Hydrophilic Films Based on Carboxymethylated Derivatives of Starch and Cellulose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CMS with High Degree of Substitution
2.3. Preparation of CMS/CMC Films
2.4. Methods
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
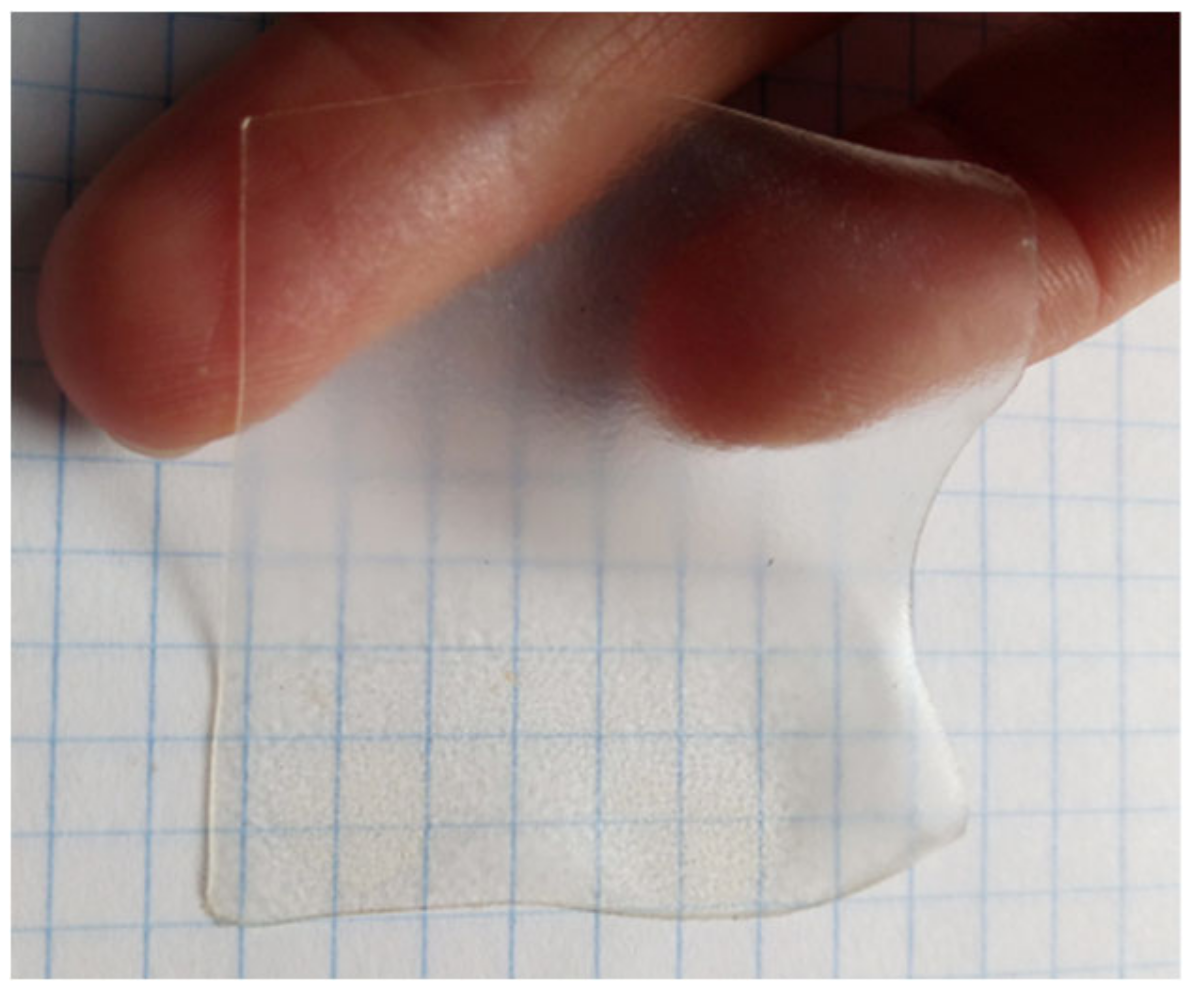
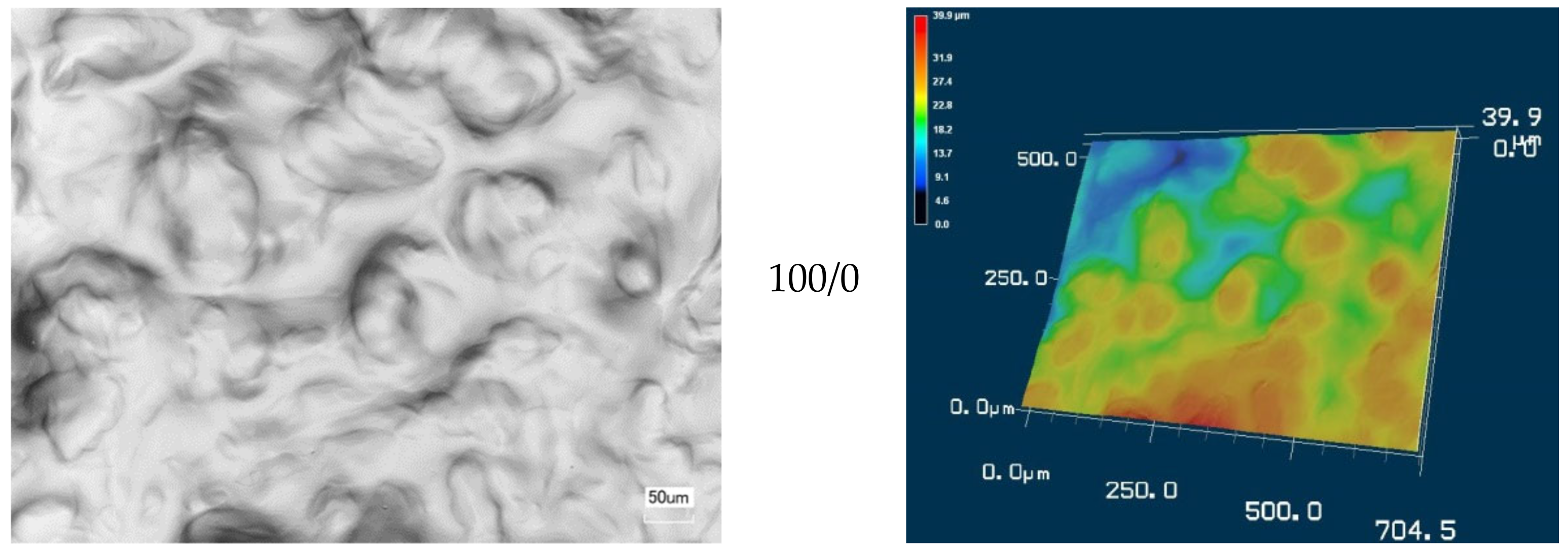
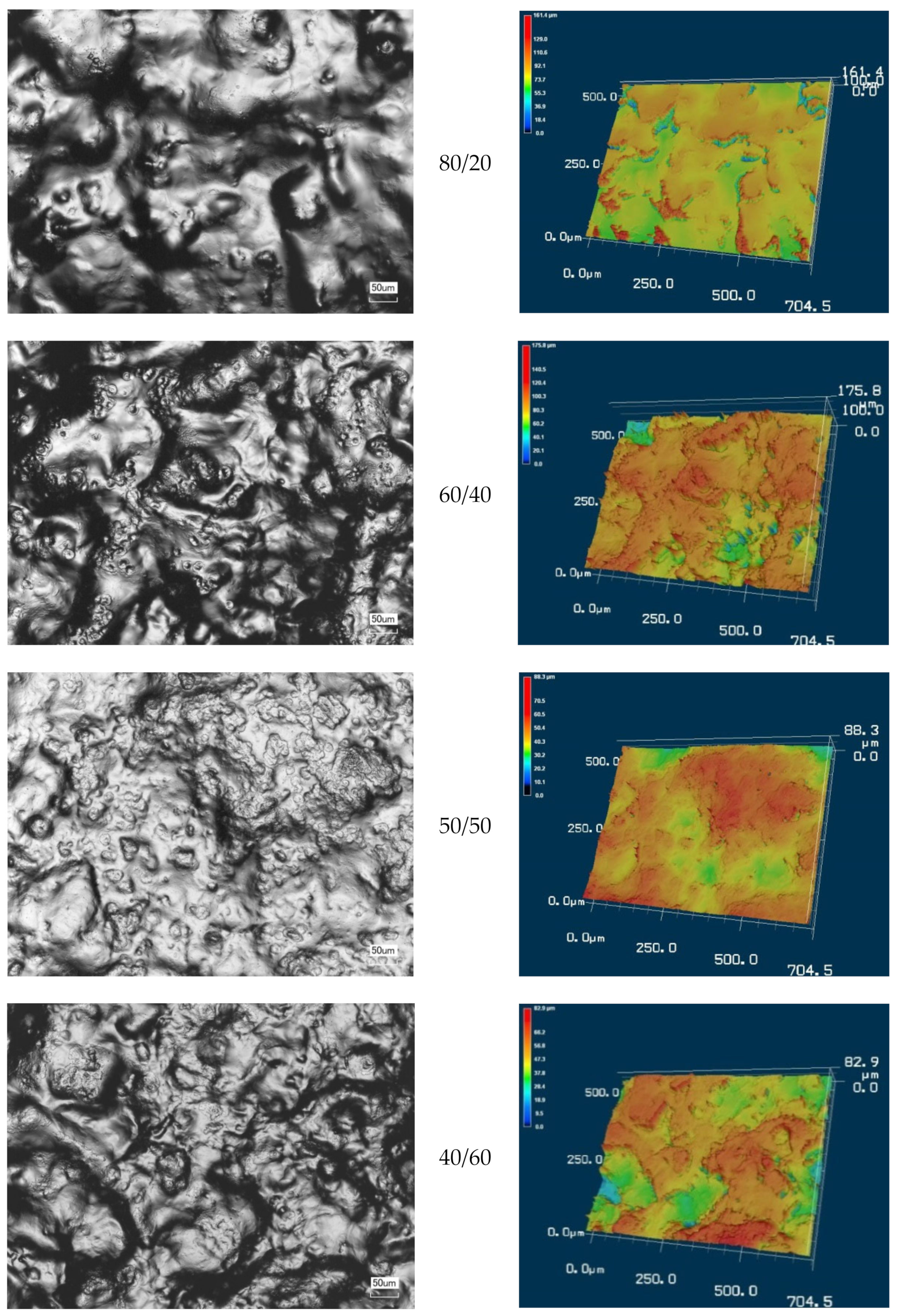
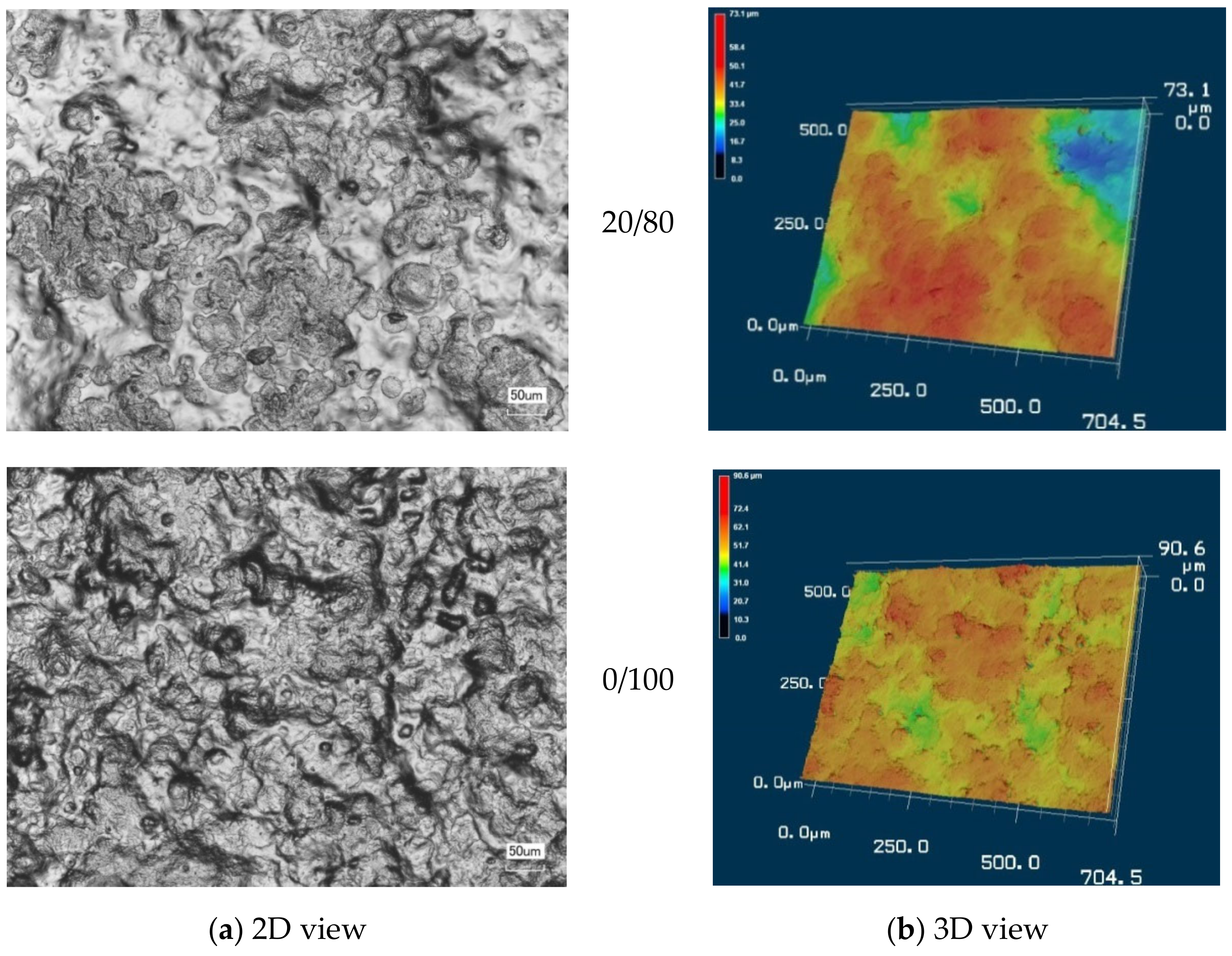
3.2. Morphology of CMS/CMC Films
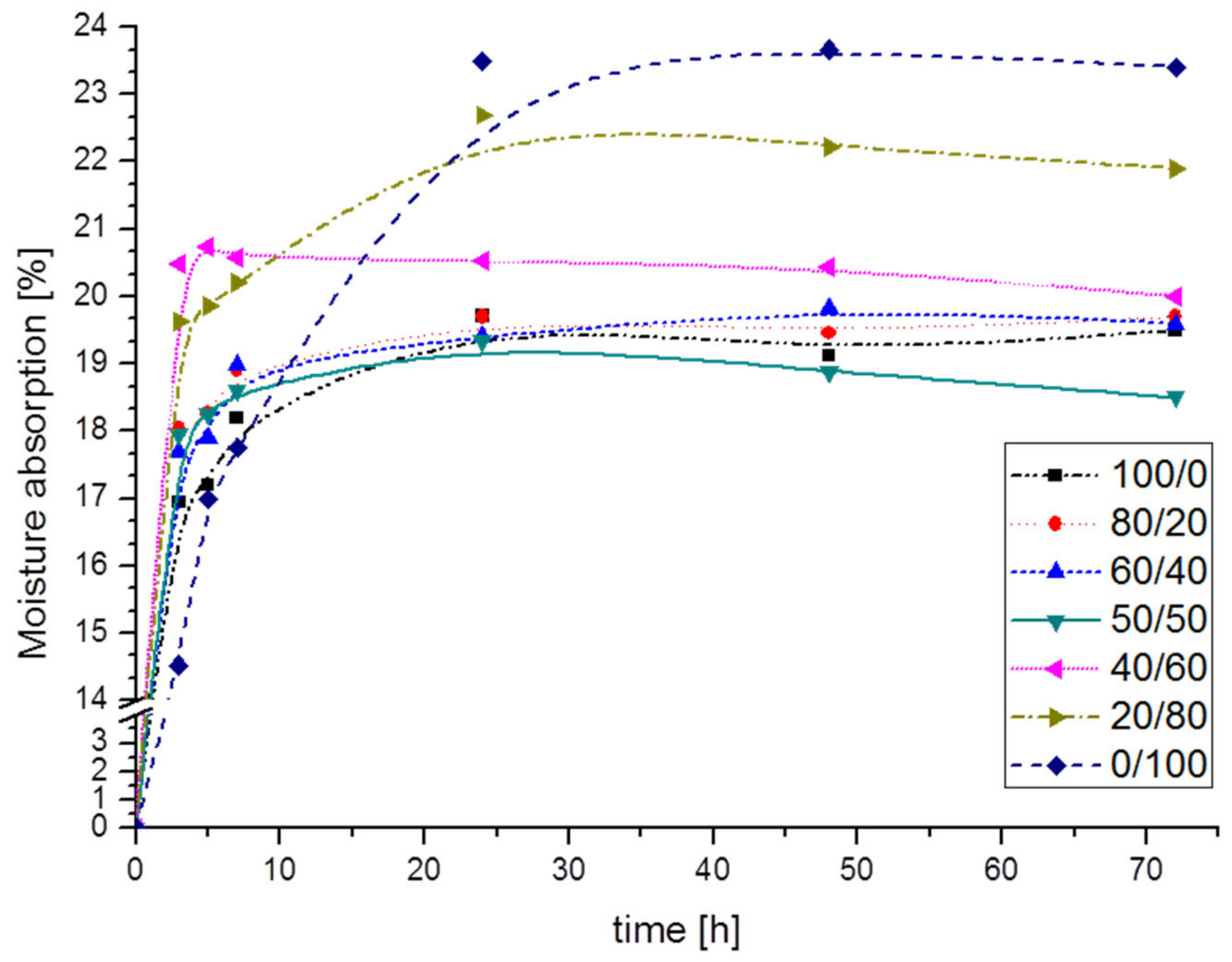
3.3. Moisture Absorption
3.4. Dynamic Mechanical Thermal Analysis (DMTA)
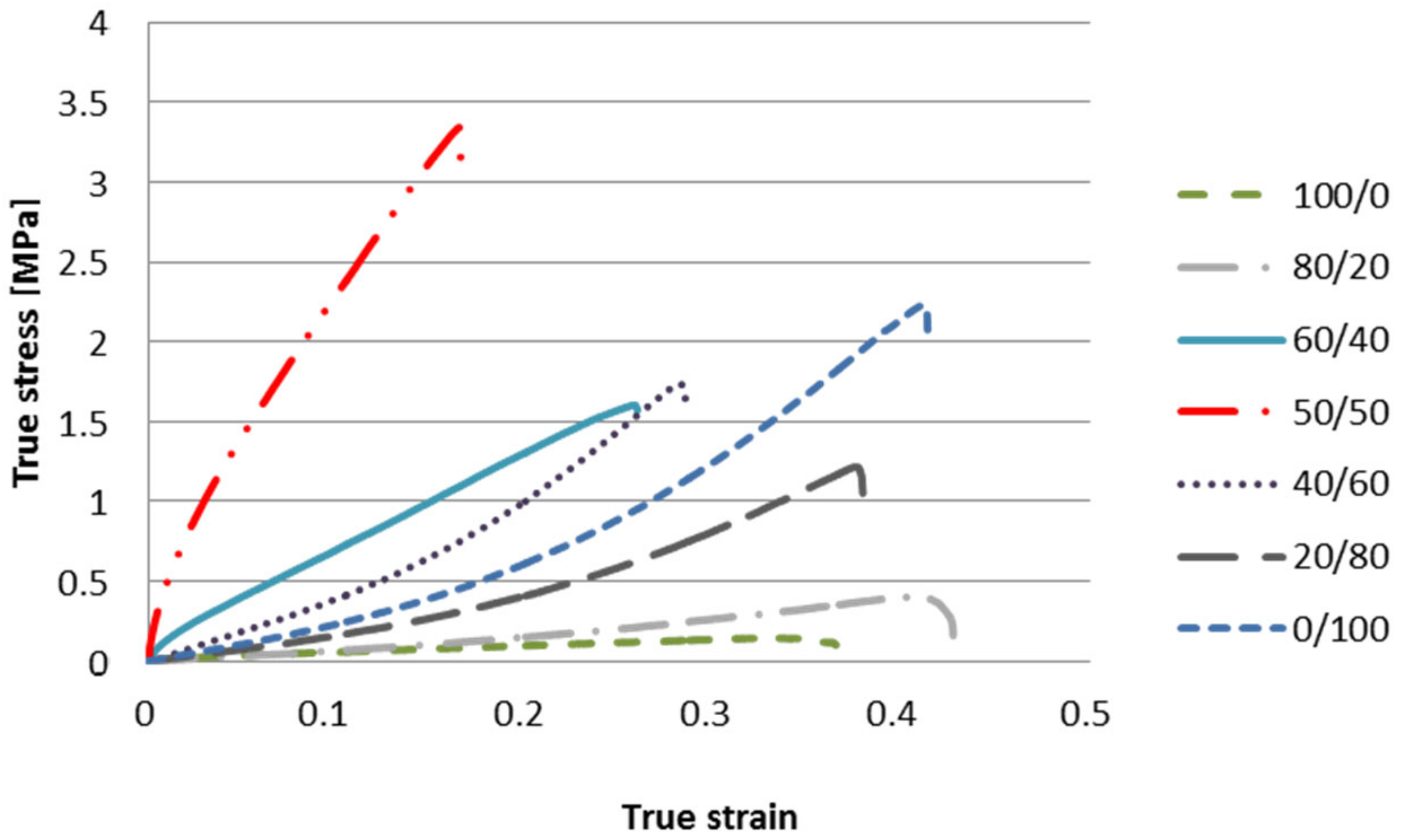
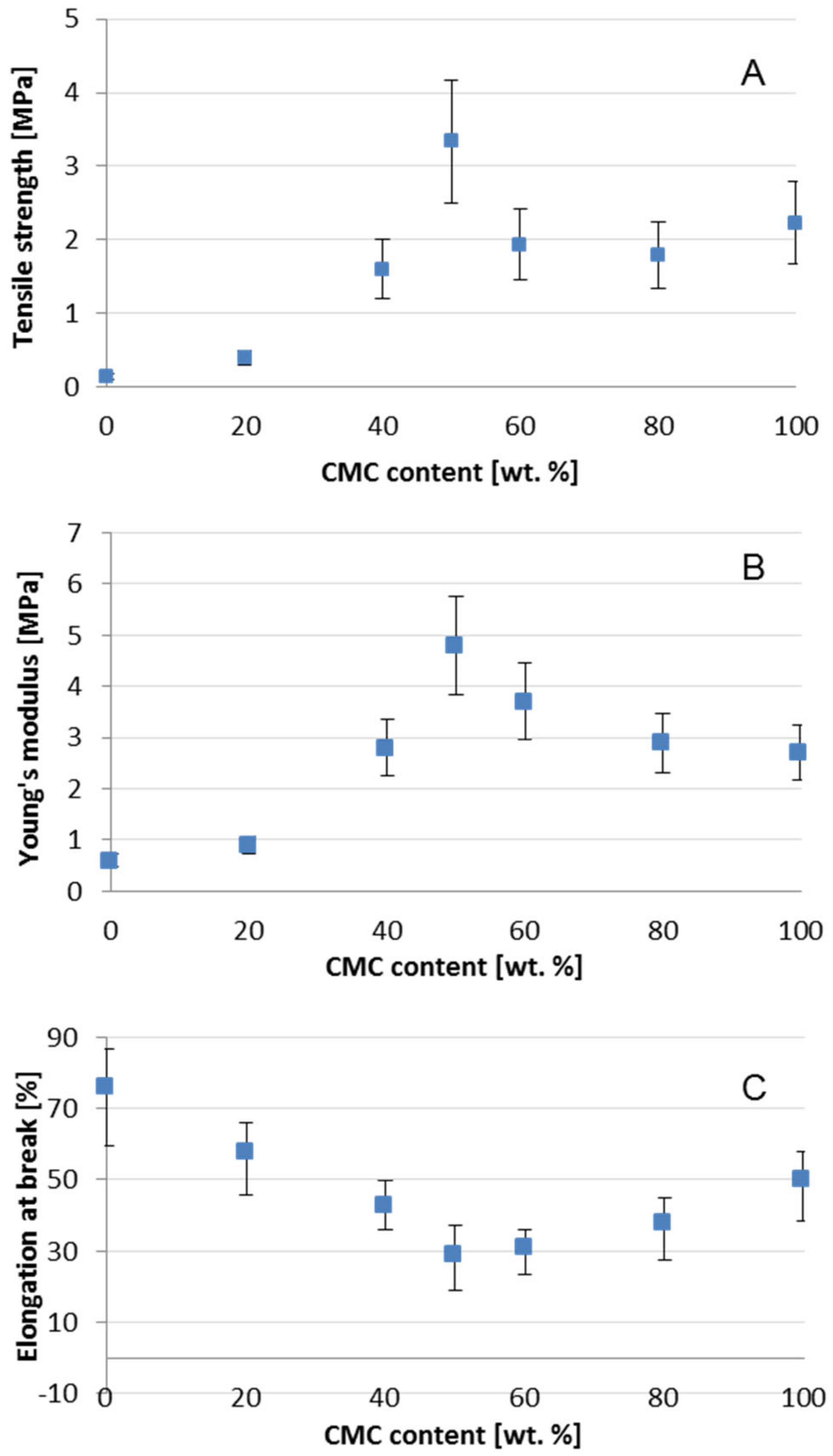
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Spychaj, T.; Wilpiszewska, K.; Zdanowicz, M. Medium and high substituted carboxymethyl starch: Synthesis, characterization and application. Starch 2013, 65, 22–33. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Kittipongpatana, O.S.; Chaitep, W.; Charumanee, S.; Kittipongpatana, N. Effects of amylose content on the physicochemical properties of sodium carboxymethyl rice starches. CMU J. Nat. Sci. 2006, 5, 199–207. [Google Scholar]
