Novel Temperature/Reduction Dual-Stimulus Responsive Triblock Copolymer [P(MEO2MA-co- OEGMA)-b-PLLA-SS-PLLA-b-P(MEO2MA-co-OEGMA)] via a Combination of ROP and ATRP: Synthesis, Characterization and Application of Self-Assembled Micelles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
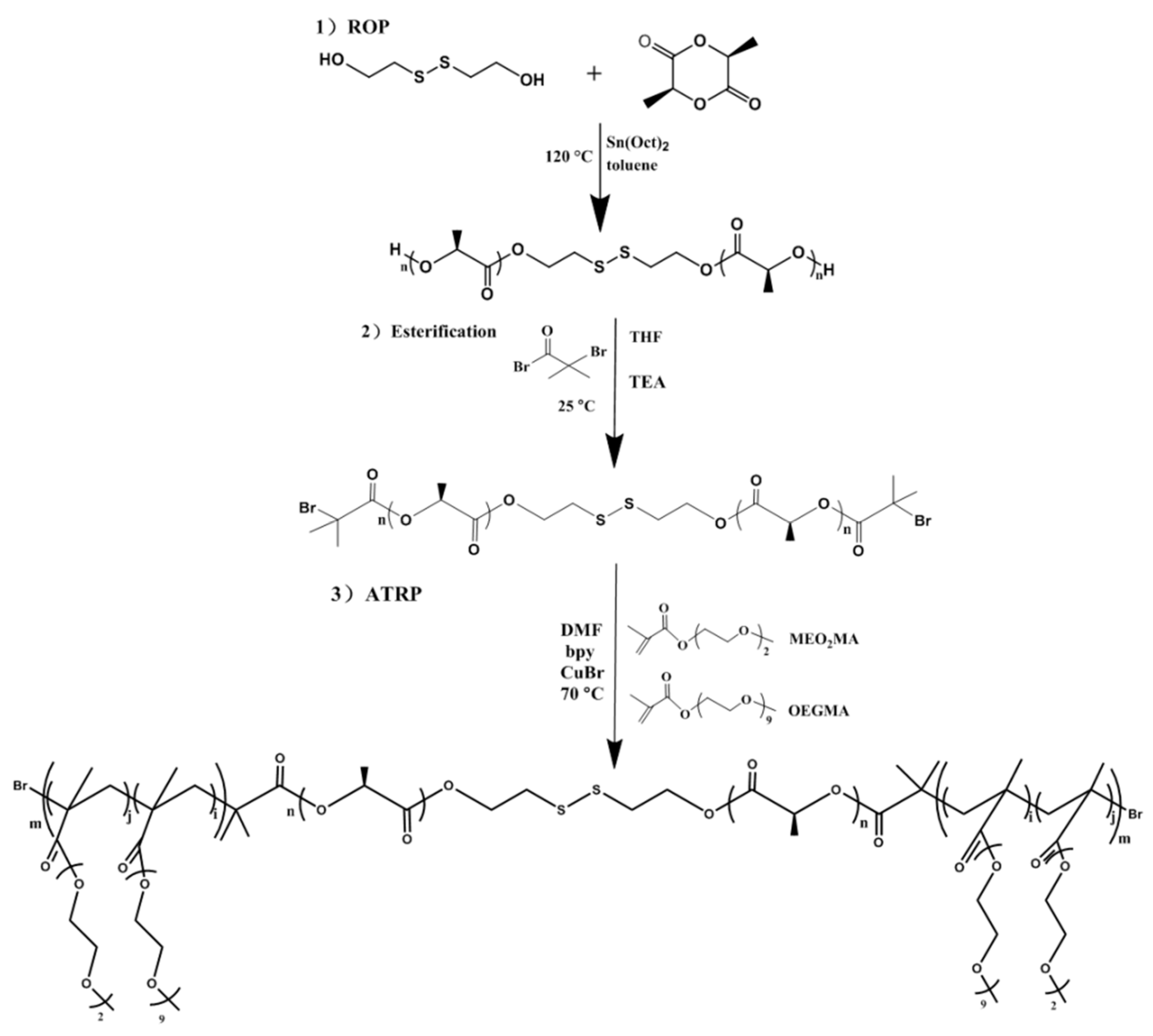
2.2.1. Synthesis of Homopolymer OH-PLLA-SS-PLLA-OH by ROP
2.2.2. Synthesis of Macromolecular Initiator iBuBr-PLLA-SS-PLLA-iBuBr
2.2.3. Synthesis of Triblock Copolymer [P(MEO2MA-co-OEGMA)-b-PLLA-SS-PLLA-b-P(MEO2MA- co-OEGMA)] by ATRP
2.3. Characterization
2.3.1. Nuclear Magnetic Resonance Spectroscopy (1H NMR)
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.3. Size Exclusive Chromatography (SEC)
2.4. The Properties of Copolymers
2.4.1. Water Solubility and Temperature Sensitivity of Copolymers
2.4.2. Low Critical Solution Temperature (LCST) of Copolymers
2.5. Properties of Copolymeric Micelles
2.5.1. Critical Micelle Concentration (CMC) of the Polymeric Micelles
2.5.2. Dynamic Light Scattering (DLS) of the Polymeric Micelles
2.5.3. Morphology of the Polymeric Micelles
2.6. Sol–Gel Transition of the Copolymers
2.7. In Vitro Release Behavior of Polymeric Drug-Loaded Micelles
2.7.1. Preparation of Polymeric Micelles Loaded with Doxorubicin (DOX)
2.7.2. Accumulative Drug Release of Polymeric Drug-Loaded Micelles
3. Results and Discussion
3.1. Synthesis and Characterization of Temperature/Reduction Dual-Stimulus Responsive Triblock Copolymers
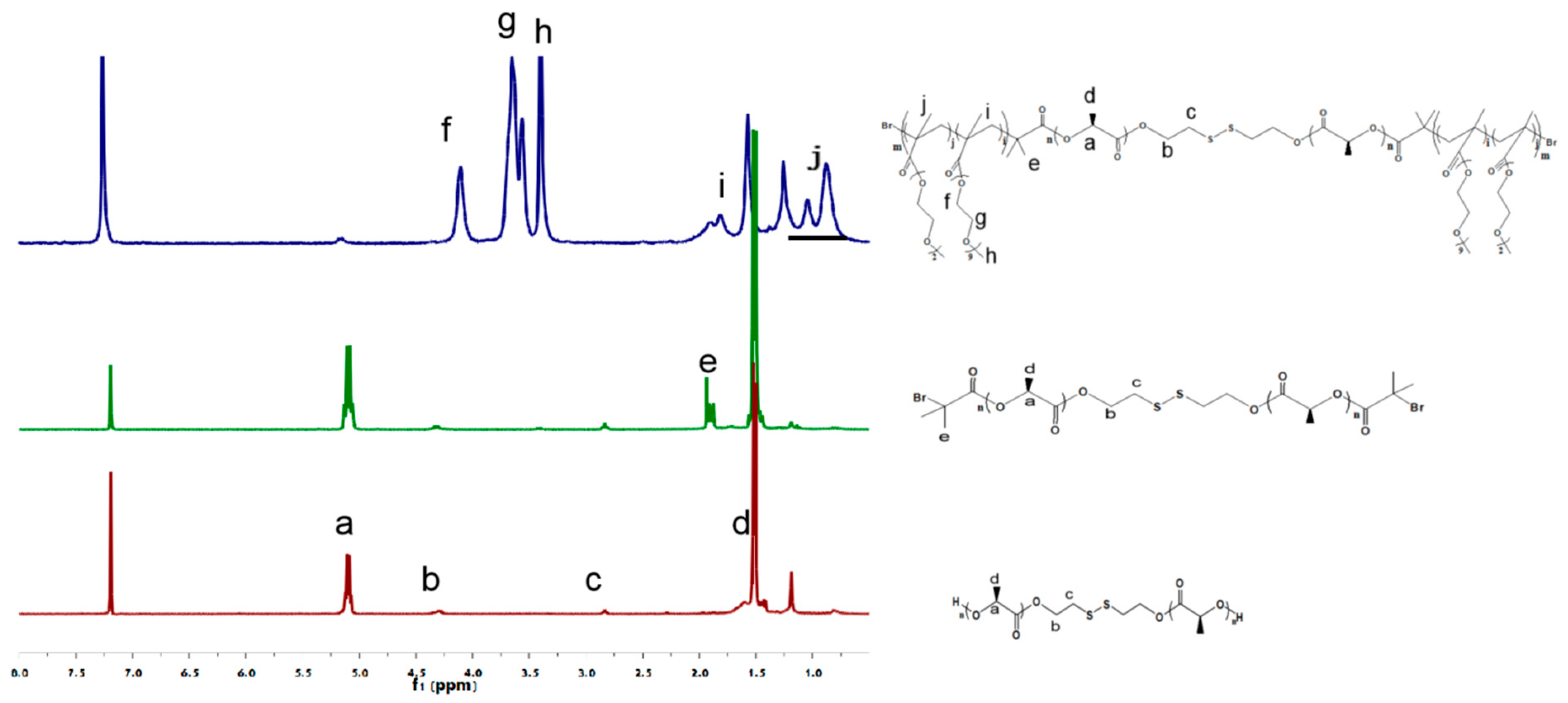
3.1.1. 1H NMR and FTIR Temperature/Reduction Dual-Stimulus Responsive Triblock Copolymers
3.1.2. Size Exclusive Chromatography (SEC) of Temperature/Reduction Dual-Stimulus Responsive Triblock Copolymers
3.2. The Properties of Temperature/Reduction Dual-Stimulus Responsive Triblock Copolymers
