A Facile Method for the Fabrication of Silver Nanoparticles Surface Decorated Polyvinyl Alcohol Electrospun Nanofibers and Controllable Antibacterial Activities
Abstract
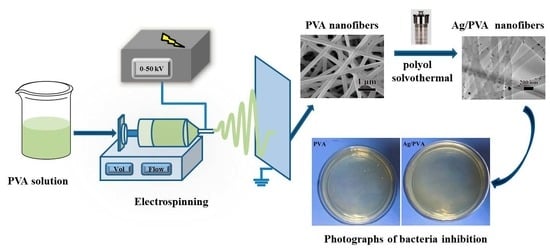
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments and Characterization
2.3. Preparation of PVA Spinning Solution
2.4. Electrospinning of PVA NFs
2.5. Growth of Ag NPs on the Surface of PVA NFs
2.6. Antimicrobial Activity Testing
3. Results and Discussion
3.1. Morphology and Structure of Ag/PVA CNFs
3.2. UV-vis and FTIR Spectra of Ag/PVA CNFs
3.3. Antibacterial Properties of Ag/PVA CNFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mei, S.; Pan, M.; Gao, S.; Song, S.; Wang, J.; Liu, G. Organic–inorganic bimetallic hybrid particles with controllable morphology for the catalytic degradation of organic dyes. New J. Chem. 2020, 44, 8366–8378. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, K.; Dicko, C.; Bülow, L.; Ye, L. Ag–Polymer Nanocomposites for Capture, Detection, and Destruction of Bacteria. ACS Appl. Nano Mater. 2019, 2, 1655–1663. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Z.; Jiang, B.; Bai, Y.; Wang, W.; Yin, Y. Reversible Assembly and Dynamic Plasmonic Tuning of Ag Nanoparticles Enabled by Limited Ligand Protection. Nano Lett. 2018, 18, 5312–5318. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, Y.; Zhao, M.; Wang, W.; Chu, Z.; Huo, R.; Hu, F.; Zhou, W.; Heb, T.; Qian, H. Ag nanoparticles decorated hybrid microspheres for superior antibacterial properties. Mater. Lett. 2020, 262, 127057. [Google Scholar] [CrossRef]
- Ni, Z.; Gu, X.; He, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Synthesis of silver nanoparticle-decorated hydroxyapatite (HA@Ag) poriferous nanocomposites and the study of their antibacterial activities. RSC Adv. 2018, 8, 41722–41730. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Münstedt, H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 2005, 26, 2081–2088. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Ouyang, Z.; Zhang, M.; Lin, Z.; Li, J.; Su, Z.; Wei, G. Nanoscale Graphene Doped with Highly Dispersed Silver Nanoparticles: Quick Synthesis, Facile Fabrication of 3D Membrane-Modified Electrode, and Super Performance for Electrochemical Sensing. Adv. Funct. Mater. 2016, 26, 2122–2134. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Lei, Y.; Yin, W.-J.; Chen, Y.-X.; Ke, Q.-F.; Guo, Y.-P.; Zhang, C.-Q. Enhanced antibacterial activity and osteoinductivity of Ag-loaded strontium hydroxyapatite/chitosan porous scaffolds for bone tissue engineering. J. Mater. Chem. B 2016, 4, 7919–7928. [Google Scholar] [CrossRef]
- Hahm, E.; Kim, Y.-H.; Phama, X.-H.; Jun, B.-H. Highly Reproducible Surface-Enhanced Raman Scattering Detection of Alternariol Using Silver-Embedded Silica Nanoparticles. Sensors 2020, 20, 3523. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Hamid, S.H.; Vakalopoulou, E.; Tsagkalias, I.; Ioannidou, M.D.; Achilias, D. Synthesis, characterization and reaction kinetics of PMMA/silver nanocomposites prepared via in situ radical polymerization. Eur. Polym. J. 2015, 72, 256–269. [Google Scholar] [CrossRef]
