Comparative Study of Green and Synthetic Polymers for Enhanced Oil Recovery
Abstract
:1. Introduction
2. Polyacrylamides and Polysaccharides
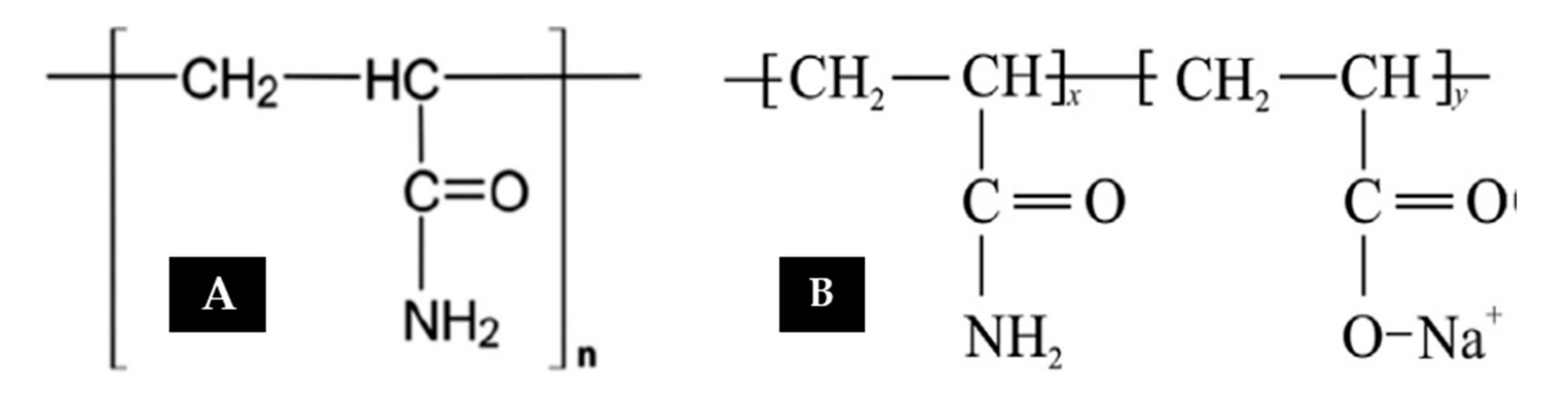
2.1. Polyacrylamide
2.2. Polysaccharide
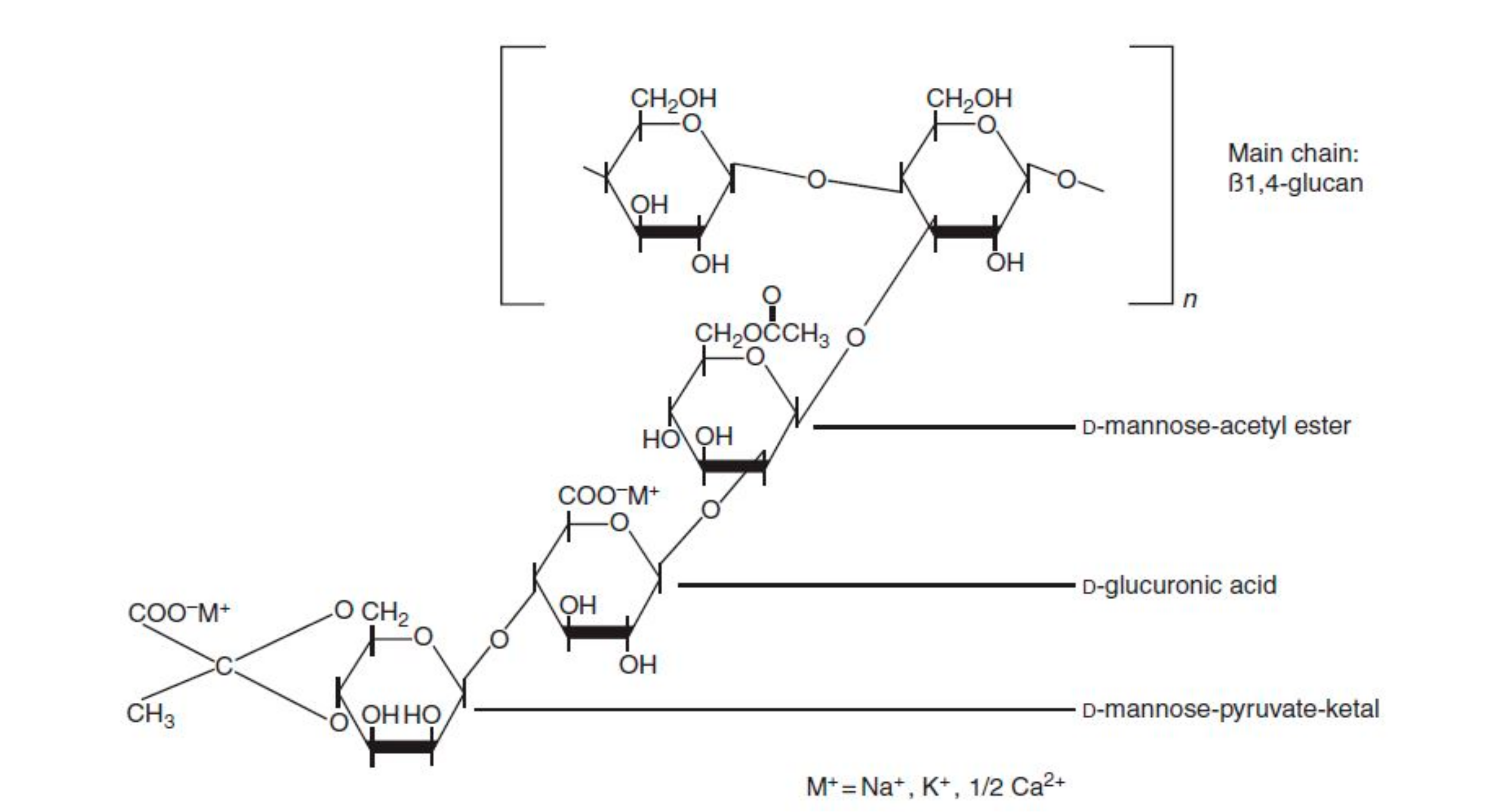
2.2.1. Xanthan Gum
2.2.2. Scleroglucan
2.2.3. Schizophyllan
3. Physical and Chemical Characteristics of Green Polymers over HPAM
3.1. Rheological Properties: Shear Rate, Shear Stress, and Viscosity
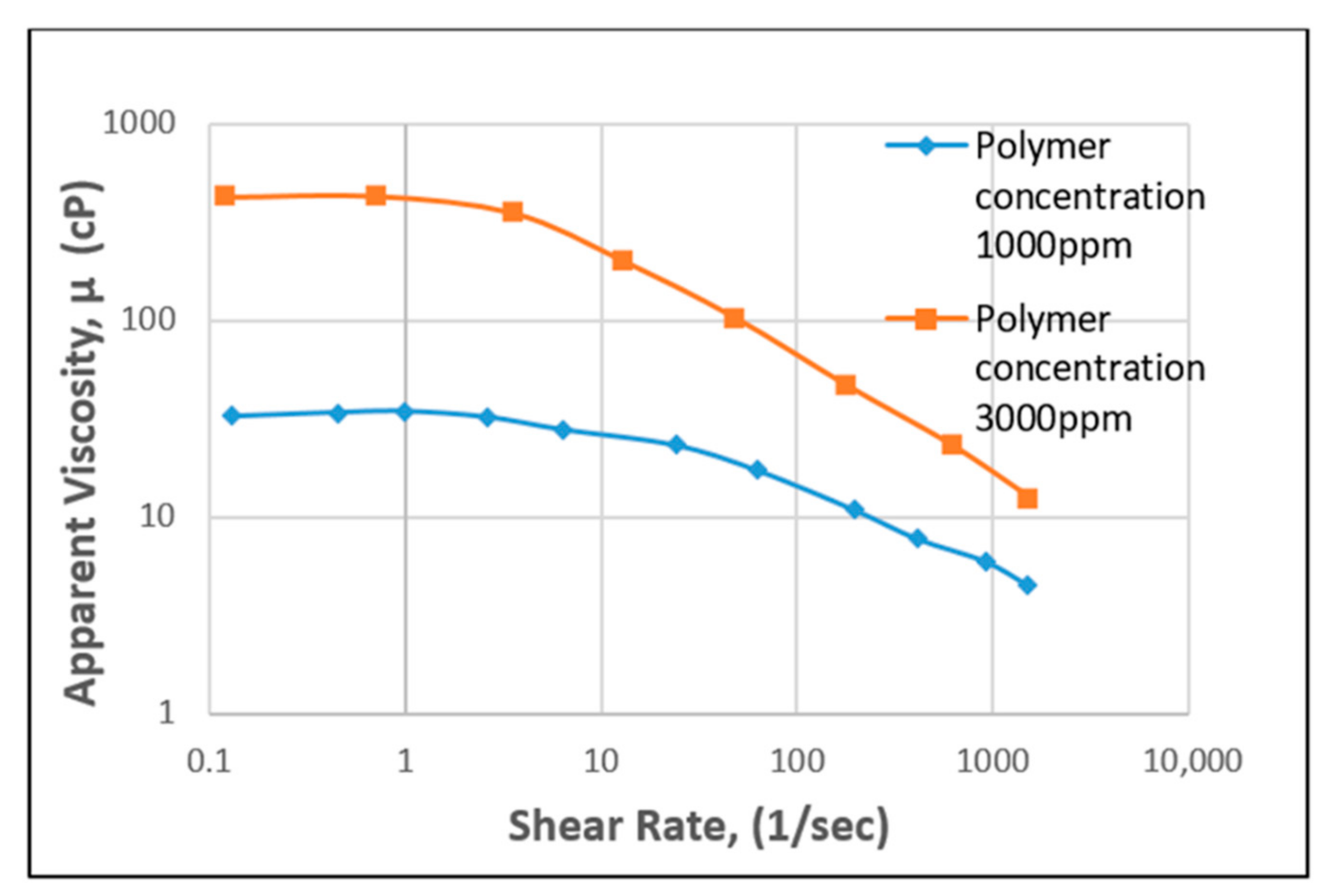
3.1.1. Effect of Shear Rate
3.1.2. Effect of Salinity and Temperature
3.2. Polymer Flow Properties
3.2.1. Permeability Reduction and Polymer Mobility
3.2.2. Retention and Adsorption of Polymer
3.2.3. Viscous Fingering and Its Effect
3.3. Cost (Per Kilogram)
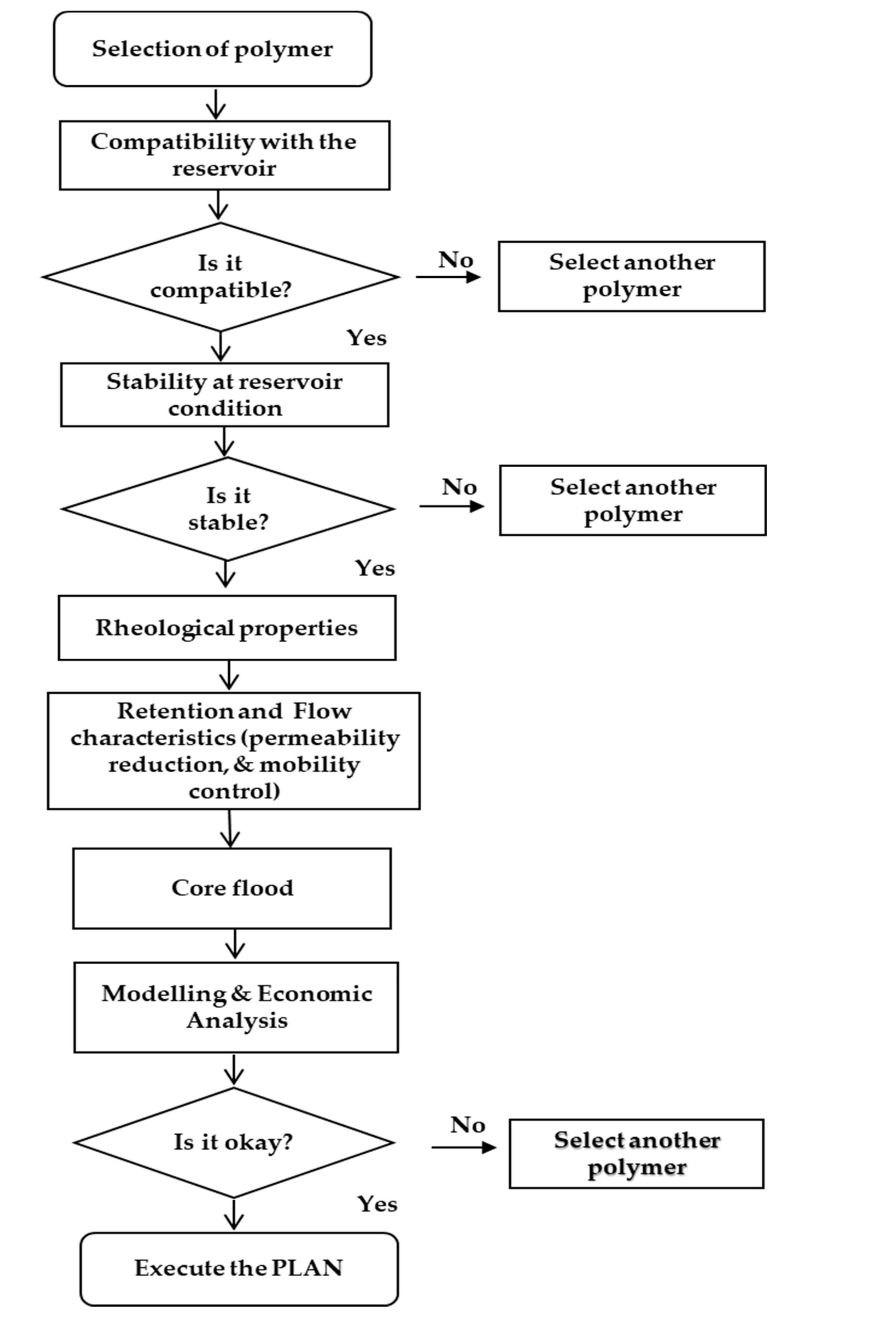
4. Screening, Design Criteria and Optimum Polymer Concentration
4.1. Screening Criteria
4.2. Design Criteria
4.3. Determination of Optimum Polymer Concentration
5. Conditions for Polymer Applications
5.1. Lab-Scale Application
5.2. Pilot-Scale Application
5.3. Field-Scale Application
5.4. Oil Fields in the Middle East
6. Summary of Comparison
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohsenatabar Firozjaii:, A.; Saghafi, H.R. Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 2019. [Google Scholar] [CrossRef]
- Green, D.W.; Willhite, P. Enhanced Oil Recovery; Doherty Memorial Fund of AIME, Society of Petroleum Engineers: The Woodlands, TX, USA, 1998; ISBN 1555630774. [Google Scholar]
- Stosur, G.J.; Hite, J.R.; Carnahan, N.F.; Miller, K. The Alphabet Soup of IOR, EOR and AOR: Effective Communication Requires a Definition of Terms. Proc. SPE Int. Improv. Oil Recover. Conf. Asia Pacific 2003, 445–451. [Google Scholar] [CrossRef]
- Al Bahri, M.S.; Vazquez, O.; Beteta, A.; Al Kalbani, M.M.; Mackay, E.J. Modelling the Impact of Low Salinity Waterflooding, Polymer Flooding and Thermally Activated Polymer on Produced Water Composition. SPE Int. Oilfield Scale Conf. Exhib. 2020. [Google Scholar] [CrossRef]
- Gao, C. Viscosity of partially hydrolyzed polyacrylamide under shearing and heat. J. Pet. Explor. Prod. Technol. 2013, 3, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.Q.; Morrow, N.R. Salinity, Temperature, Oil Composition, and Oil Recovery by Waterflooding. SPE Reserv. Eng. Soc. Pet. Eng. 1997, 12, 269–276. [Google Scholar] [CrossRef]
- Delshad, M.; Kim, D.H.; Magbagbeola, O.A.; Huh, C.; Pope, G.A.; Tarahhom, F. Mechanistic interpretation and utilization of viscoelastic behavior of polymer solutions for improved polymer-flood efficiency. Proc. SPE Symp. Improv. Oil Recover. 2008, 2, 1051–1065. [Google Scholar] [CrossRef]
- Weiss, W.W.; Baldwin, R.W. Planning and Implementing a Large-Scale Polymer Flood. JPT J. Pet. Technol. 1985, 37, 720–730. [Google Scholar] [CrossRef]
- Mohsenatabar Firozjaii, A.; Zargar, G.; Kazemzadeh, E. An investigation into polymer flooding in high temperature and high salinity oil reservoir using acrylamide based cationic co-polymer: experimental and numerical simulation. J. Pet. Explor. Prod. Technol. 2019, 9, 1485–1494. [Google Scholar] [CrossRef] [Green Version]
- Abidin, A.Z.; Puspasari, T.; Nugroho, W.A. Polymers for Enhanced Oil Recovery Technology. Procedia Chem. 2012, 4, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on polymer flooding: Rheology, adsorption, stability, and field applications of various polymer systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Seright, R.S.; Wavrik, K.E.; Zhang, G.; AlSofi, A.M. Stability and Behavior in Carbonate Cores for New Enhanced-Oil-Recovery Polymers at Elevated Temperatures in Hard Saline Brines. SPE Reserv. Eval. Eng. 2020, 1–18. [Google Scholar] [CrossRef]
- Al Quraishi, A.A.; Alsewailem, F.D. Adsorbtion of guar, xanthan and xanthan-guar mixtures on high salinity, high temperature reservoirs. In Proceedings of the Offshore Mediterranean Conference and Exhibition, Ravenna, Italy, 23–25 March 2011; pp. 1–15. [Google Scholar]
- Alfazazi, U.; AlAmeri, W.; Hashmet, M.R. Screening of new HPaM base polymers for applications in high temperature and high salinity carbonate reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 12–15 November 2019; pp. 1–17. [Google Scholar] [CrossRef]