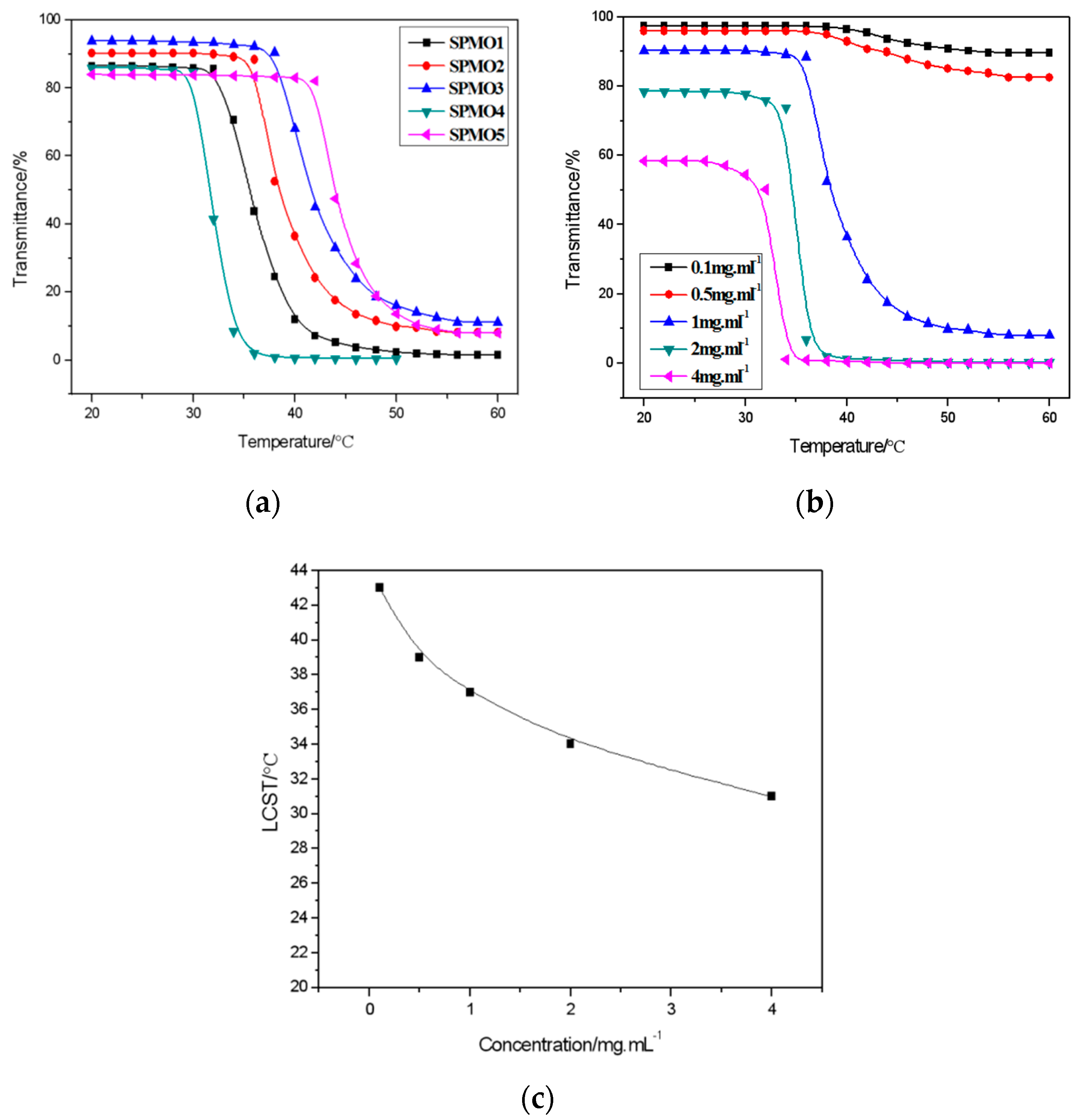
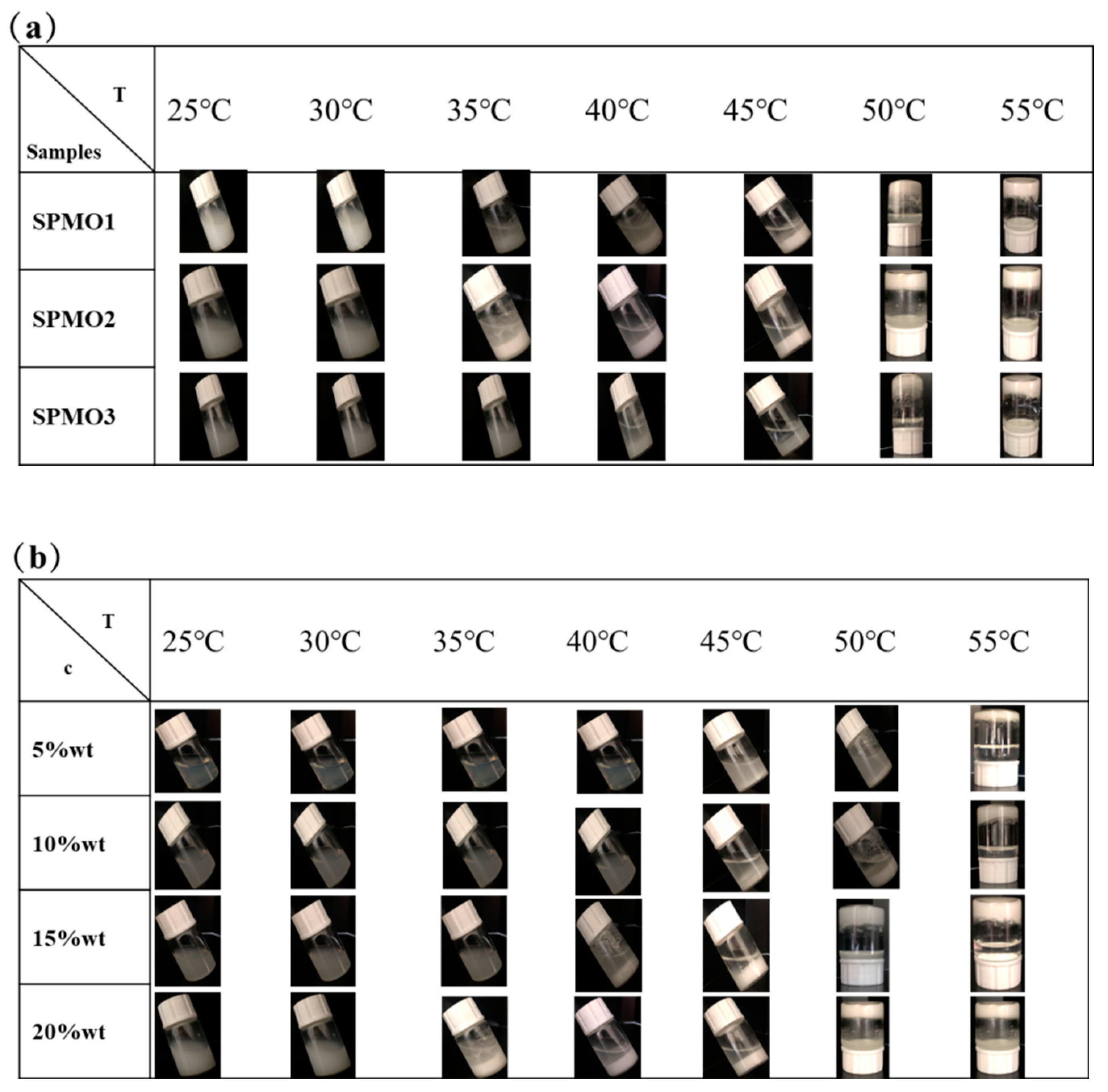
3.2.1. Water Solubility and Temperature Sensitivity of Copolymers
3.2.2. Low Critical Solution Temperature (LCST) of Copolymers
3.3. The Properties of Temperature/Reduction Dual-Stimulus Responsive Triblock Polymeric Micelles
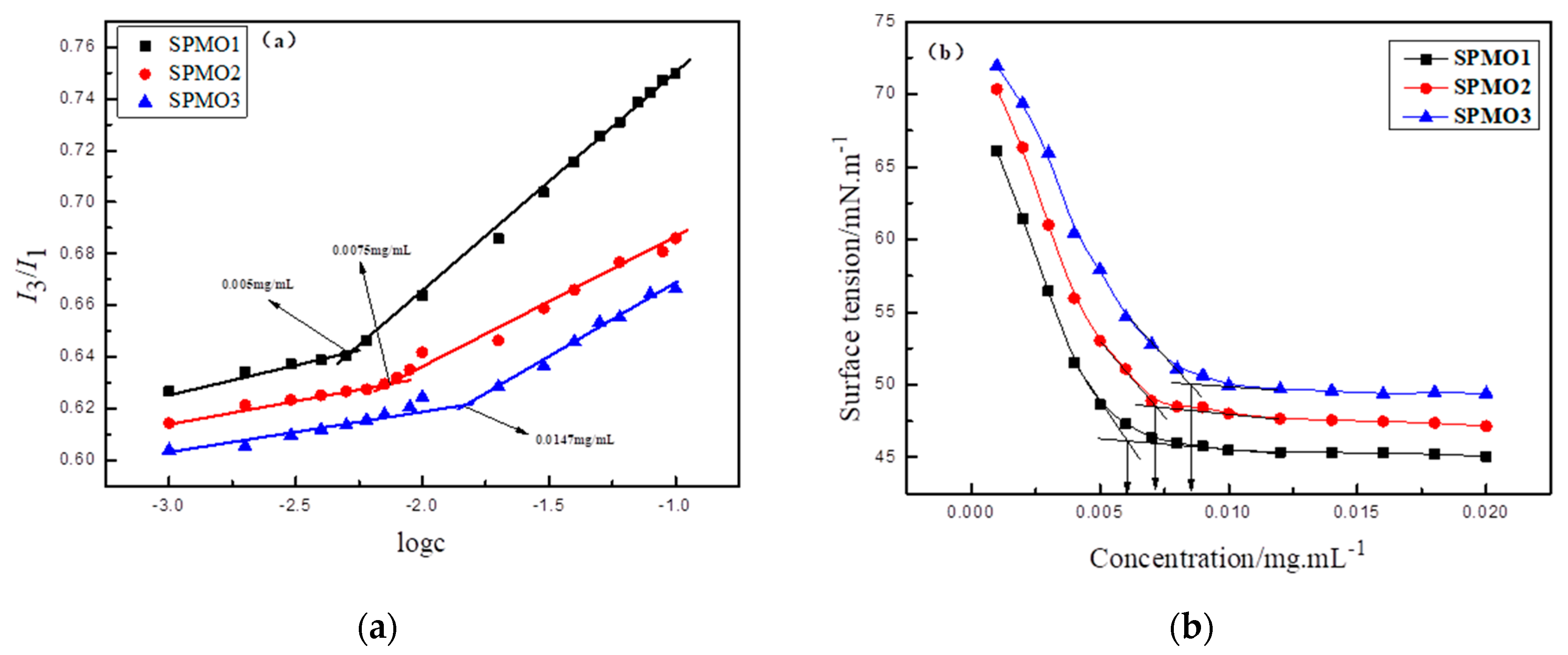
3.3.1. Critical Micelle Concentration (CMC) of the Polymeric Micelles
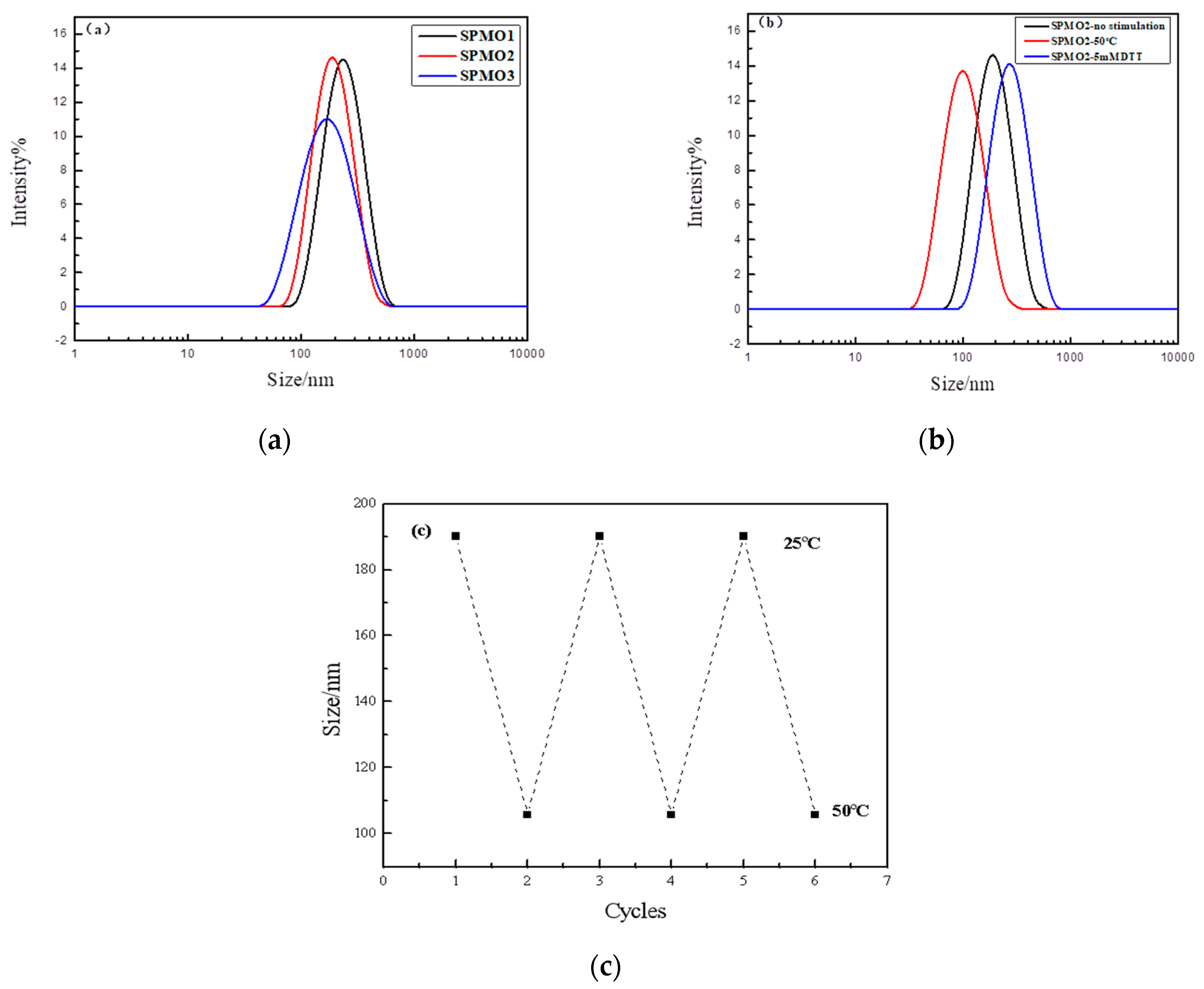
3.3.2. Dynamic Light Scattering (DLS) of the Polymeric Micelles
3.3.3. Morphology of the Polymeric Micelles
3.4. Sol–Gel Transition of the Temperature/Reduction Dual-Stimulus Responsive Triblock Copolymers
3.5. Drug Release Behavior of the Temperature/Reduction Dual-Stimulus Responsive Triblock Copolymers
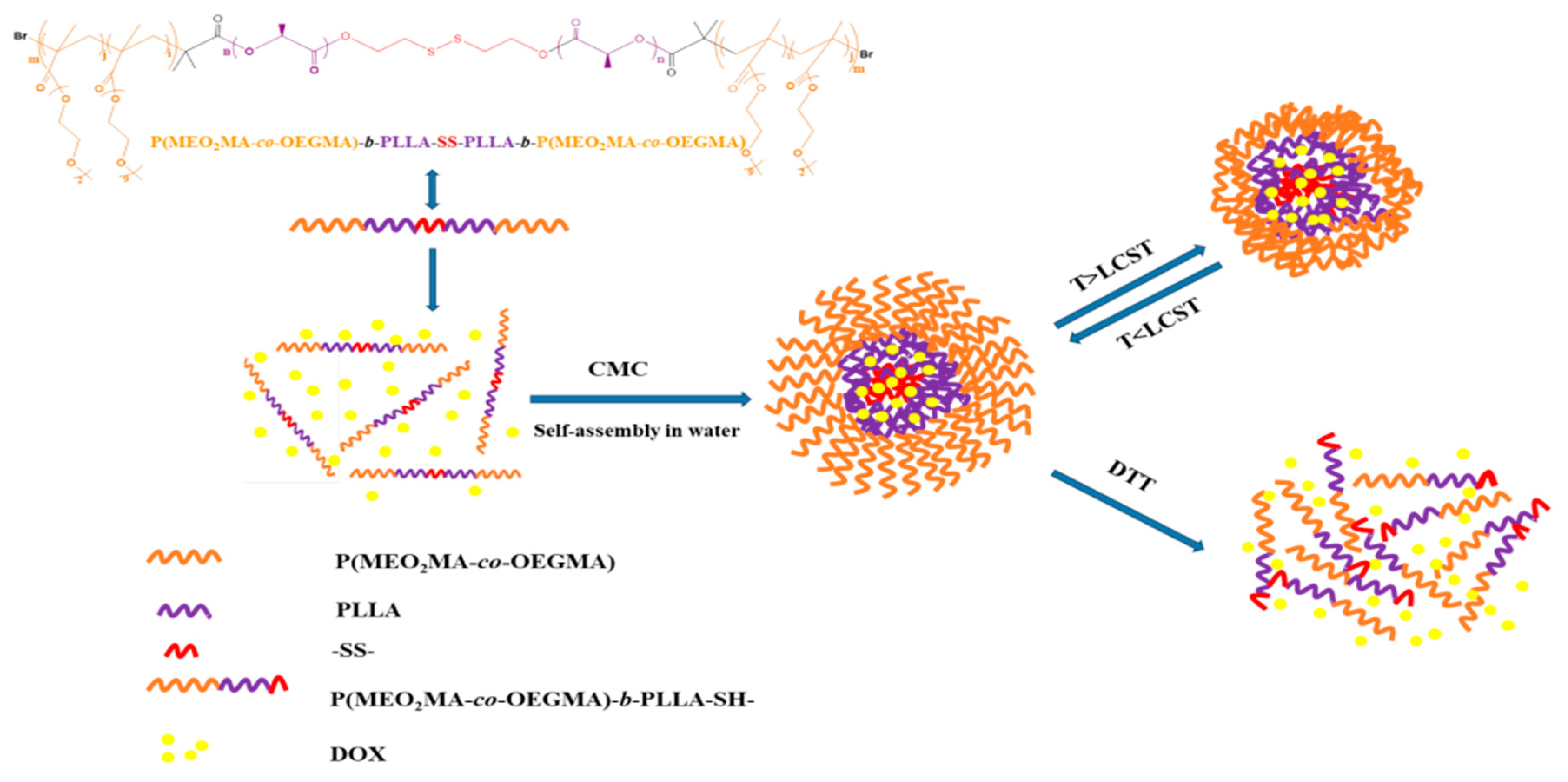
3.6. Self-Assembly Behavior of the Temperature/Reduction Dual-Stimulus Responsive Triblock Copolymers and the Drug Release Mechanism of Micelles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wan, L.Y.; Chu, L.Y.; Zhang, Y.Q.; Wu, J.F. ‘Smart’ nanoparticles as drug delivery systems for applications in tumor therapy. Expert Opin. Drug Deliv. 2015, 12, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ko, N.R.; Oh, J.K. Recent advances in stimuli-responsive degradable block copolymer micelles: Synthesis and controlled drug delivery applications. Chem. Commun. 2012, 48, 7542–7552. [Google Scholar] [CrossRef] [PubMed]
- Pawar, P.V.; Gohil, S.V.; Jain, J.P.; Kumar, N. Functionalized polymersomes for biomedical applications. Polym. Chem. 2013, 4, 3160–3176. [Google Scholar] [CrossRef]