- Kim, K.W.; Ko, C.J.; Park, H.J. Mechanical properties, water vapor permeabilities and solubilities of highly carboxymethylated starch-based edible films. J. Food Sci. 2002, 67, 218–222. [Google Scholar] [CrossRef]
- Kittipongpatana, O.S.; Chaichanasak, N.; Kanchongkittipoan, S.; Panturat, A.; Taekanmark, T.; Kittipongpatana, N. An aqueous film-coating formulation based on sodium carboxymethyl mungbean starch. Starch 2006, 58, 587–589. [Google Scholar] [CrossRef]
- Kittipongpatana, N.; Janta, S.; Kittipongpatana, O. Preparation of cross-linked carboxymethyl jackfruit starch and evaluation as tablet disintegrant. Pak. J. Pharm. Sci. 2011, 24, 415–420. [Google Scholar]
- Anirudhan, T.S.; Parvathy, J. Novel semi-IPN based on crosslinked carboxymethyl starch and clay for the in vitro release of theophylline. Int. J. Biol. Macromol. 2014, 67, 238–245. [Google Scholar] [CrossRef]
- Kittipongpatana, N.; Kittipongpatana, O.S. Cross-linked carboxymethyl mung bean starch as pharmaceutical gelling agent and emulsion stabilizer. Int. J. Pharm. Pharm. Sci. 2015, 7, 403–407. [Google Scholar]
