. Crystallographic structure and solid-state fluorescence enhancement behavior of a 2-(9-anthryl)phenanthroimidazole-type clathrate host upon inclusion of amine molecules. Dyes Pigm. 2003, 59, 43–52. [Google Scholar] [CrossRef]
- Boydston, A.J.; Vu, P.D.; Dykhno, O.L.; Chang, V.; Wyatt, A.R.; Stockett, A.S.; Ritschdorff, E.T.; Shear, J.B.; Bielawski, C.W. Modular fluorescent benzobis(imidazolium) salts: Syntheses, photophysical analyses, and applications. J. Am. Chem. Soc. 2008, 130, 3143–3156. [Google Scholar] [CrossRef] [PubMed]
- Jayabharathi, J.; Thanikachalam, V.; Devi, K.B.; Srinivasan, N. Physicochemical studies of some novel y-shaped imidazole derivatives a sensitive chemisensor. Spectrochim. Acta A 2011, 82, 513–520. [Google Scholar] [CrossRef]
- Xie, N.; Chen, Y. Synthesis and photophysical properties of 1,4-bis(4,5-diarylimidazol) benzene dyes. J. Photochem. Photobiol. A 2007, 189, 253–257. [Google Scholar] [CrossRef]
- Zhou, J.X.; Luo, X.S.; Liu, X.; Qiao, Y.; Wang, P.; Mecerreyes, D.; Bogliotti, N.; Chen, S.L.; Huang, M.H. Azo-linked porous organic polymers: Robust and time-efficient synthesis via NaBH4-mediated reductive homocoupling on polynitro monomers and adsorption capacity towards aniline in water. J. Mater. Chem. A 2018, 6, 5608–5612. [Google Scholar] [CrossRef]
- Xie, S.; Wong, A.Y.H.; Kwok, R.T.K.; Li, Y.; Su, H.; Lam, J.W.Y.; Chen, S.; Tang, B.Z. Fluorogenic Ag+–Tetrazolate Aggregation Enables Efficient Fluorescent Biological Silver Staining. Angew. Chem. Int. Ed. 2018, 57, 5750–5753. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Zou, D.; Cui, P.; Ren, H.; Zhu, G. Facile synthesis of cost-effective porous aromatic materials with enhanced carbon dioxide uptake. J. Mater. Chem. A 2013, 1, 13926–13931. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lu, J.; Zhang, J. Porosity Enhancement of Carbazolic Porous Organic Frameworks Using Dendritic Building Blocks for Gas Storage and Separation. Chem. Mater. 2014, 26, 4023–4029. [Google Scholar] [CrossRef]
- Yao, S.; Yang, X.; Yu, M.; Zhang, Y.; Jiang, J.X. High surface area hypercrosslinked microporous organic polymer networks based on tetraphenylethylene for CO2 capture. J. Mater. Chem. A 2014, 2, 8054–8059. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Shen, C.; Ju, Z.; Yuan, D. A facile synthesis of microporous organic polymers for efficient gas storage and separation. J. Mater. Chem. A 2015, 3, 3051–3058. [Google Scholar] [CrossRef]