- Li, L.Y.; Raghupathi, K.; Song, C.F.; Prasad, P.; Thayumanavan, S. Self-assembly of random copolymers. Chem. Commun. 2014, 50, 13417–13432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, F.; Yu, Y.Y.; Wang, C.C.; Yang, S. Consecutive morphological transitions in nanoaggregates assembled from amphiphilic random copolymer via water-driven micellization and light-triggered dissociation. Macromolecules 2008, 41, 3385–3388. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Zou, H.; Gou, W.; Shen, T.X.; Ren, J. Supramolecular micelles with dual temperature and redox responses for multi-controlled drug release. Polym. Chem. 2013, 4, 2658–2661. [Google Scholar]
- Camelia Elena, I.T.; Monica, S.C.; Violeta, P.; Marcel, P.; Oana, M.D.; Leonard, I.A.; Lacramioara, O. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450–1468. [Google Scholar]
- Chen, J.; Qiu, X.Z.; Ouyang, J.; Kong, J.M.; Xing, M.M.Q. pH and reduction dual-sensitive copolymeric micelles for intracellular doxorubicin delivery. Biomacromolecules 2011, 12, 3601–3611. [Google Scholar]
- Li, S.S.; Chen, G.X.; Zhou, Z.; Li, Q.F. Stimuli-induced multiple dissociation and micellization transitions of random copolymers. RSC Adv. 2015, 5, 65847–65855. [Google Scholar] [CrossRef]
- Wang, C.Y.; Qi, P.L.; Lu, Y.; Zhang, Y.N.; Sheng, Q.L.; Wang, T.S.; Zhang, M.Y.; Wang, R.; Song, S.Y. Bicomponent polymeric micelles for pH-controlled delivery of doxorubicin. Drug Deliv. 2020, 27, 344–357. [Google Scholar] [CrossRef]
- Liu, Z.H.; Jiao, Y.P.; Wang, Y.F.; Zhou, C.G.; Zhang, Z.Y. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Feng, K.; Xie, N.; Chen, B.; Zhang, L.P.; Tung, C.H.; Wu, L.Z. Reversible light-triggered transition of amphiphilic random copolymers. Macromolecules 2012, 45, 5596–5603. [Google Scholar] [CrossRef]
- Zhang, K.H.; Liu, J.T.; Ma, X.; Lei, L.; Li, Y.; Lei, Z.L. Temperature, pH, and reduction triple-stimuli-responsive inner-layer crosslinked micelles as nanocarriers for controlled release. J. Appl. Polym. Sci. 2008, 135, 46714–46723. [Google Scholar] [CrossRef]