- Lawal, O.S.; Storz, J.; Storz, H.; Lohmann, D.; Lechner, D.; Kulicke, W.M. Hydrogels based on carboxymethyl cassava starch cross-linked with di- or polyfunctional carboxylic acids: Synthesis, water absorbent behavior and rheological characterizations. Eur. Polym. J. 2009, 45, 3399–3408. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Antosik A., K.; Spychaj, T. Novel hydrophilic carboxymethyl starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2015, 128, 85–89. [Google Scholar] [CrossRef]
- Wilpiszewska, K. Hydrophilic films based on starch and carboxymethyl starch. Pol. J. Chem. Technol. 2019, 21, 26–30. [Google Scholar] [CrossRef]
- Takahashi, K.; Ogata, A.; Yang, W.H.; Hattori, M. Increased hydrophobicity of carboxymethyl starch film by conjugation with zein. Biosci. Biotechnol. Biochem. 2002, 66, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Ngamekaue, N.; Chitprasert, P. Effects of beeswax-carboxymethyl cellulose composite coating on shelf-life stability and intestinal delivery of holy basil essential oil-loaded gelatin microcapsules. Int. J. Biol. Macromol. 2019, 135, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.E.; Aziz, M.S.A.; Alsehli, M. Carboxymethyl cellulose/sodium alginate/chitosan biguanidine hydrochloride ternary system for edible coatings. Int. J. Biol. Macromol. 2019, 139, 614–620. [Google Scholar] [CrossRef]
- Putri, D.A.; Setiawan, A.; Anggraini, P.D. Physical properties of edible sorghum starch film added with carboxymethyl cellulose. J. Phys. Sci. 2018, 29, 185–194. [Google Scholar] [CrossRef]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J.; Dhane, N.S.; Ghorpade, V.S. Citric acid crosslinked carboxymethyl cellulose-based composite hydrogel films for drug delivery. Ind. J. Pharm. Sci. 2018, 80, 657–667. [Google Scholar] [CrossRef]
- Wahyuningtyas, D.; Dinata, A. Combination of carboxymethyl cellulose (CMC)—Corn starch edible film and glycerol plasticizer as a delivery system of diclofenac. AIP Conf. Proc. 2018, 1977, 030032. [Google Scholar]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch–CMC–nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, R. Utilization