- Li, T.; Yan, X.; Liu, Y.; Zhang, W.D.; Fu, Q.T.; Zhu, H.; Li, Z.; Gu, Z.G. A 2D covalent organic framework involving strong intramolecular hydrogen bonds for advanced supercapacitors. Polym. Chem. 2020, 11, 47–52. [Google Scholar] [CrossRef]
- Shu, G.; Zhang, C.; Li, Y.; Jiang, J.X.; Wang, X.; Li, H.; Wang, F. Hypercrosslinked silole-containing microporous organic polymers with N-functionalized pore surfaces for gas storage and separation. J. Appl. Polym. Sci. 2018, 135, 45907–45916. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, H.; Chen, L.; Chen, Y. N,N’-Bicarbazole: A Versatile Building Block toward the Construction of Conjugated Porous Polymers for CO2 Capture and Dyes Adsorption. Macromolecules 2017, 50, 4993–5003. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.M.; Liu, T.E.; Kuo, S.W. Direct synthesis of nitrogen-doped mesoporous carbons from triazine-functionalized resol for CO2 uptake and highly efficient removal of dyes. J. Hazard. Mater. 2020, 391, 122163. [Google Scholar] [CrossRef]
- Wang, S.; Shao, L.; Sang, Y.; Huang, J. Hollow Hyper-Cross-Linked Polymer Microspheres for Efficient Rhodamine B Adsorption and CO2 Capture. J. Chem. Eng. Data 2019, 64, 1662–1670. [Google Scholar] [CrossRef]
- Huang, J.H.; Huang, K.L.; Liu, S.Q.; Wang, A.W.; Yan, C. Adsorption of Rhodamine B and methyl orange on a hypercrosslinked polymeric adsorbent in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 55–61. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorbtion of gases on plane surfaces of glass mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Schreivoge, A.; Maurer, J.; Winter, R.; Baro, A.; Laschat, S. Synthesis and Electrochemical Properties of Tetrasubstituted Tetraphenylethenes. Eur. J. Org. Chem. 2006, 2006, 3395–3404. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Yang, F.; Ji, L.; Xu, L.; Zhang, C. Polytriphenylamine derivative with high free radical density as the novel organic cathode for lithium ion batteries. J. Mater. Chem. A 2014, 2, 20083–20088. [Google Scholar] [CrossRef]
- Su, C.; He, H.; Xu, L.; Zhao, K.; Zheng, C.; Zhang, C. A mesoporous conjugated polymer based on a high free radical density polytriphenylamine derivative: Its preparation and electrochemical performance as a cathode material for Li-ion batteries. J. Mater. Chem. A 2017, 5, 2701–2709. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Young, C.; Kim, J.; You, J.; Yamauchi, Y.; Kuo, S.W. Hollow Microspherical and Microtubular [3 + 3] Carbazole-Based Covalent Organic Frameworks and Their Gas and Energy Storage Applications. ACS Appl. Mater. Interfaces 2019, 11, 9343–9354. [Google Scholar] [CrossRef] [PubMed]