- Falireas, P.G.; Vamvakaki, M. Triple-responsive block copolymer micelles with synergistic pH and temperature response. Macromolecules 2018, 51, 6848–6858. [Google Scholar] [CrossRef]
- Chang, X.H.; Wang, C.; Shan, G.R.; Bao, Y.Z.; Pan, P.J. Thermoresponsivity, micelle structure, and thermal-induced structural transition of an amphiphilic block copolymer tuned by terminal multiple H-bonding units. Langmuir 2020, 36, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, W.; Delia, M.I.; Leonard, I.A.; Slim, S.; Christelle, D.; Gerard, R. Micellization of novel biocompatible thermo-sensitive graft copolymers based on poly(ε-caprolactone), poly(N-vinylcaprolactam) and poly(N-vinylpyrrolidone). Eur. Polym. J. 2019, 119, 74–82. [Google Scholar]
- Marwah, N.M.; Kamal, B.Y.; Jun, H.B.H.S. Poly(N-vinyl caprolactam) thermoresponsive polymer in novel drug delivery systems: A review. Mater. Express 2018, 8, 21–34. [Google Scholar]
- Cao, Y.; Zhu, X.X.; Luo, J.T.; Liu, H.Y. Effects of substitution groups on the RAFT polymerization of N-alkylacrylamides in the preparation of thermosensitive block copolymers. Macromolecules 2007, 40, 6481–6488. [Google Scholar] [CrossRef]
- Li, Y.T.; Lokitz, B.S.; McCormick, C.L. Thermally responsive vesicles and their structural “locking” through polyelectrolyte complex formation. Angew. Chem. Int. Ed. 2006, 45, 5792–5795. [Google Scholar] [CrossRef]