- Gaballah, S.T.; El-Nazer, H.A.; Abdel-Monem, R.A.; El-Liethy, M.A.; Hemdan, B.A.; Rabie, S.T. Synthesis of novel chitosan-PVC conjugates encompassing Ag nanoparticles as antibacterial polymers for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 707–717. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Z.; Liu, K.; Dong, B. Direct evidence of plasmonic enhancement on catalytic reduction of 4-nitrophenol over silver nanoparticles supported on flexible fibrous networks. Appl. Catal. B. Environ. 2016, 188, 245–252. [Google Scholar] [CrossRef]
- Zhang, R.; Hong, Y.; Reinhard, B.M.; Liu, P.; Wang, R.; Negro, L.D. Plasmonic Nanotrough Networks for Scalable Bacterial Raman Biosensing. ACS Appl. Mater. Interfaces 2018, 10, 27928–27935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gong, X.; Bao, Y.; Zhao, Y.; Xi, M.; Jiang, C.; Fong, H. Electrospun Nanofibrous Membranes Surface-Decorated with Silver Nanoparticles as Flexible and Active/Sensitive Substrates for Surface-Enhanced Raman Scattering. Langmuir 2012, 28, 14433–14440. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.; Sheu, C.; Chen, C.-H.; Chen, S.-H.; Jose, G.; Kuo, C.-Y.; Chen, J.-P. Multi-functional electrospun antibacterial core-shell nanofibrous membranes for prolonged prevention of post-surgical tendon adhesion and inflammation. Acta Biomater. 2018, 72, 121–136. [Google Scholar] [CrossRef]
- Mondal, K.; Ali, A.; Singh, C.; Sumana, G.; Malhotra, B.D.; Sharma, A. Highly sensitive porous carbon and metal/carbon conducting nanofiber based enzymatic biosensors for triglyceride detection. Sens. Actuators B Chem. 2017, 246, 202–214. [Google Scholar] [CrossRef]
- Wei, X.; Cai, J.; Lin, S.; Li, F.; Tian, F. Controlled release of monodisperse silver nanoparticles via in situ cross-linked polyvinyl alcohol as benign and antibacterial electrospun nanofibers. Colloids Surf. B Biointerfaces 2020, 197, 111370. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, R.; Xie, J.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Electrospun antibacterial poly(vinyl alcohol)/Ag nanoparticles membrane grafted with 3,3′,4,4′-benzophenone tetracarboxylic acid for efficient air filtration. Appl. Surf. Sci. 2020, 533, 147516. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Fabrication of silver nanoparticles embedded into polyvinyl alcohol (Ag/PVA) composite nanofibrous films through electrospinning for antibacterial and surface-enhanced Raman scattering (SERS) activities. Mater. Sci. Eng. C 2016, 69, 462–469. [Google Scholar] [CrossRef]
- Yang, T.; Yang, H.; Zhen, S.J.; Huang, C.Z. Hydrogen-Bond-Mediated in Situ Fabrication of AgNPs/Agar/PAN Electrospun Nanofibers as Reproducible SERS Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1586–1594. [Google Scholar] [CrossRef]
- Sekar, A.D.; Muthukumar, H.; Chandrasekaran, N.I.; Matheswaran, M. Photocatalytic degradation of naphthalene using calcined Fe ZnO/ PVA nanofibers. Chemosphere 2018, 205, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huang, Y.; Miao, Y.E.; Tjiu, W.W.; Liu, T. Polydopamine-coated electrospun poly(vinyl alcohol)/poly(acrylic acid) membranes as efficient dye adsorbent with good recyclability. J. Hazard. Mater. 2015, 283, 730–739. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, P.; Liu, G.; He, X.; Qi, B.; Zeng, G.; Wang, W.; Sun, Y.; Cui, F. Ultrahigh adsorption capacity of anionic dyes with sharp selectivity through the cationic charged hybrid nanofibrous membranes. Chem. Eng. J. 2017, 313, 957–966. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Wu, L.; Yuan, X.; Sheng, J. Immobilization of cellulase in nanofibrous PVA membranes by electrospinning. J. Membr. Sci. 2005, 250, 167–173. [Google Scholar] [CrossRef]