- Rivenq, R.C.; Donche, A.; Nolk, C. Improved Scleroglucan for polymer flooding under harsh reservoir conditions. SPE Reserv. Eng. 1992. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure-property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Stability and Utility of Pyridyl Disulfide Functionality in RAFT and Conventional Radical Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar] [CrossRef]
- Sabhapondit, A.; Borthakur, A.; Haque, I. Water soluble acrylamidomethyl propane sulfonate (AMPS) copolymer as an enhanced oil recovery chemical. Energy Fuels 2003, 17, 683–688. [Google Scholar] [CrossRef]
- Jamshidi, H.; Rabiee, A. Synthesis and characterization of acrylamide-based anionic copolymer and investigation of solution properties. Adv. Mater. Sci. Eng. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Morgan, S.E.; McCormick, C.L. Water-soluble polymers in enhanced oil recovery. Prog. Polym. Sci. 1990, 15, 103–145. [Google Scholar] [CrossRef]
- Al-Murayri, M.T.; Kamal, D.S.; Garcia, J.G.; Al-Tameemi, N.; Driver, J.; Hernandez, R.; Fortenberry, R.; Britton, C. Stability of biopolymer and partially hydrolyzed polyacrylamide in presence of H2S and oxygen. Proc. SPE Annu. Tech. Conf. Exhib. 2018, 2018. [Google Scholar] [CrossRef]
- JOUENNE, S. Polymer flooding in high temperature, high salinity conditions: Selection of polymer type and polymer chemistry, thermal stability. J. Pet. Sci. Eng. 2020, 195, 107545. [Google Scholar] [CrossRef]
- Nasr, S.; Soudi, M.R.; Haghighi, M. Xanthan production by a native strain of X. campestris and evaluation of application in EOR. Pakistan J. Biol. Sci. 2007, 10, 3010–3013. [Google Scholar]
- Hublik, G. Xanthan. Polym. Sci. A Compr. Ref. 10 Vol. Set 2012, 10, 221–229. [Google Scholar] [CrossRef]
- Becker, A.; Katzen, F.; Pühler, A.; Ielpi, L. Xanthan gum biosynthesis and application: A biochemical/genetic perspective. Appl. Microbiol. Biotechnol. 1998, 50, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Dhiaa, A.H. The temperature effect on the viscosity and density of Xanthan Gum solution. Kufa J. Eng. 2012, 3, 31–45. [Google Scholar]
- Dentini, M.; Crescent, V.; Blasi, D. Conformational properties of xanthan derivatives in dilute aqueous-solution. Int. J. Biol. 1984, 6, 93–98. [Google Scholar] [CrossRef]
- Gulrez, S.K.H.; Al-Assaf, S.; Fang, Y.; Phillips, G.O.; Gunning, A.P. Revisiting the conformation of xanthan and the effect of industrially relevant treatments. Carbohydr. Polym. 2012, 90, 1235–1243. [Google Scholar] [CrossRef]
- Teckentrup, J.; Al-Hammood, O.; Steffens, T.; Bednarz, H.; Walhorn, V.; Niehaus, K.; Anselmetti, D. Comparative analysis of different xanthan samples by atomic force microscopy. J. Biotechnol. 2017, 257, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.M.; Schols, H.A.; Delahaije, R.J.B.M.; Sworn, G.; Wierenga, P.A.; Gruppen, H. The influence of the primary and secondary xanthan structure on the enzymatic hydrolysis of the xanthan backbone. Carbohydr. Polym. 2013, 97, 368–375. [Google Scholar] [CrossRef]
- Li, R.; Feke, D.L. Rheological and kinetic study of the ultrasonic degradation of xanthan gum in aqueous solution: Effects of pyruvate group. Carbohydr. Polym. 2015, 124, 216–221. [Google Scholar] [CrossRef]
- Scott, A.J.; Romero-Zerón, L.; Penlidis, A. Evaluation of Polymeric Materials for Chemical Enhanced Oil Recovery. Processes 2020, 8, 361. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.T.; Barnabe, N.; Greaney, K. Biodegradation of xanthan by salt-tolerant aerobic microorganisms. J. Ind. Microbiol. 1986, 1, 31–37. [Google Scholar] [CrossRef]
- Sutherl, I.W. An enzyme system hydrolysing the polysaccharides of Xanthomonas species. J. Appl. Bacteriol. 1982, 53, 385–393. [Google Scholar] [CrossRef]
- Ghoumrassi-Barr, S.; Aliouche, D. Characterisation and rheological study of xanthan polymer for enhanced oil recovery (EOR) application. In Proceedings of the Offshore Mediterranean Conference and Exhibition, Ravenna, Italy, 25–27 March; pp. 1–16.