- Park, C.; Heo, J.; Lee, J.; Kim, T.; Kim, S.Y. Well-defifined dual light- and thermo-responsive rod-coil block copolymers containing an azobenzene, MEO2MA and OEGMA. Polymers 2020, 12, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.F.; Darcos, V.; Monge, S.; Li, S.M. Thermo-responsive drug release from self-assembled micelles of brush-like PLA/PEG analogues block copolymers. Int. J. Pharm. 2015, 491, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.L.; Tam, K.C. Self-assembly of thermo-responsive poly(oligo(ethylene glycol) methyl ether methacrylate)-C60 in water-methanol mixtures. Polymer 2011, 52, 3769–3775. [Google Scholar] [CrossRef]
- Raita, G.; Sho, M.; Satoshi, U.; Hideaki, Y.; Kohzo, I.; Takashi, I. Surface characterization of amphiphilic block copolymers possessing polyisoprene and poly[tri(ethylene glycol) methacrylate] segments and the effect of side chain ω-function on surface energy. Polymer 2020, 190, 122257–122264. [Google Scholar]
- Goseki, R.; Hong, L.; Inutsuka, M.; Yokoyama, H.; Ito, K.; Ishizone, T. Synthesis and surface characterization of well-defifined amphiphilic block copolymers composed of polydimethylsiloxane and poly[oligo(ethylene glycol) methacrylate]. RSC Adv. 2017, 7, 25199–25207. [Google Scholar] [CrossRef] [Green Version]
- Lutz, J.F.; Weichenhan, K.; Akdemir, Ö.; Hoth, A. About the phase transitions in aqueous solutions of thermoresponsive copolymers and hydrogels based on 2-(2-methoxyethoxy) ethyl methacrylate and oligo(ethylene glycol) methacrylate. Macromolecules 2007, 40, 2503–2508. [Google Scholar] [CrossRef]
- Sun, S.T.; Wu, P.Y. On the thermally reversible dynamic hydration behavior of oligo(ethylene glycol) methacrylate-based polymers in water. Macromolecules 2013, 46, 236–246. [Google Scholar] [CrossRef]