- Mansu, H.S.; Orefice, R.L.; Mansur, A.A.P. Characterization of poly (vinyl alcohol)/poly (ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Chen, S.Y.; Drehmel, J.R.; Penn, R.L. Facile synthesis of monodispersed Ag NPs in ethylene glycol using mixed capping gents. ACS Omega 2020, 5, 6069–6073. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Balankura, T.; Fichthorn, K.A.; Rioux, R.M. Revisiting the Polyol Synthesis of Silver Nanostructures: Role of Chloride in Nanocube Formation. ACS Nano 2019, 13, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, Y.; Wei, F.; Luo, G.; Qian, W. Elastic deformation of multiwalled carbon nanotubes in electrospun MWCNTs–PEO and MWCNTs–PVA nanofibers. Polymer 2005, 46, 12689–12695. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Wang, Z.; Zhang, X.; Zhao, Y.; Sun, L. Electrospinning of Ag Nanowires/polyvinyl alcohol hybrid nanofibers for their antibacterial properties. Mater. Sci. Eng. C 2017, 78, 706–714. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. A facile method to prepare size-tunable silver nanoparticles and its antibacterial mechanism. Adv. Powder Technol. 2018, 29, 407–415. [Google Scholar] [CrossRef]
- Bhat, N.; Nate, M.; Kurup, M.; Bambole, V.; Sabharwal, S. Effect of γ-radiation on the structure and morphology of polyvinyl alcohol films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2005, 237, 585–592. [Google Scholar] [CrossRef]
- Salari, M.; Khiabani, M.S.; Mokarram, R.R.; Ghanbarzadeh, B.; Kafil, H.S. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient catalytic degradation of dyes using kappa-carrageenan stabilized silver nanoparticles nanocatalyst. Carbohydr. Polym. 2020, 230, 115597. [Google Scholar] [CrossRef]
- Hemmati, S.; Rashtiani, A.; Zangeneh, M.M.; Mohammadi, P.; Zangeneh, A.; Veisi, H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron 2019, 158, 8–14. [Google Scholar] [CrossRef]
- Gao, M.; Sun, L.; Wang, Z.; Zhao, Y. Controlled synthesis of Ag nanoparticles with different morphologies and their antibacterial properties. Mater. Sci. Eng. C 2013, 33, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Bahrami, S.H.; Joghataei, M. Fabrication of novel nanofiber scaffolds from gum tragacanth/poly(vinyl alcohol) for wound dressing application: In vitro evaluation and antibacterial properties. Mater. Sci. Eng. C 2013, 33, 4935–4943. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, E.; Huang, Z.; Ning, D.; Wang, J. Combustion and emission characteristics of a spray guided direct-injection spark-ignition engine fueled with natural gas-hydrogen blends. Int. J. Hydrog. Energy 2011, 36, 11155–11163. [Google Scholar] [CrossRef]