- Gorin, P.A.J.; Spencer, J.F.T. Structural chemistry of fungal polysaccharides. M. L. Wolfrom R. S. Tipson Adv. Carbohydr. Chem. 1968, 367–417. [Google Scholar]
- Pu, W.; Shen, C.; Wei, B.; Yang, Y.; Li, Y. A comprehensive review of polysaccharide biopolymers for enhanced oil recovery (EOR) from flask to field. J. Ind. Eng. Chem. 2018, 61, 1–11. [Google Scholar] [CrossRef]
- Fariña, J.I.; Siñeriz, F.; Molina, O.E.; Perotti, N.I. Isolation and physicochemical characterization of soluble scleroglucan from Sclerotium rolfsii. Rheological properties, molecular weight and conformational characteristics. Carbohydr. Polym. 2001, 44, 41–50. [Google Scholar] [CrossRef]
- Quadri, S.M.R.; Shoaib, M.; AlSumaiti, A.M.; Alhassan, S.M. Screening of Polymers for EOR in High Temperature, High Salinity and Carbonate Reservoir Conditions. In Proceedings of the International Petroleum Technology Conference; International Petroleum Technology Conference, Doha, Qatar, 6–9 December 2015. [Google Scholar]
- Akstinat, M.H. Polymers for Enhanced Oil Recovery in Reservoirs of Extremely High Sa1inities and High Temperatures. In Proceedings of the paper SPE 8979 presented at the SPE Inti. Symposium on Oilfield and Geothermal Chemistry, Stanford, CA, USA, 28–30 May 1979; pp. 28–30. [Google Scholar]
- Davison, P.; Mentzer, E. Polymer Flooding in North Sea Reservoirs. Soc. Pet. Eng. J. 1982, 22, 353–362. [Google Scholar] [CrossRef]
- Kalpacki, B. Thermal Stability of Sc1eroglucaiJ. at Realistic Reservoir Conditions. In Proceedings of the paper SPE 20237 presente’d at the 1990 SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, Oklahoma, 22–25 April 1990; pp. 22–24. [Google Scholar]
- Ryles, R.G. Chemical stability limits of water-soluble polymers used in oil recovery processes. Spe Reserv. Eng. 1989, 3, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Chauveteau, G.; Kohler, N. Influence of Microgels in Polysaccharide Solutions on Their Flow Behavior Through Porous Media. Soc. Pet. Eng. J. 1984, 24, 361–368. [Google Scholar] [CrossRef]
- Troung, N.D.; Gadioux, J. Purification du Sc1eroglucane. French Patent 87-15663, 1987. (In French). [Google Scholar]
- Enomoto, H.; Einaga, Y.; Teramoto, A. Viscosity of Aqueous Solutions of a Triple-Helical Polysaccharide Schizophyllan. Macromolecules 1984, 17, 1573–1577. [Google Scholar] [CrossRef]
- Yanaki, T.; Norisuye, T.; Fujita, H. Triple Helix of Schizophyllum commune Polysaccharide in Dilute Solution. 3. Hydrodynamic Properties in Water. Macromolecules 1980, 13, 1462–1466. [Google Scholar] [CrossRef]
- Fang, Y.; Nishinari, K. Gelation Behaviors of schizophyllan-sorbital aqueous solutions. Biopolym. Orig. Rese. Biomol. 2004, 73, 44–60. [Google Scholar]
- Fang, Y.; Takahashi, R.; Nishinari, K. Rheological characterization of Schizophyllan aqueous solutions after denaturation-renaturation treatment. Biopolymers 2004, 74, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Grisel, M.; Muller, G. Rheological properties of schizophyllan in presence of borate ions. Prog. Colloid Polym. Sci. 1996, 102, 32–37. [Google Scholar] [CrossRef]
- Leonhardt, B.; Ernst, B.; Reimann, S.; Steigerwald, A.; Lehr, F. Field testing the Polysaccharide schizophyllan: Results of the first year. SPE DOE Improv. Oil Recover. Symp. Proc. 2014, 1, 57–72. [Google Scholar] [CrossRef]
- Sanada, Y.; Matsuzaki, T.; Mochizuki, S.; Okobira, T.; Uezu, K.; Sakurai, K. β-1,3-d-glucan schizophyllan/Poly(dA) triple-helical complex in dilute solution. J. Phys. Chem. B 2012, 116, 87–94. [Google Scholar] [CrossRef]
- Ogezi, O.; Strobel, J.; Egbuniwe, D.; Leonhardt, B. Operational aspects of a biopolymer flood in a Mature Oilfield. SPE DOE Improv. Oil Recover. Symp. Proc. 2014, 3, 1735–1751. [Google Scholar] [CrossRef]
- Mungan, N. Shear Viscosities of Ionic Polyacrylamide Solutions. Soc Pet Eng J 1972, 12, 469–473. [Google Scholar] [CrossRef]
- Audibert, A.; Noik, C.; Lecourtier, J. Behaviour of Polysaccharides Under Harsh Conditions. J. Can. Pet. Technol. 1993, 32. [Google Scholar] [CrossRef]
- Holzwarth, G. Conformation of the Extracellular Polysaccharide of Xanthomonas campestris. Biochemistry 1976, 15, 4333–4339. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Romero-Zerón, L.; Rodrigue, D. Mechanical properties and flow behavior of polymers for enhanced oil recovery. J. Macromol. Sci. Part B Phys. 2014, 53, 625–644. [Google Scholar] [CrossRef]
- Ferreira, V.H.S.; Moreno, R.B.Z.L. Polyacrylamide Adsorption and Readsorption in Sandstone Porous Media. SPE J. 2020, 25, 497–514. [Google Scholar] [CrossRef]