- Lutz, J.F. Thermo-switchable materials prepared using the OEGMA-platform. Adv. Mater. 2011, 23, 2237–2243. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, Z.G.; Lim, C.W.; Lee, Y.H.; Dongbang, S.; Kang, C.; Kim, J.S. Disulfide-cleavage-triggered chemosensors and their biological applications. Chem. Rev. 2013, 113, 5071–5109. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Xu, Z.G.; Chen, Z.J.; Liu, X.Y.; Hou, C.L.; Zhang, X.Y.; Zhang, H.X. Fabrication of single-hole glutathione-responsive degradable hollow silica nanoparticles for drug delivery. Acs Appl. Mater. Interfaces 2014, 6, 12600–12608. [Google Scholar] [CrossRef]
- Liu, L.; Liu, P. Synthesis strategies for disulfide bond-containing polymer-based drug delivery system for reduction-responsive controlled release. Front. Mater. Sci. 2015, 9, 211–226. [Google Scholar] [CrossRef]
- Liu, C.Y.; Yuan, J.; Luo, X.M.; Chen, M.H.; Chen, Z.J.; Zhao, Y.C.; Li, X.H. Folate-decorated and reduction-sensitive micelles assembled from amphiphilic polymer-camptothecin conjugates for intracellulsar drug delivery. Mol. Pharm. 2014, 11, 4258–4269. [Google Scholar] [CrossRef]
- Onaca, O.; Enea, R.; Hughes, D.W.; Meier, W. Stimuli-responsive polymersomes as nanocarriers for drug and gene delivery. Macromol. Biosci. 2009, 9, 129–139. [Google Scholar] [CrossRef]
- Stewart, D.J. Mechanisms of resistance to cisplatin and carboplatin. Crit. Rev. Oncol. Hematol. 2007, 63, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Du, H.L.; Liu, J.Y.; Zhai, G.X. Advanced nanocarriers based on heparin and its derivatives for cancer management. Biomacromolecules 2015, 16, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.Q.; Zhang, Q.; Zhao, D.; Xu, J.Q.; Zhong, Z.L.; Zhuo, R.X.; Li, F. Preparation of pH and redox dual-sensitive core crosslinked micelles for overcoming drug resistance of DOX. Polym. Chem. 2016, 7, 1719–1729. [Google Scholar] [CrossRef]