- Mbhele, Z.H.; Salemane, M.G.; Van Sittert, C.G.C.E.; Nedeljković, J.M.; Djoković, V.; Luyt, A.S. Fabrication and Characterization of Silver−Polyvinyl Alcohol Nanocomposites. Chem. Mater. 2003, 15, 5019–5024. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, X.; Song, C. Single-molecule technique: A revolutionary approach to exploring fundamental questions in plant science. New Phytol. 2019, 223, 508–510. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhao, J.; Tang, N.; Sun, H.; Huang, J. Horizontal Gene Transfer from Bacteria and Plants to the Arbuscular Mycorrhizal Fungus Rhizophagus irregularis. Front. Plant Sci. 2018, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Zhao, H.; Wang, Z.; Zhao, Y.; Sun, L. Preparation of Ag@PDA@SiO2 electrospinning nanofibrous membranes for direct bacteria SERS detection and antimicrobial activities. Mater. Res. Express 2020, 7, 095012. [Google Scholar] [CrossRef]
- Song, Y.; Miao, Y.; Song, C.-P. Behind the scenes: The roles of reactive oxygen species in guard cells. New Phytol. 2013, 201, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Chan, Z.; Yokawa, K.; Kim, W.-Y.; Song, C.-P. Editorial: ROS Regulation during Plant Abiotic Stress Responses. Front. Plant Sci. 2016, 7, 1536. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.-P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.-K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [Green Version]






| AgNO3 Concentration (mol/L) | Diluent Ratio and Colony Count | Total Numbers of the Bacteria (CFU/mL) | Antibacterial Rate (%) | ||
|---|---|---|---|---|---|
| 10 | 102 | 103 | |||
| Neat PVA | Innumerable | 59 | 7 | 1,180,000 | —— |
| 0.016 | Innumerable | 32 | 3 | 640,000 | 46 |
| 0.033 | Innumerable | 18 | 1 | 360,000 | 70 |
| 0.049 | 32 | 4 | 0 | 64,000 | 95 |
| 0.066 | 9 | 2 | 0 | 20,000 | 98 |
| 0.098 | Innumerable | 12 | 0 | 24,000 | 98 |
| 0.132 | Innumerable | 15 | 0 | 30,000 | 97 |
| AgNO3 Concentration (mol/L) | Diluent Ratio and Colony Count | Total Numbers of the Bacteria (CFU/mL) | Antibacterial Rate (%) | ||
|---|---|---|---|---|---|
| 10 | 102 | 103 | |||
| Neat PVA | Innumerable | 14 | 2 | 280,000 | —— |
| 0.016 | Innumerable | 8 | 0 | 160,000 | 43 |
| 0.033 | 21 | 1 | 0 | 42,000 | 85 |
| 0.049 | 8 | 0 | 0 | 16,000 | 94 |
| 0.066 | 2 | 0 | 0 | 0 | >99 |
| 0.098 | 15 | 1 | 0 | 20,000 | 93 |
| 0.132 | 18 | 2 | 0 | 40,000 | 85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, Z.; Wan, M.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. A Facile Method for the Fabrication of Silver Nanoparticles Surface Decorated Polyvinyl Alcohol Electrospun Nanofibers and Controllable Antibacterial Activities. Polymers 2020, 12, 2486. https://doi.org/10.3390/polym12112486
Yang Y, Zhang Z, Wan M, Wang Z, Zou X, Zhao Y, Sun L. A Facile Method for the Fabrication of Silver Nanoparticles Surface Decorated Polyvinyl Alcohol Electrospun Nanofibers and Controllable Antibacterial Activities. Polymers. 2020; 12(11):2486. https://doi.org/10.3390/polym12112486
Chicago/Turabian StyleYang, Yan, Zhijie Zhang, Menghui Wan, Zhihua Wang, Xueyan Zou, Yanbao Zhao, and Lei Sun. 2020. "A Facile Method for the Fabrication of Silver Nanoparticles Surface Decorated Polyvinyl Alcohol Electrospun Nanofibers and Controllable Antibacterial Activities" Polymers 12, no. 11: 2486. https://doi.org/10.3390/polym12112486
APA StyleYang, Y., Zhang, Z., Wan, M., Wang, Z., Zou, X., Zhao, Y., & Sun, L. (2020). A Facile Method for the Fabrication of Silver Nanoparticles Surface Decorated Polyvinyl Alcohol Electrospun Nanofibers and Controllable Antibacterial Activities. Polymers, 12(11), 2486. https://doi.org/10.3390/polym12112486