- Stokes, R.J.; Evans, D.F. Fundamentals of Interfacial Engineering; Wiley-VCH: New York, NY, USA, 1997. [Google Scholar]
- Seright, R.S.; Campbell, A.; Mozley, P.; Han, P. Stability of Partially Hydrolyzed Polyacrylamides at Elevated Temperatures in the Absence of Divalent Cations. SPE J. 2010, 15, 341–348. [Google Scholar] [CrossRef]
- Dautzenberg, H. Polyelectrolyte complex formation in highly aggregating systems. 1. Effect of salt: Polyelectrolyte complex formation in the presence of NaCl. Macromolecules 1997, 30, 7810–7815. [Google Scholar] [CrossRef]
- Norton, I.T.; Goodall, D.M.; Frangou, S.A.; Morris, E.R.; Rees, D.A. Mechanism and dynamics of conformational ordering in xanthan polysaccharide. J. Mol. Biol. 1984, 175, 371–394. [Google Scholar] [CrossRef]
- Zhong, L.; Oostrom, M.; Truex, M.J.; Vermeul, V.R.; Szecsody, J.E. Rheological behavior of xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J. Hazard. Mater. 2013, 244, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Saleh, L.D.; Wei, M.; Zhang, Y.; Bai, B. Data analysis for polymer flooding that is based on a comprehensive database. SPE Reserv. Eval. Eng. 2017, 20, 876–893. [Google Scholar] [CrossRef]
- Sheng, J.J.; Leonhardt, B.; Gmbh, W.H. Status of Polymer-Flooding Technology. J. Can. Petroleum Technol. 2015. [Google Scholar] [CrossRef]
- Muller, G. Thermal stability of high-molecular-weight polyacrylamide aqueous solutions. Polym. Bull. 1981, 5, 31–37. [Google Scholar] [CrossRef]
- Moradi-Araghi, A.; Doe, P.H. Hydrolysis and Precipitation of Polyacrylamides in Hard Brines At Elevated Temperatures. SPE Reserv. Eng. Soc. Pet. Eng. 1987, 2, 189–198. [Google Scholar] [CrossRef]
- Kierulf, C.; Sutherland, I.W. Thermal Stability of Xanthan Preparations. Carbohydr. Polym. 1988, 9, 185–194. [Google Scholar] [CrossRef]
- Rellegadla, S.; Prajapat, G.; Agrawal, A. Polymers for enhanced oil recovery: fundamentals and selection criteria. Appl. Microbiol. Biotechnol. 2017, 101, 4387–4402. [Google Scholar] [CrossRef]
- Wang, D.; Seright, R.S.; Shao, Z.; Wang, J. Key aspects of project design for polymer flooding at the Daqing Oil Field. SPE Reserv. Eval. Eng. 2008, 11, 1117–1124. [Google Scholar] [CrossRef]
- Kohler, N.; Chauveteau, G. Xanthan Polysaccharide Plugging Behavior in Porous Media - Preferential Use of Fermentation Broth. JPT, J. Pet. Technol. 1981, 33, 349–358. [Google Scholar] [CrossRef]
- Mishra, S.; Bera, A.; Mandal, A. Effect of Polymer Adsorption on Permeability Reduction in Enhanced Oil Recovery. J. Pet. Eng. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Seright, R.S.; Henrici, B.J. Xanthan Stability At Elevated Temperatures. Soc. Pet. Eng. AIME, SPE 1986, 2, 285–299. [Google Scholar] [CrossRef]
- Shupe, R.D. Chemical Stability of Polyacrylamide Polymers. JPT, J. Pet. Technol. 1981, 33, 1513–1529. [Google Scholar] [CrossRef]
- Jang, H.Y.; Zhang, K.; Chon, B.H.; Choi, H.J. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J. Ind. Eng. Chem. 2015, 21, 741–745. [Google Scholar] [CrossRef]
- Buciak, J.; Fondevila Sancet, G.; Del Pozo, L. Polymer-flooding-pilot learning curve: Five-plus years’ experience to reduce cost per incremental barrel of oil. SPE Reserv. Eval. Eng. 2015, 18, 11–19. [Google Scholar] [CrossRef]
- Sofia, G.B.; Djamel, A. A Rheological Study of Xanthan Polymer for Enhanced Oil Recovery. J. Macromol. Sci. Part B Phys. 2016, 55, 793–809. [Google Scholar] [CrossRef]
- de Moura, M.R.V.; Moreno, R.B.Z.L. Concentration, brine salinity and temperature effects on xanthan gum solutions rheology. Appl. Rheol. 2019, 29, 69–79. [Google Scholar] [CrossRef]
- Zou, J.; Rezaee, R.; Liu, K. Effect of Temperature on Methane Adsorption in Shale Gas Reservoirs. Energy Fuels 2017, 31, 12081–12092. [Google Scholar] [CrossRef]
- Zaotoun, A.; Kohler, N. Two-Phase Flow Through Porous Media: Effect of Polymer Layer. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 2–5 October 1988. [Google Scholar]
- Vela, S.; Peaceman, D.W.; Sandvik, E.I. Evaluation of Polymer Flooding in a Layered Reservoir With Crossflow, Retention, and Degradation. Soc. Pet. Eng. AIME J. 1976, 16, 82–96. [Google Scholar] [CrossRef]
- Liang, J.; Seright, R.S. Wall-effect/gel-droplet model of disproportionate permeability reduction. SPE J. 2001, 6, 268–272. [Google Scholar] [CrossRef]
- Manichand, R.N.; Seright, R.S. Field vs. Laboratory Polymer-Retention Values for a Polymer Flood in the Tambaredjo Field. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, Ok, USA, 12–16 April 2014. [Google Scholar]