- Khattak, A.M.; Ghazi, Z.A.; Liang, B.; Khan, N.A.; Iqbal, A.; Li, L.; Tang, Z. A redox-active 2D covalent organic framework with pyridine moieties capable of faradaic energy storage. J. Mater. Chem. A 2016, 4, 16312–16317. [Google Scholar] [CrossRef]
- Liu, S.; Yao, L.; Lu, Y.; Hua, X.; Liu, J.; Yang, Z.; Wei, H.; Mai, Y. All-organic covalent organic framework/polyaniline composites as stable electrode for high-performance supercapacitors. Mater. Lett. 2019, 236, 354–357. [Google Scholar] [CrossRef]
- DeBlase, C.R.; Silberstein, K.E.; Truong, T.T.; Abruña, H.D.; Dichtel, W.R. β-Ketoenamine-Linked Covalent Organic Frameworks Capable of Pseudocapacitive Energy Storage. J. Am. Chem. Soc. 2013, 135, 16821–16824. [Google Scholar] [CrossRef]

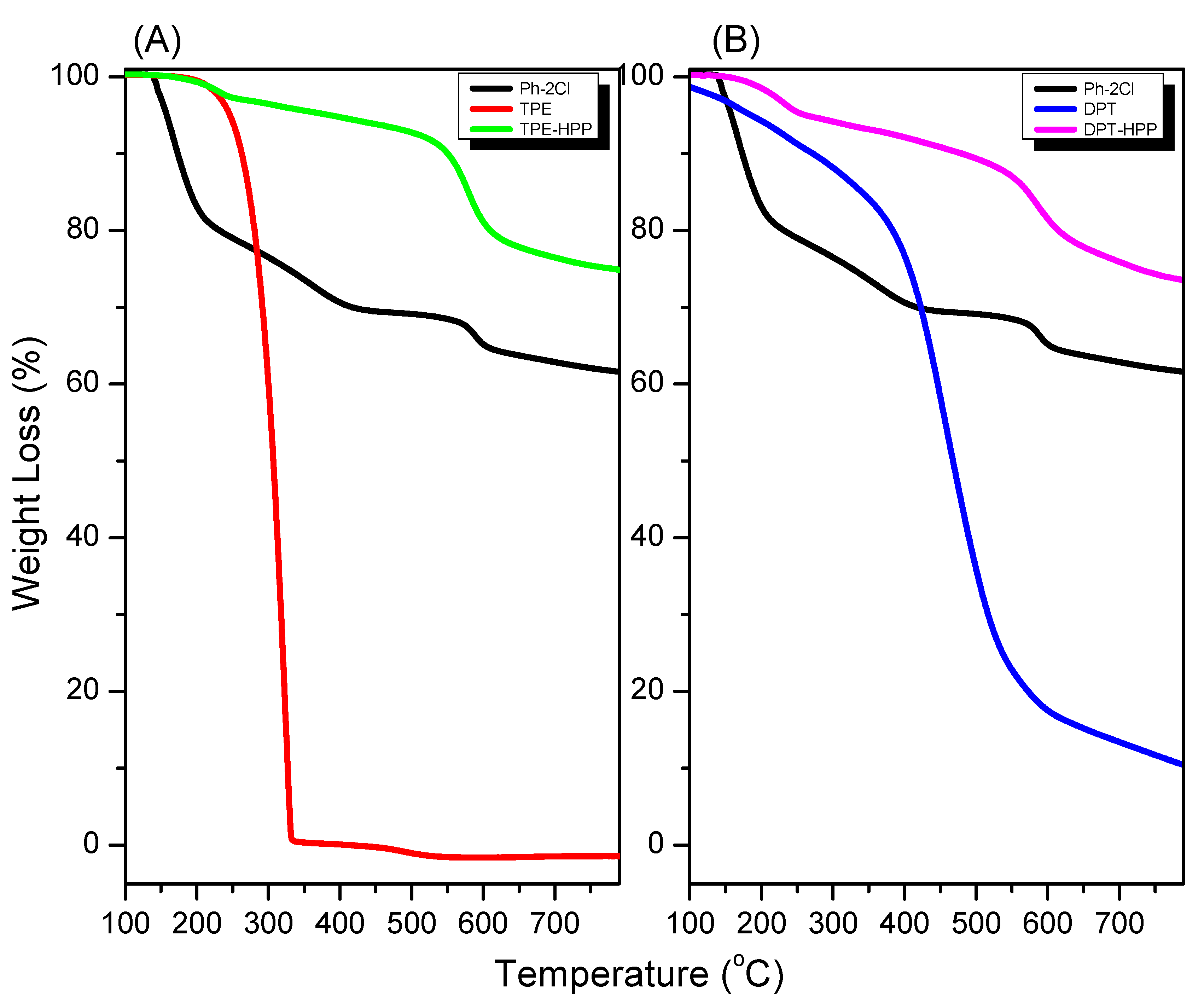

| Sample | Td5 (°C) | Td10 (°C) | Char Yield (wt.%) | Surface Area (m2 g−1) | Pore Size (nm) |
|---|---|---|---|---|---|
| Ph-2Cl | 158 | 273 | 62 | - | - |
| TPE | 246 | 263 | 0 | - | - |
| DPT | 186 | 272 | 11 | - | - |
| TPE-HPP | 387 | 551 | 75 | 922 | 1.01–1.98 |
| DPT-HPP | 263 | 481 | 74 | 1230 | 1.21–2.15 |
| Qm (mg g−1) | KL | RL2 | |
|---|---|---|---|
| TPE-HPP | 107.41 | 0.5649 | 0.99266 |
| DPT-HPP | 256.40 | 0.6250 | 0.96436 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.G.; EL-Mahdy, A.F.M.; Meng, T.-S.; Samy, M.M.; Kuo, S.-W. Multifunctional Hypercrosslinked Porous Organic Polymers Based on Tetraphenylethene and Triphenylamine Derivatives for High-Performance Dye Adsorption and Supercapacitor. Polymers 2020, 12, 2426. https://doi.org/10.3390/polym12102426
Mohamed MG, EL-Mahdy AFM, Meng T-S, Samy MM, Kuo S-W. Multifunctional Hypercrosslinked Porous Organic Polymers Based on Tetraphenylethene and Triphenylamine Derivatives for High-Performance Dye Adsorption and Supercapacitor. Polymers. 2020; 12(10):2426. https://doi.org/10.3390/polym12102426
Chicago/Turabian StyleMohamed, Mohamed Gamal, Ahmed. F. M. EL-Mahdy, Tso-Shiuan Meng, Maha Mohamed Samy, and Shiao-Wei Kuo. 2020. "Multifunctional Hypercrosslinked Porous Organic Polymers Based on Tetraphenylethene and Triphenylamine Derivatives for High-Performance Dye Adsorption and Supercapacitor" Polymers 12, no. 10: 2426. https://doi.org/10.3390/polym12102426
APA StyleMohamed, M. G., EL-Mahdy, A. F. M., Meng, T.-S., Samy, M. M., & Kuo, S.-W. (2020). Multifunctional Hypercrosslinked Porous Organic Polymers Based on Tetraphenylethene and Triphenylamine Derivatives for High-Performance Dye Adsorption and Supercapacitor. Polymers, 12(10), 2426. https://doi.org/10.3390/polym12102426