of carboxymethyl cellulose from durian rind agricultural waste to improve physical properties and stability of rice starch-based film. J. Polym. Environ. 2019, 27, 286–298. [Google Scholar] [CrossRef]
- Tavares, K.M.; de Campos, A.; Mitsuyuki, M.C.; Luchesi, B.R.; Marconcini, J.M. Corn and cassava starch with carboxymethyl cellulose films and its mechanical hydrophobic properties. Carbohydr. Polym. 2019, 223, 115055. [Google Scholar] [CrossRef]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem. Cent. J. 2011, 5, 1–8. [Google Scholar] [CrossRef]
- Veronese, A.F.; de Souza Rocha, T.; Franco, C.M.L.; Costa, M.S.; Grossmann, M.V.E. Starch-carboxymethyl cellulose (CMC) mixtures processed by extrusion. Starch 2018, 70, 1700336. [Google Scholar] [CrossRef]
- Xiong, J.; Li, Q.; Shi, Z.; Ye, J. Interactions between wheat starch and cellulose derivatives in short-term retrogradation: Rheology and FTIR study. Food Res. Int. 2017, 100, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Putri, D.A.; Setiawan, A.; Anggraini, P.D. Effect of carboxymethyl cellulose (CMC) as biopolymers to edible film sorghum starch hydrophobicity characteristics. AIP Conf. Proc. 2017, 1818, 020044. [Google Scholar]
- Antosik, A.K.; Piątek, A.; Wilpiszewska, K. Carboxymethylated starch and cellulose derivatives-based film as human skin equivalent for adhesive properties testing. Carbohydr. Polym. 2019, 222, 115014. [Google Scholar] [CrossRef] [PubMed]
- Antosik, A.K.; Wilpiszewska, K.; Czech, Z. Carboxymethylated polysaccharide-based films as carriers for acrylic pressure-sensitive adhesives. Int. J. Adhes. Adhes. 2017, 73, 75–79. [Google Scholar] [CrossRef]
- Kessel, H. Determination of the functional group and the degree of substitution of carboxymethyl starch. Starch 1985, 37, 334–336. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Schmutz, M.; Avérous, L. New approach to elaborate exfoliated starch-based nanobiocomposites. Biomacromol 2008, 9, 896–900. [Google Scholar] [CrossRef]
- Pushpadass, H.A.; Marx, D.B.; Hanna, M.A. Effects of extrusion temperature and plasticizers on the physical and functional properties of starch. Starch 2008, 60, 527–538. [Google Scholar] [CrossRef]