- Zhang, G.; Seright, R.S. Effect of concentration on HPAM retention in porous media. SPE J. 2014, 19, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Torrealba, V.A.; Hoteit, H. Improved polymer flooding injectivity and displacement by considering compositionally-tuned slugs. J. Pet. Sci. Eng. 2019, 178, 14–26. [Google Scholar] [CrossRef]
- Seright, R.S. Disproportionate permeability reduction with pore-filling gels. SPE J. 2009, 14, 5–13. [Google Scholar] [CrossRef]
- Liang, J.T.; Sun, H.; Seright, R.S. Why do gels reduce water permeability more than oil permeability? SPE Reserv. Eng. Society Pet. Eng. 1995, 10, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Wenjie, S.; Li, F.; Qu, Y.; Yang, Y. Novel method for characterizing single-phase polymer flooding. SPE J. 2014, 19, 695–702. [Google Scholar] [CrossRef]
- Lindner, A.; Bonn, D.; Poiré, E.C.; Amar, M.B.; Meunier, J. Viscous fingering in non-Newtonian fluids. J. Fluid Mech. 2002, 469, 237–256. [Google Scholar] [CrossRef]
- Saffman, P.G. Viscous fingering in Hele-Shaw cells. J. Fluid Mech. 1986, 0, 73–94. [Google Scholar]
- Li, H.; Yu, C.; Chen, R.; Li, J.; Li, J. Novel ionic liquid-type Gemini surfactants: Synthesis, surface property and antimicrobial activity. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 116–124. [Google Scholar] [CrossRef]
- Abdul Hamid, S.A.; Muggeridge, A.H. Analytical solution of polymer slug injection with viscous fingering. Comput. Geosci. 2018, 22, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Foraie, J.; Huang, S.; Chatzis, I. Analysis of immiscible Water-Alternating-Gas (WAG) injection using micromodel tests. J. Can. Pet. Technol. 2005, 44, 17–24. [Google Scholar] [CrossRef]
- Moussa, E.O.; Attia, A.M. Optimum Polymer Concentration in EOR. Iarjset 2016, 3, 4–15. [Google Scholar] [CrossRef]
- Chang, H.L. Polymer flooding technology—Yesterday, today and tomorrow. J. Petroleum Technol. 1978. [Google Scholar] [CrossRef]
- Llano, V.; Henthorne, L.; Walsh, J. Water Management for EOR Applications—Sourcing, Treating, Reuse and Recycle. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2013; pp. 1–13. [Google Scholar] [CrossRef] [Green Version]
- Aspinall, G.O. The Polysaccharides; Academic Press Inc.: Cambridge, MA, USA, 1983. [Google Scholar]
- Donaldson, E.C.; Chilingar, G.V.; Yen, T.F. Enhanced Oil Recovery; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S. Review on Surfactant Flooding: Phase Behavior, Retention, IFT, and Field Applications. Energy Fuels 2017, 31, 7701–7720. [Google Scholar] [CrossRef]
- Knight, B.L. Reservoir Stability of Polymer Solutions. J. Pet. Technol. 1973, 25, 618–626. [Google Scholar] [CrossRef]
- Wang, G.C.; Caudle, B.H. Effects of polymer concentrations, slug size and permeability stratification in viscous waterfloods. In Proceedings of the Fall Meeting of the Society of Petroleum Engineers of AIME, Houston, TX, USA, 4–7 October 1970. [Google Scholar]
- Sarem, A.M. On the theory of polymer solution flooding process. In Proceedings of the Fall Meeting of the Society of Petroleum Engineers of AIME, Houston, TX, USA, 4–7 October 1970. [Google Scholar]
- Omar, A.E. Effect of Polymer Adsorption on Mobility Ratio. In Proceedings of the Middle East Oil Technical Conference and Exhibition, Manama, Bahrain, 14–17 March 1983. [Google Scholar]
- Seright, R.S.; Seheult, M.; Talashek, T. Injectivity characteristics of EOR polymers. SPE Reserv. Eval. Eng. 2009, 12, 783–792. [Google Scholar] [CrossRef]
- Xu, L.; Xu, G.; Yu, L.; Gong, H.; Dong, M.; Li, Y. The displacement efficiency and rheology of welan gum for enhanced heavy oil recovery. Polym. Adv. Technol. 2014, 25, 1122–1129. [Google Scholar] [CrossRef]
- Choi, S.K.; Sharma, M.M.; Bryant, S.L.; Huh, C. pH-sensitive polymers for novel conformance-control and polymer-flood applications. SPE Reserv. Eval. Eng. 2010, 13, 926–939. [Google Scholar] [CrossRef] [Green Version]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Yusuff, A.S. An Overview of Chemical Enhanced Oil Recovery: Recent Advances and Prospects; Springer: Berlin/Heidelberg, Germany, 2019; Volume 9, ISBN 0123456789. [Google Scholar]
- Seright, R.S. Potential for polymer flooding reservoirs with viscous oils. SPE Reserv. Eval. Eng. 2010, 13, 730–740. [Google Scholar] [CrossRef] [Green Version]
- Standnes, D.C.; Skjevrak, I. Literature review of implemented polymer field projects. J. Pet. Sci. Eng. 2014, 122, 761–775. [Google Scholar] [CrossRef]