- Garofalo, C.; Capuano, G.; Sottile, R.; Tallerico, R.; Adami, R.; Reverchon, E.; Carbone, E.; Izzo, L.; Pappalardo, D. Different insight into amphiphilic PEG-PLA copolymers: Influence of macromolecular architectureon the micelle formation and cellular uptake. Biomacromolecules 2014, 15, 403–415. [Google Scholar] [CrossRef]
- Wu, X.H.; Ghzaoui, E.A.; Li, S.M. Anisotropic self-assembling micelles prepared by the direct dissolution of PLA/PEG block copolymers with a high PEG fraction. Langmuir 2011, 27, 8000–8008. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, H.Q.; Dai, Y. Biodegradable amphiphilic graft polymer synthesized via the combination of ring-opening polymerization (ROP) and atom transfer radical polymerization (ATRP). Mater. Lett. 2017, 198, 144–147. [Google Scholar] [CrossRef]
- Yu, C.; Wang, L.; Xu, Z.Z.; Teng, W.Q.; Wu, Z.M.; Xiong, D. Smart micelles self-assembled from four-arm star polymers as potential drug carriers for pH-triggered DOX release. J. Polym. Res. 2020, 27, 111–120. [Google Scholar] [CrossRef]
- Tian, K.; Jia, X.; Zhao, X.B.; Liu, P. Biocompatible reduction and pH dual-responsive core cross-linked micelles based on multifunctional amphiphilic linear−hyperbranched copolymer for controlled anticancer drug delivery. Mol. Pharm. 2017, 14, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Cunningham, A.; Oh, J.K. New design of thiol-responsive degradable polylactide-based block copolymer micelles. Macromol. Rapid Commun. 2013, 34, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ko, N.R.; Yao, K.J.; Tang, C.B.; Oh, J.K. Synthesis and thiol-responsive degradation of polylactide-based block copolymers having disulfide junctions using ATRP and ROP. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3071–3080. [Google Scholar] [CrossRef]