- Rachtanapun, P. Blended films of carboxymethyl cellulose from papaya peel (CMCp) and corn starch. Kasetsart J. (Nat. Sci.) 2009, 43, 259–266. [Google Scholar]
- Olsson, E.; Menzel, M.; Johansson, C.; Andersson, R.; Koch, K.; Järnström, L. The effect of pH on hydrolysis, cross-linking, and barrier properties of starch barriers containing citric acid. Carbohydr. Polym. 2013, 98, 1505–1513. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Buksa, K.; Szumny, A.; Zięba, T.; Gryszkin, A. Analysis of molecular structure of starch citrate obtained by a well-stablished method. LWT Food Sci. Technol. 2016, 69, 334–341. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Physical properties of edible modified starch/carboxymethyl cellulose films. Innov. Food Sci. Emer. Technol. 2010, 11, 697–702. [Google Scholar] [CrossRef]
- Su, J.F.; Huang, Z.; Yuan, X.Y.; Wang, X.Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Li, M.C.; Mei, C.; Xu, X.; Lee, S.; Wu, Q. Cationic Surface modification of cellulose nanocrystals: Toward tailoring dispersion and interface in carboxymethyl cellulose films. Polymer 2016, 107, 200–210. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilpiszewska, K.; Antosik, A.K.; Schmidt, B.; Janik, J.; Rokicka, J. Hydrophilic Films Based on Carboxymethylated Derivatives of Starch and Cellulose. Polymers 2020, 12, 2447. https://doi.org/10.3390/polym12112447
Wilpiszewska K, Antosik AK, Schmidt B, Janik J, Rokicka J. Hydrophilic Films Based on Carboxymethylated Derivatives of Starch and Cellulose. Polymers. 2020; 12(11):2447. https://doi.org/10.3390/polym12112447
Chicago/Turabian StyleWilpiszewska, Katarzyna, Adrian Krzysztof Antosik, Beata Schmidt, Jolanta Janik, and Joanna Rokicka. 2020. "Hydrophilic Films Based on Carboxymethylated Derivatives of Starch and Cellulose" Polymers 12, no. 11: 2447. https://doi.org/10.3390/polym12112447
APA StyleWilpiszewska, K., Antosik, A. K., Schmidt, B., Janik, J., & Rokicka, J. (2020). Hydrophilic Films Based on Carboxymethylated Derivatives of Starch and Cellulose. Polymers, 12(11), 2447. https://doi.org/10.3390/polym12112447