- Pye, D.J. Improved Secondary Recovery by Control of Water Mobility. J. Pet. Technol. 1964, 16, 911–916. [Google Scholar] [CrossRef]
- Sandiford, B.B. Laboratory and Field Studies of Water Floods Using Polymer Solutions to Increase Oil Recoveries. J. Pet. Technol. 1964, 16, 917–922. [Google Scholar] [CrossRef]
- Jones, M.A. Waterflood Mobility Control: A Case History. J. Pet. Technol. 1966, 18, 1151–1156. [Google Scholar] [CrossRef]
- Ustick, R.E. Comparison of Polymer Flooding and Waterflooding at Huntington Beach, California. J. Pet. Technol. 1967, 19, 1103–1111. [Google Scholar] [CrossRef]
- Corlay, P.; Lemouzy, P.; Eschard, R.; Zhang, L.R. Fully integrated reservoir study and numerical forecast simulations of two-polymer pilots in Daqing field. In Proceedings of the International Meeting on Petroleum engineering, Beijing, China, 24–27 March 1992; pp. 331–340. [Google Scholar] [CrossRef]
- Littmann, W.; Kleinitz, W.; Christensen, B.E.; Stokke, B.T. Late results of a polymer pilot test. Performance, simulation adsorption, and xanthan stability in the reservoir. Eighth Symp. Enhanc. Oil Recover. 1992, 177–188. [Google Scholar] [CrossRef]
- Putz, A.; Pedron, B.; Bazin, B. Commercial polymer injection in the Courtenay Field, 1993 update. Geol. Soc. Spec. Publ. 1995, 84, 239–249. [Google Scholar] [CrossRef]
- Shahin, G.T.; Thigpen, D.R. Injecting Polyacrylamide into Gulf Coast Sands: The White Castle Q Sand Polymer-Injectivity Test. SPE Reserv. Eng. Soc. Pet. Eng. 1996, 11, 174–179. [Google Scholar] [CrossRef]
- Delamaide, E.; Zaitoun, A.; Renard, G.; Tabary, R. Pelican lake field: First successful application of polymer flooding in a heavy-oil reservoir. SPE Reserv. Eval. Eng. 2014, 17, 340–354. [Google Scholar] [CrossRef]
- He, J.; Song, Z.Y.; Qiu, L.; Xie, F.; Tan, Z.L.; Yue, Q.S.; Li, X.N. High temperature polymer flooding in thick reservoir in Shuanghe oilfield. In Proceedings of the International Oil and Gas Conference and Exhibition in China, Beijing, China, 2–6 November 1998. [Google Scholar]
- de Melo, M.A.; da Silva, I.P.G.; de Godoy, G.M.R.; Sanmartim, A.N. Polymer Injection Projects in Brazil: Dimensioning, Field Application and Evaluation. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, Oklahoma, 13–17 April 2002; pp. 1–11. [Google Scholar] [CrossRef]
- Liu, B.; Sun, X.S.; Wang, K.; Xu, H.; Liu, Q.; Liu, X.; Song, S. Flooded by High Concentration Polymer Doubled Oil Recovery of Common Polymer on Field Test with 20% Closed to the Result of Lab Test in Daqing. In Proceedings of the International Oil Conference and Exhibition in Mexico; Society of Petroleum Engineers, Veracruz, Mexico, 27–30 June 2007. [Google Scholar]
- Tiwari, D.; Marathe, R.V.; Patel, N.K.; Ramachandran, K.P.; Maurya, C.R.; Tewari, P.K. Performance of polymer flood in Sanand Field, India-A case study. SPE Asia Pacific Oil Gas Conf. Exhib. 2008 Gas Now Deliv. Expect. 2008, 1, 372–380. [Google Scholar] [CrossRef]
- Wassmuth, F.R.; Arnold, W.; Green, K.; Cameron, N. Polymer flood application to improve heavy oil recovery at East Bodo. J. Can. Pet. Technol. 2009, 48, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Morel, D.C.; Vert, M.; Jouenne, S.; Gauchet, R.; Bouger, Y. First Polymer Injection in Deep Offshore Field Angola: Recent Advances in the Dalia/Camelia Field Case. Oil Gas Facil. 2012, 1, 43–52. [Google Scholar] [CrossRef]
- Moe Soe Let, K.P.; Manichand, R.N.; Seright, R.S. Polymer flooding a ∼500-cp oil. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- BP. BP Statistical Review of World Energy; BP: London, UK, 2014. [Google Scholar]
- Jabbar, M.; Xiao, R.; Teletzke, G.F.; Willingham, T.; Al Obeidli, A.; Al Sowaidi, A.; Britton, C.; Delshad, M.; Li, Z. Polymer EOR Assessment Through Integrated Laboratory and Simulation Evaluation for an Offshore Middle East Carbonate Reservoir. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference; Society of Petroleum Engineers, Abu Dhabi, UAE, 12–15 November 2018. [Google Scholar]
- Jabbar, M.Y.; Al Sowaidi, A.; Al Obeidli, A.; Willingham, T.W.; Britton, C.; Adkins, S.; Delshad, M.; Xiao, R.; Teletzke, G.F. Chemical formulation design in high salinity, high temperature carbonate reservoir for a super giant offshore field in middle east. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 13–16 November 2017. [Google Scholar] [CrossRef]