- Lutz, J.F.; Hoth, A. Preparation of ideal PEG analogues with a tunable thermosensitivity by controlled radical copolymerization of 2-(2-Methoxyethoxy)ethyl methacrylate and oligo(ethylene glycol) methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]





| Samples | [LLA]/[SS-DOH] (n:n) | [M]/[O](n:n) | DP | Mn, Theory(g/mol) | Mn,SEC(g/mol) (g/mol) | ÐM(Mw/Mn) | |
|---|---|---|---|---|---|---|---|
| SP | 20 | - | - | 3034 | 3632 | 1.47 | |
| SPBr | 20 | - | - | 3332 | 3447 | 1.34 | |
| SPMO1 | 20 | 95:5 | 212 | 46,230 | 47,048 | 1.12 | |
| SPMO2 | 20 | 95:5 | 264 | 56,753 | 57,457 | 1.03 | |
| SPMO3 | 20 | 95:5 | 316 | 67,275 | 67,573 | 1.06 | |
| SPMO4 | 20 | 97:3 | 264 | 55,237 | 55,671 | 1.10 | |
| SPMO5 | 20 | 92:8 | 264 | 59,026 | 60,486 | 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, F.; Wang, Z.; Gao, W.; Fu, Y.; Wu, Q.; Liu, S. Novel Temperature/Reduction Dual-Stimulus Responsive Triblock Copolymer [P(MEO2MA-co- OEGMA)-b-PLLA-SS-PLLA-b-P(MEO2MA-co-OEGMA)] via a Combination of ROP and ATRP: Synthesis, Characterization and Application of Self-Assembled Micelles. Polymers 2020, 12, 2482. https://doi.org/10.3390/polym12112482
Song F, Wang Z, Gao W, Fu Y, Wu Q, Liu S. Novel Temperature/Reduction Dual-Stimulus Responsive Triblock Copolymer [P(MEO2MA-co- OEGMA)-b-PLLA-SS-PLLA-b-P(MEO2MA-co-OEGMA)] via a Combination of ROP and ATRP: Synthesis, Characterization and Application of Self-Assembled Micelles. Polymers. 2020; 12(11):2482. https://doi.org/10.3390/polym12112482
Chicago/Turabian StyleSong, Fei, Zhidan Wang, Wenli Gao, Yu Fu, Qingrong Wu, and Shouxin Liu. 2020. "Novel Temperature/Reduction Dual-Stimulus Responsive Triblock Copolymer [P(MEO2MA-co- OEGMA)-b-PLLA-SS-PLLA-b-P(MEO2MA-co-OEGMA)] via a Combination of ROP and ATRP: Synthesis, Characterization and Application of Self-Assembled Micelles" Polymers 12, no. 11: 2482. https://doi.org/10.3390/polym12112482