- Rodriguez, L.; Giovannetti, B.; Dupuis, G.; Gaillard, N.; Jouenne, S.; Bourdarot, G.; Morel, D.; Zaitoun, A.; Grassl, B. A new thermally stable synthetic polymer for harsh conditions of Middle East reservoirs. In Proceedings of the Research and development Petroleum Conference and Exhibition, Abu Dhabi, UAE, 9–10 May 2018; pp. 16–19. [Google Scholar] [CrossRef]
- Gao, C.H. Scientific research and field applications of polymer flooding in heavy oil recovery. J. Pet. Explor. Prod. Technol. 2011, 1, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Diab, W.N.; Al-Shalabi, E.W. Recent developments in polymer flooding for carbonate reservoirs under harsh conditions. In Proceedings of the Offshore technology conference Brasil, 29–31 October 2020; Brasil, Rio de Janeiro. [Google Scholar] [CrossRef]
- Delamaide, E. Polymers and their limits in temperature, salinity and hardness: Theory and practice. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Brisbane, Australia, 23–25 October 2018. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Zentz, F.; Verchère, J.F.; Muller, G. Thermal denaturation and degradation of schizophyllan. Carbohydr. Polym. 1992, 17, 289–297. [Google Scholar] [CrossRef]






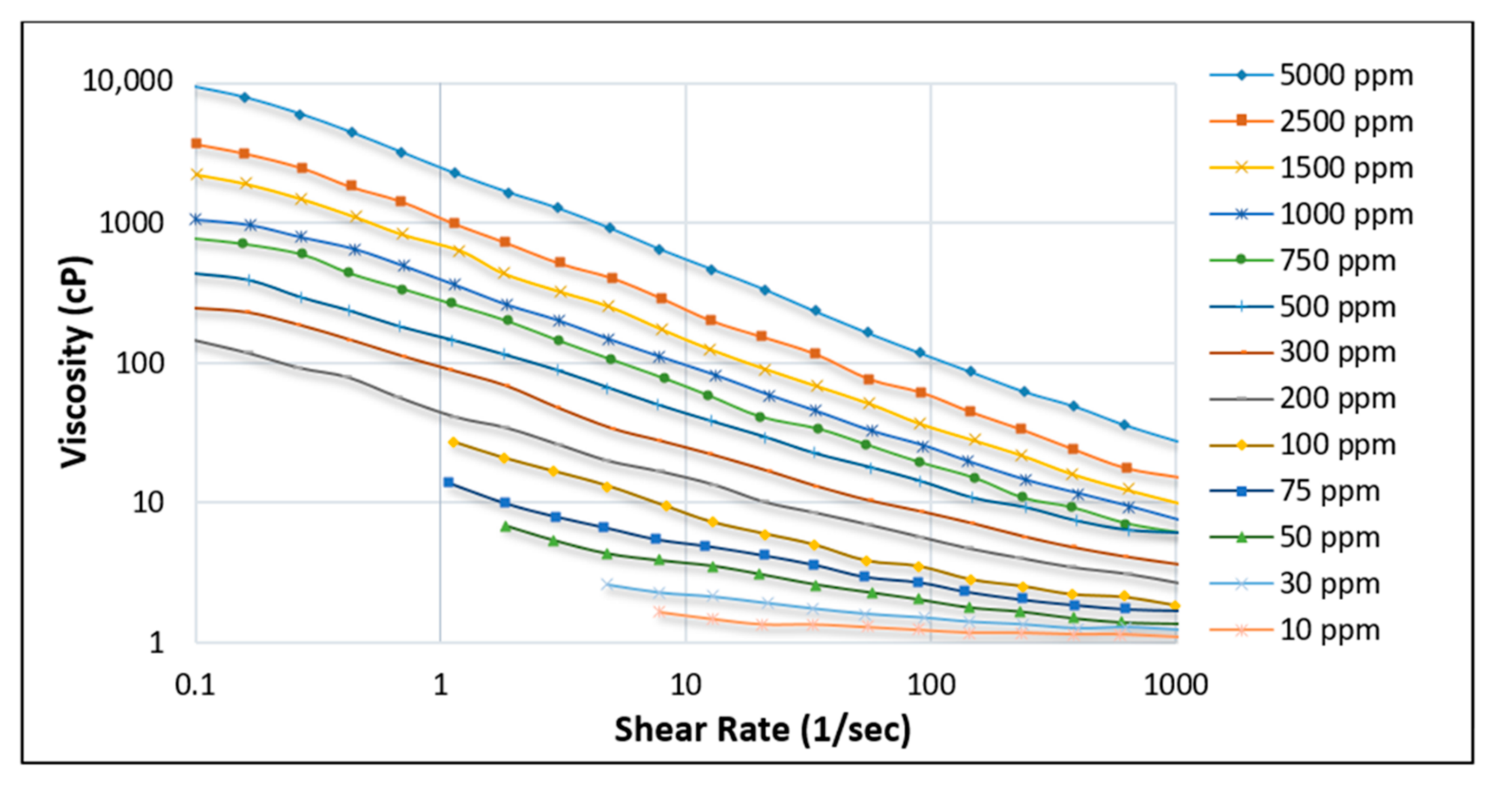
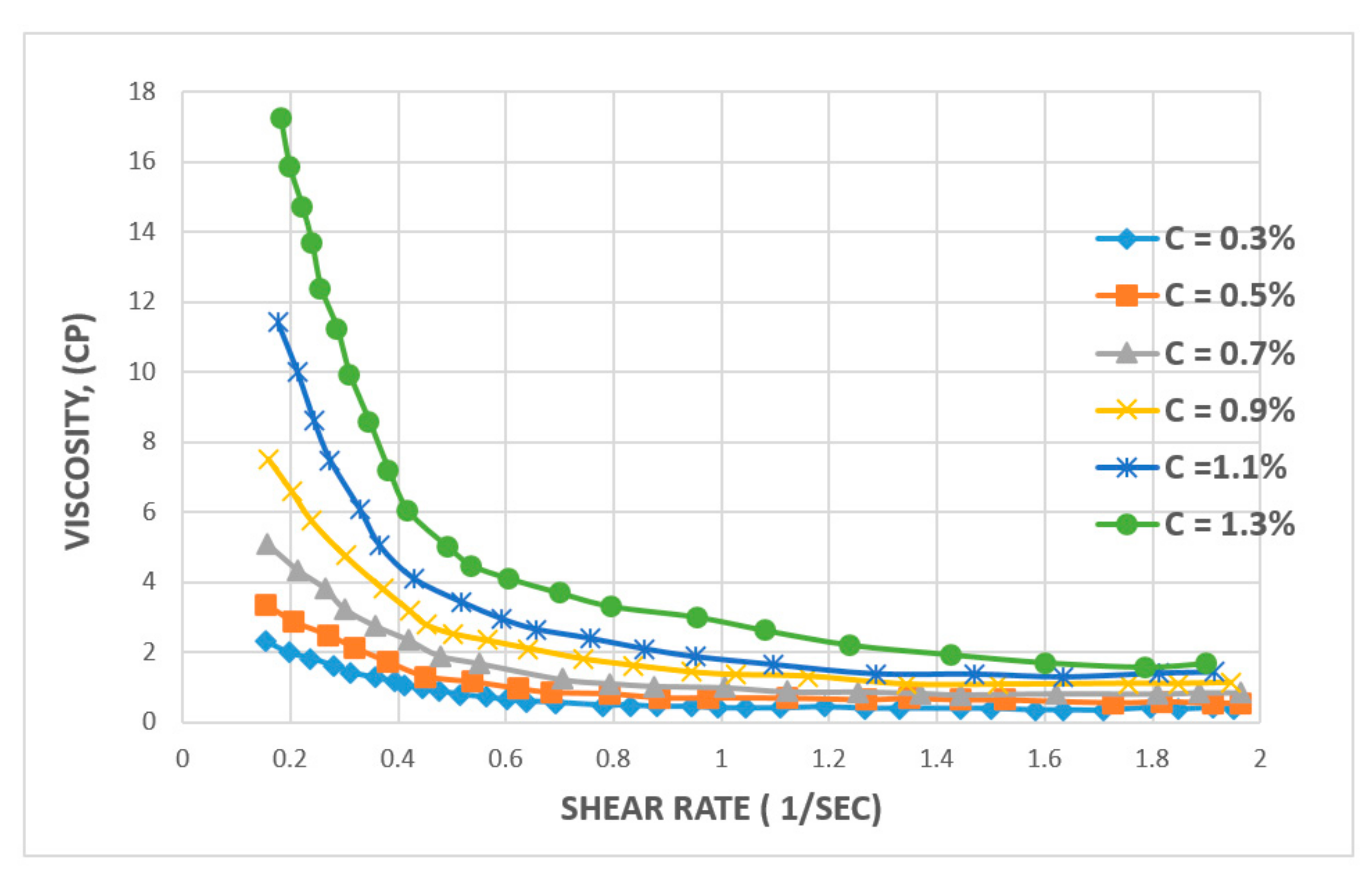


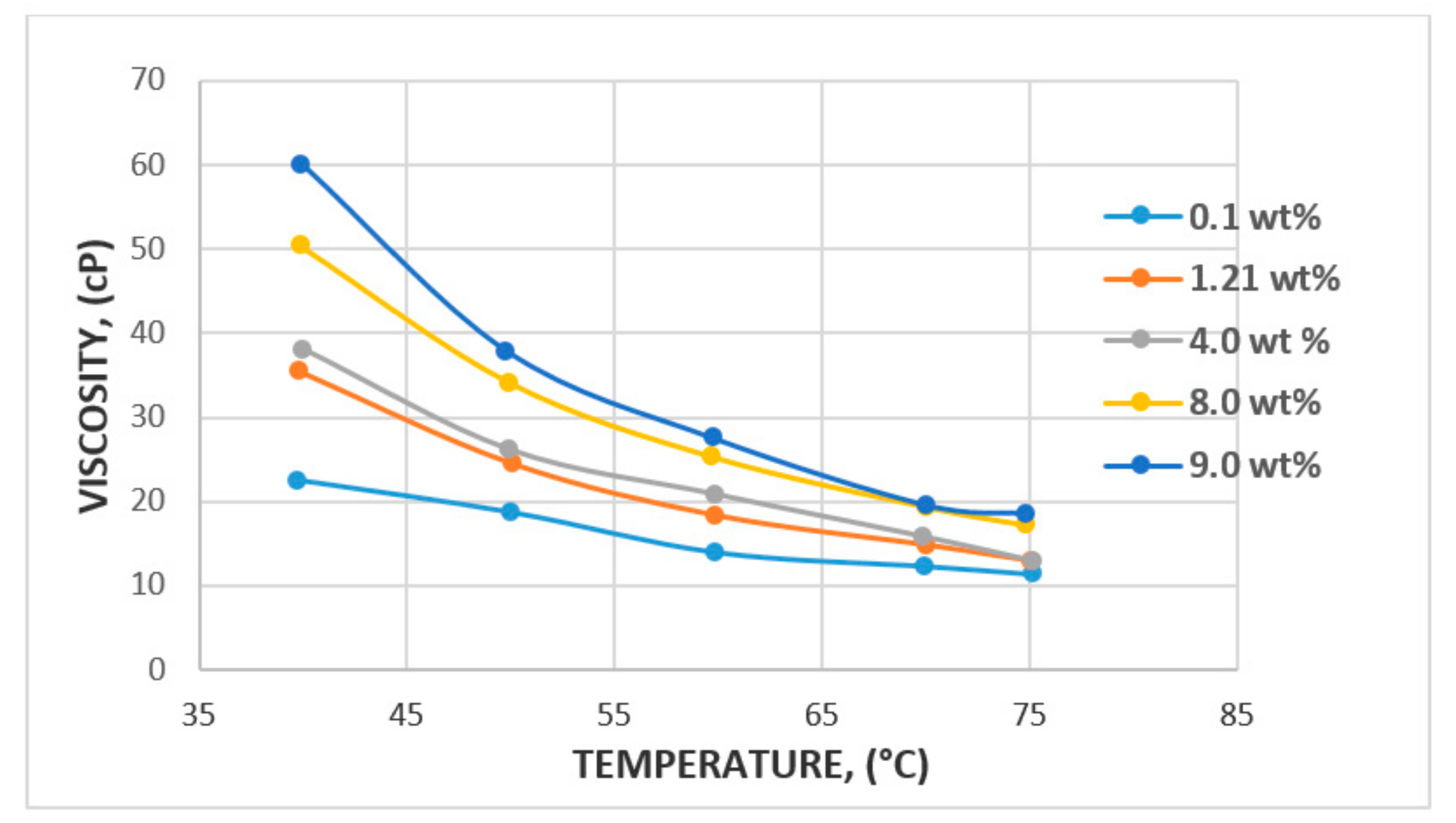
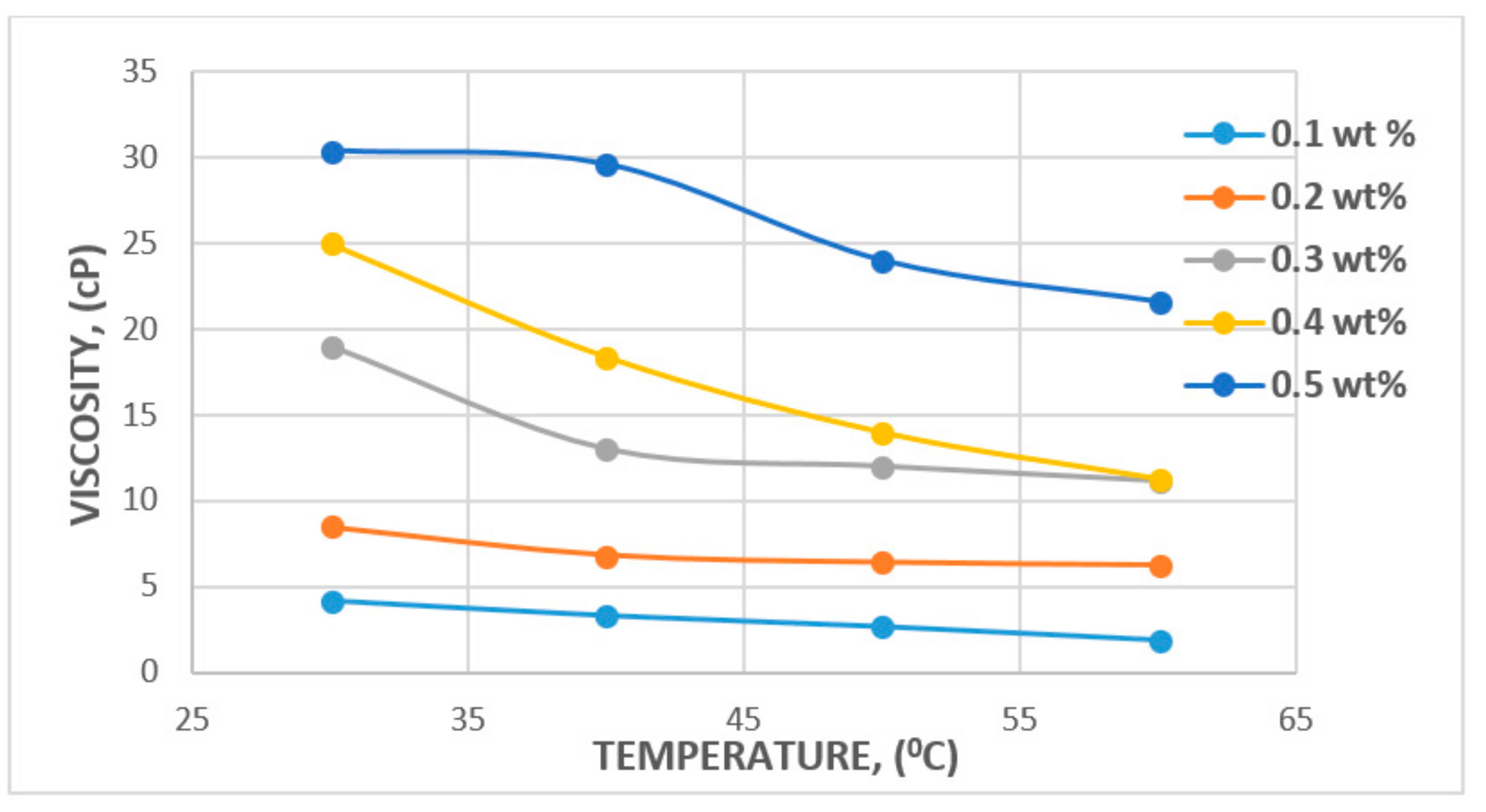
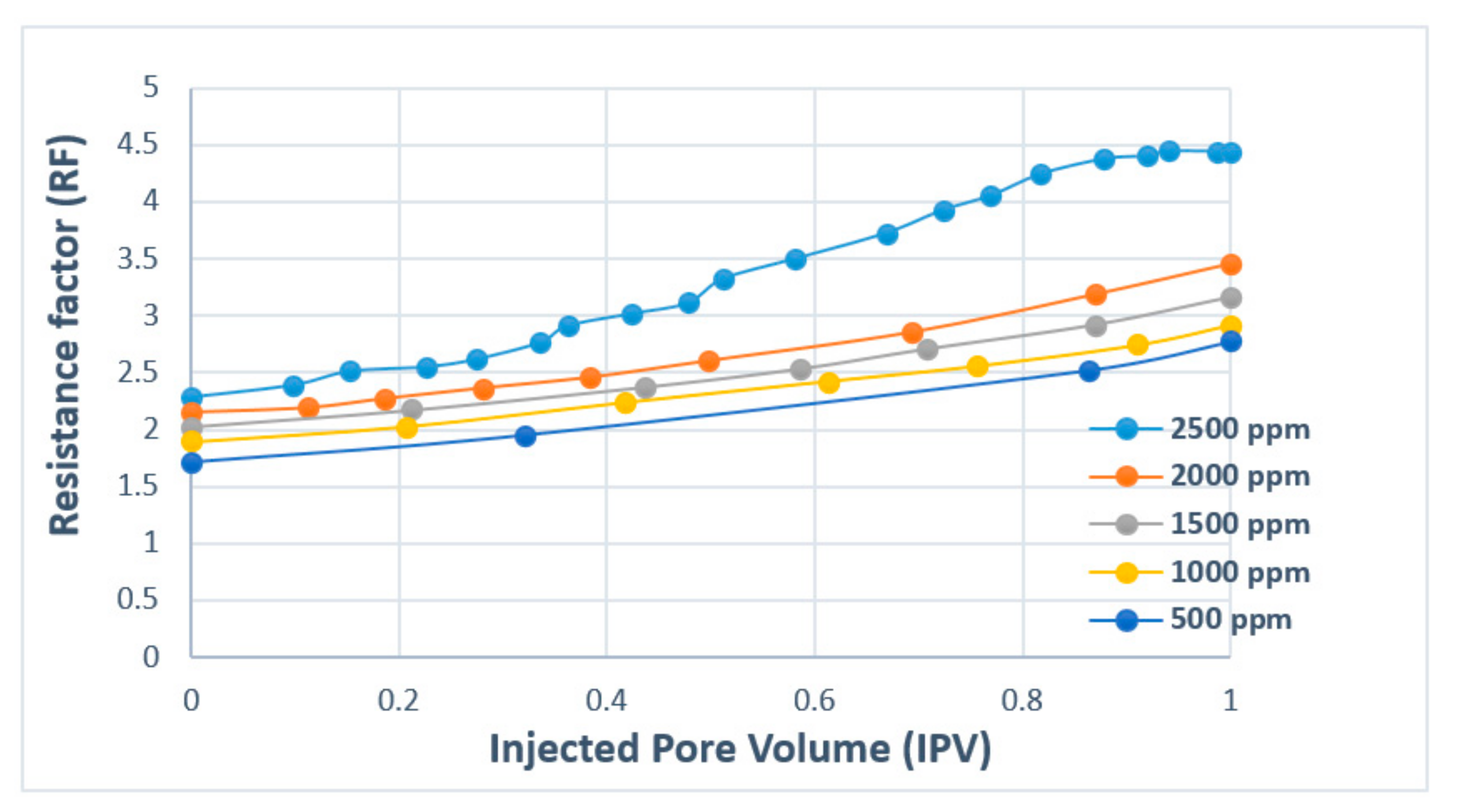
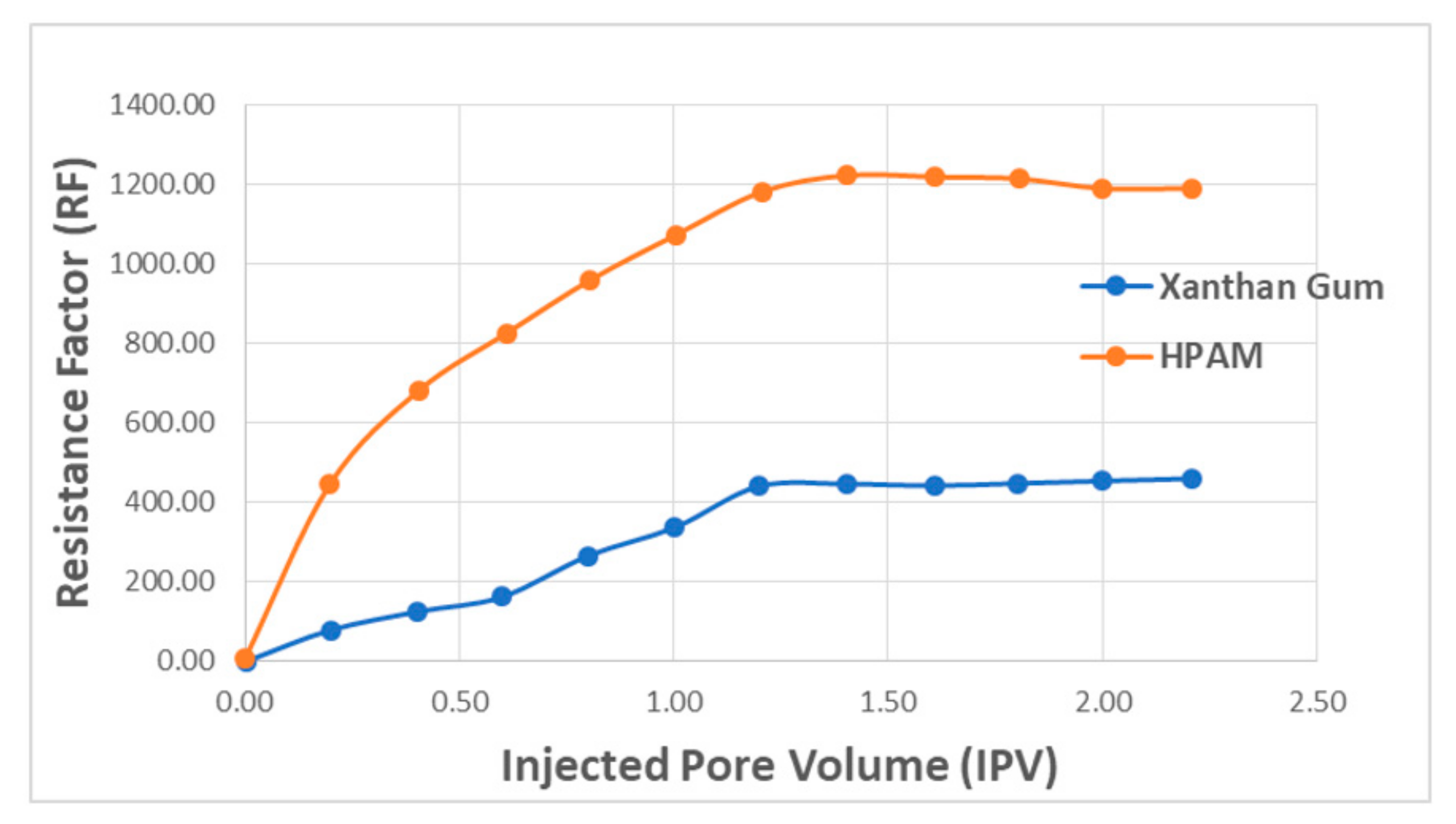

| Polymer Type | Polymer Concentration (ppm) | Temperature(°C) | NaCl Salinity (ppm) | IPV (PV/Year) | Reference |
|---|---|---|---|---|---|
| XG | 500–3000 | 25–65 | 3000 | NC | [13] |
| XG and Scleroglucan | 3000 | 90–120 | 1500 | NC | [55] |
| HPAM | 1000–2000 | NC | 3000–7000 | 0.14–0.20 | [70] |
| HPAM | 1500 | 55–90 | NC | NC | [5] |
| XG | 1000–5000 | 68–70 | NC | NC | [35] |
| XG | 400–500 | NC | 5000 | 2 | [71] |
| HPAM | 1000–2500 | 30 | 40,000 | 0.16–1.8 | [72] |
| HPAM | 500–2500 | 22 | 20,000 | NC | [54] |
| HPAM | 800 | <93.3 | <100 | 0.75 | [65] |
| XG | 1500 | 75–80 | 3000–30,000 | NC | [73] |
| HPAM | 1000 | 100–160 | 3000–30,000 | 0.5–1 | [60] |
| PAM | 500–5000 | 46–105 | 1230 | NC | [74] |
| HPAM | 10,000 | 25 | NC | 1.2 | [57] |
| HMSPAM | 7000 | 25 | NC | 1.4 | [57] |
| XG | 4000 | 25 | NC | 1.2 | [57] |
| XG | 300–5000 | 150 | 600 ** | NC | [63] |
| HPAM | 5000 | 20–30 | 5000 | 0.034–1 | [64] |
| HPAM | 1500 | 25–85 | 30,000 | 0.6 | [75] |
| XG | 1800 | 25–85 | 30,000 | 0.6 | [75] |
| HPAM | 1500 | 50 | 15,000 | NC | [76] |
| XG | 250–2000 | 20–120 | NC | NC | [77] |
| NVP-HPAM | 1000–4000 | 120 | 167,000 | NC | [14] |
| XG | 5000 | 23–77 | 20,000 | NC | [78] |
| PROPERTY | VALUES |
|---|---|
| Gravity | >25 °API |
| Viscosity | <150 cP |
| Composition | Not critical |
| Oil Saturation | >10% PV |
| Type of Formation | Sandstone preferred but can be used in carbonates |
| Net Thickness | Not critical |
| Average Permeability | >15 mD |
| Depth | <9000 ft |
| Temperature | <200 °F |
| Salinity | <100,000 ppm |
| Parameters | XG | HPAM | Other Associated (OA) |
|---|---|---|---|
| MW of polymer (g/mol) | 1 × 106–20 × 106 | 1 × 106–25 × 106 | 1.3 × 106–20 × 106 |
| Concentration of polymer (ppm) | 30–2000 | 50–10,000 | 500–3000 |
| Brine salinity (ppm) | 661–350,000 | 250–133,480 | 5000–186,000 |
| Viscosity of oil (cP) | 8–129 | 1.7–5500 | 140–18,700 |
| Porosity (%) | 10–48 | 10–45 | 21–40 |
| Permeability (mD) | 18–6000 | 2.5–13,000 | 30–12,600 |
| Temperature (°C) | 20–100 | 22–120 | 22–93 |
| Parameters | Range |
|---|---|
| MW of polymer (g/mol) | 5 × 106–37 × 106 |
| Polymer concentration (ppm) | 200–2500 |
| Polymer viscosity (cP) | 1.35–40 |
| Brine salinity (ppm) | 500–120,000 |
| Viscosity of oil (cP) | 0.2–10,000 |
| Porosity (%) | 11–34 |
| Permeability (mD) | 3.9–15,000 |
| Temperature (°C) | 22–90 |
| PARAMETERS | RANGE |
|---|---|
| Polymer type | HPAMs and XG |
| Porosity (%) | 4–38 |
| Permeability (mD) | >50 |
| Temperature (°C) | <90 |
| Formation brine salinity (ppm) | <50,000 |
| Divalent ion concentrations (ppm) | <100 |
| Lithology | Sandstone preferred but can be used in carbonates |
| Water cut for the reservoir to initiate polymer flood (%) | ≥95 |
| Clay content level in the reservoir rock | Relatively low |
| Oil viscosity (cP) | <5000 |
| Oil saturation (%) | >22 |
| Aquifer type | None to weak |
| Gas cap type | None to weak |
| The pattern for injection well during the completion | Large hole diameter, high density, and deep penetration |
| MW of polymer (g/mol) with respective permeability (mD) | For 12 × 106–16 × 106 k ≥ 100 For 17 × 106–25 × 106 k ≥ 100 |
| Polymer injection rate (PV/year) with respective well spacing (m) | For 0.14–0.16 PV/Yr, spacing = 250 m For 0.14–0.20 PV/Yr, spacing = (150–176) m |
| Addition of oxygen scavengers to polymer solutions | Alcohol, thiourea, sodium sulphite, and tri-or pentachlorophenol |
| Addition of biocides to control biological degradation | Formaldehyde |
| Field Name/Segment | Polymer Type | Lith. | Temp. (°C) | Formation Water TDS (ppm) | Well Spacing(m) | Polymer Injection Viscosity (cP) | Oil Viscosity (cP) | Recovery Method | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Niagara | PAM | Sand | NR | NR | 30 | 1.35 | 16 | Sec. | [110] |
| West Cat Canyon | HPAM | Sand | 63 | NR | 200–400 | 12 (12 rpm at 24 °C) | 110 | Sec. | [111] |
| Vernon/Upper Squirrel | HPAM | Sand | 24 | NR | 90–240 | 1.4 | 75 | Tert. | [112] |
| Huntington Beach/Garfield | HPAM | Sand | 52 | 31,801 | 300 | NR | 76 | Tert. | [113] |
| East Coalinga | XG and Kelzan | Sand | 38 | NR | 150 | NR | 25 (res.) | Tert. | [109] |
| Vorhop-Knesebeck/Dogger | Xanthan (state oil) | Sand | 56 | 220,000 | NR | 4 | 4 | Tert. | [109] |
| Daqing/Putuahoa form, PO | HPAM (10 MDa) | Sand | 45 | 60,000 | 100 | NR | 9.5 | Tert. | [114] |
| Eddesse-Nord | Xanthan | Sand | 22 | 120,000 | 60 | 12 | 7 | Tert. | [115] |
| Chateaurenard/Courtnay | HPAM | Sand | 30 | 400 | 400 | 10 | 40 @ 30 °C | Tert. | [116] |
| West Castle/Reservoir AQ | HPAM (NaCl 3857) | Sand | NR | NR | 66 | 4 | NR | NR | [117] |
| Daqing/Saertu form | PAM (10 MDa, 22–25% hydro) | Sand | 45 | 7000 | 200 | 28 | 9.5 | Tert. | [109] |
| Captain | HPAM (30% hydro) | Sand | 31 | 13,000–18,000 | NR | 4.5 (500 ppm) | 88 @ 31°C | NR | [109] |
| Pelican Lake/Wabiskaw | HPAM (13.6 MDa, 32% hydro) | Sand | 23 | 6853 | 150 | 30 | 600–1000 | Tert. | [118] |
| Shuang He/Layer II | HPAM (14.3 MDa, 23% hydro) | Sand | 72 | 4356 | 250 | 58 (well head) | 7.8 | Tert. | [119] |
| Carmopolis | HPAM (Flopam SNF) | Sand | 50 | 17,091 | NR | 30 | 50 | Tert. | [120] |
| Daqing/ZQXB | HPAM | Sand | 65 | NR | NR | 200–250 | 9.5 | Tert. | [121] |
| Sanand/KS-III | HPAM | Sand | 85 | NR | 200 | NR | 20 | Tert. | [122] |
| East Bodo/Lloydminster | HPAM (F3630) | Sand | NR | 29,000 | NR | 60 | 600–2000 | Tert. | [123] |
| Dalia, Camelia | HPAM (18–20 MDa) | Sand | 45–56 | 117,700 | 1000–1500 | 3.3 | 1–11 | NR | [124] |
| Tambaredjo | HPAM | Sand | 38 | 5000 | 100–600 | 45 (well head) | 300–600 | Tert. | [125] |
| Reservoir Property | Saudi Arabia | |
|---|---|---|
| Northern Area | Southern Area | |
| Depth (ft) | 4100–6800 | 5200–8000 |
| Lithology | Sandstone | Carbonate |
| Thickness (ft) | 20–200 | 100–300 |
| Porosity (%) | 20–29 | 14–22 |
| Permeability (mD) | 1000–3000 | 100–500 |
| Oil Gravity (API) | 27–34 | 34–37 |
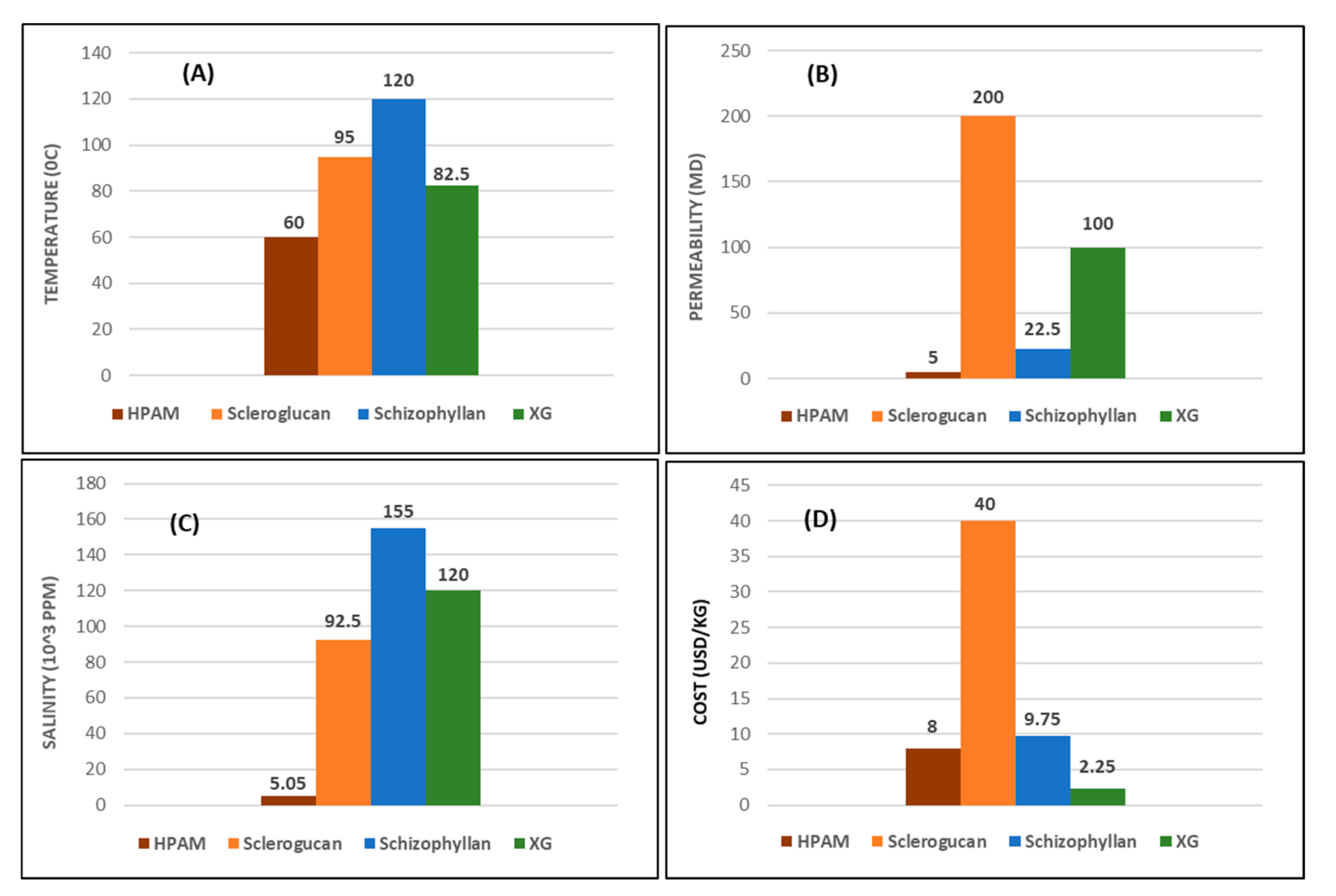
| Polymer Chemistry | Polymer Family | Synthetic | Biopolymer | ||
|---|---|---|---|---|---|
| Polymer Type | HPAM | Scleroglucan | Schizophyllan | Xanthan Gum | |
| Range of application | Highest temperature limit (°C) | 60 | 95 | 120 | 75–90 |
| Lowest permeability limit (mD) | 5 | 200 | 150–300 | 100 | |
| Pros and cons involved | 0.1–10 | 20–165 | 30–280 | 20–220 | |
| Tolerance for shear resistance | |||||
| Tolerance to biodegradation | |||||
| Industrial availability | |||||
| Cost economics (USD/kg) | 5–11 | 30–50 | 8.5–11 | 1.5–3 | |
| Logistics and handling | |||||
| Application cases | Lab (Experiment) | ||||
| Pilot | |||||
| Field | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammed, N.S.; Haq, M.B.; Al-Shehri, D.; Rahaman, M.M.; Keshavarz, A.; Hossain, S.M.Z. Comparative Study of Green and Synthetic Polymers for Enhanced Oil Recovery. Polymers 2020, 12, 2429. https://doi.org/10.3390/polym12102429
Muhammed NS, Haq MB, Al-Shehri D, Rahaman MM, Keshavarz A, Hossain SMZ. Comparative Study of Green and Synthetic Polymers for Enhanced Oil Recovery. Polymers. 2020; 12(10):2429. https://doi.org/10.3390/polym12102429
Chicago/Turabian StyleMuhammed, Nasiru Salahu, Md. Bashirul Haq, Dhafer Al-Shehri, Mohammad Mizanur Rahaman, Alireza Keshavarz, and S. M. Zakir Hossain. 2020. "Comparative Study of Green and Synthetic Polymers for Enhanced Oil Recovery" Polymers 12, no. 10: 2429. https://doi.org/10.3390/polym12102429
APA StyleMuhammed, N. S., Haq, M. B., Al-Shehri, D., Rahaman, M. M., Keshavarz, A., & Hossain, S. M. Z. (2020). Comparative Study of Green and Synthetic Polymers for Enhanced Oil Recovery. Polymers, 12(10), 2429. https://doi.org/10.3390/polym12102429






