Development of Green and Sustainable Cellulose Acetate/Graphene Oxide Nanocomposite Films as Efficient Adsorbents for Wastewater Treatment
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Chemicals
2.2. Synthetic Approaches
2.2.1. Preparation of Graphene Oxide (GO)
2.2.2. Preparation of Cellulose Acetate (CA)
2.2.3. Determining the Degree of Substitution (DS)
2.2.4. Preparation of Cellulose Acetate/Graphene Oxide (CA-GO) Nanocomposite Adsorbents
2.3. Methods and Apparatus
2.4. Adsorption Evaluation of Ni2+
2.5. Kinetics Measurements
2.6. Adsorption Isotherms
2.7. Thermodynamics Measurements
3. Results and Discussion
3.1. Preparation of CA-GO Nanocomposite Adsorbents
3.2. Characterization of CA-GO
3.3. Adsorption Study
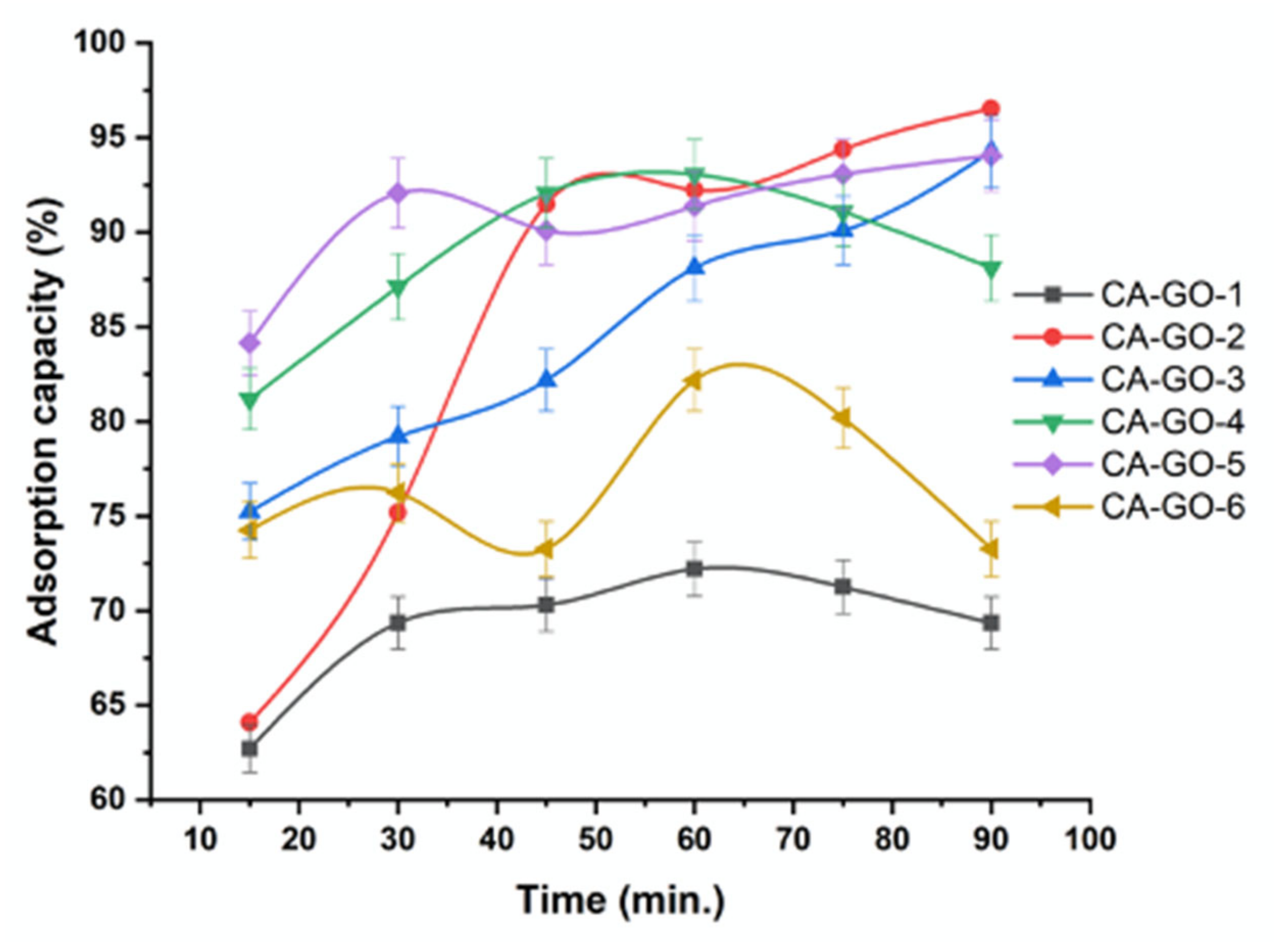
3.3.1. Effect of Time
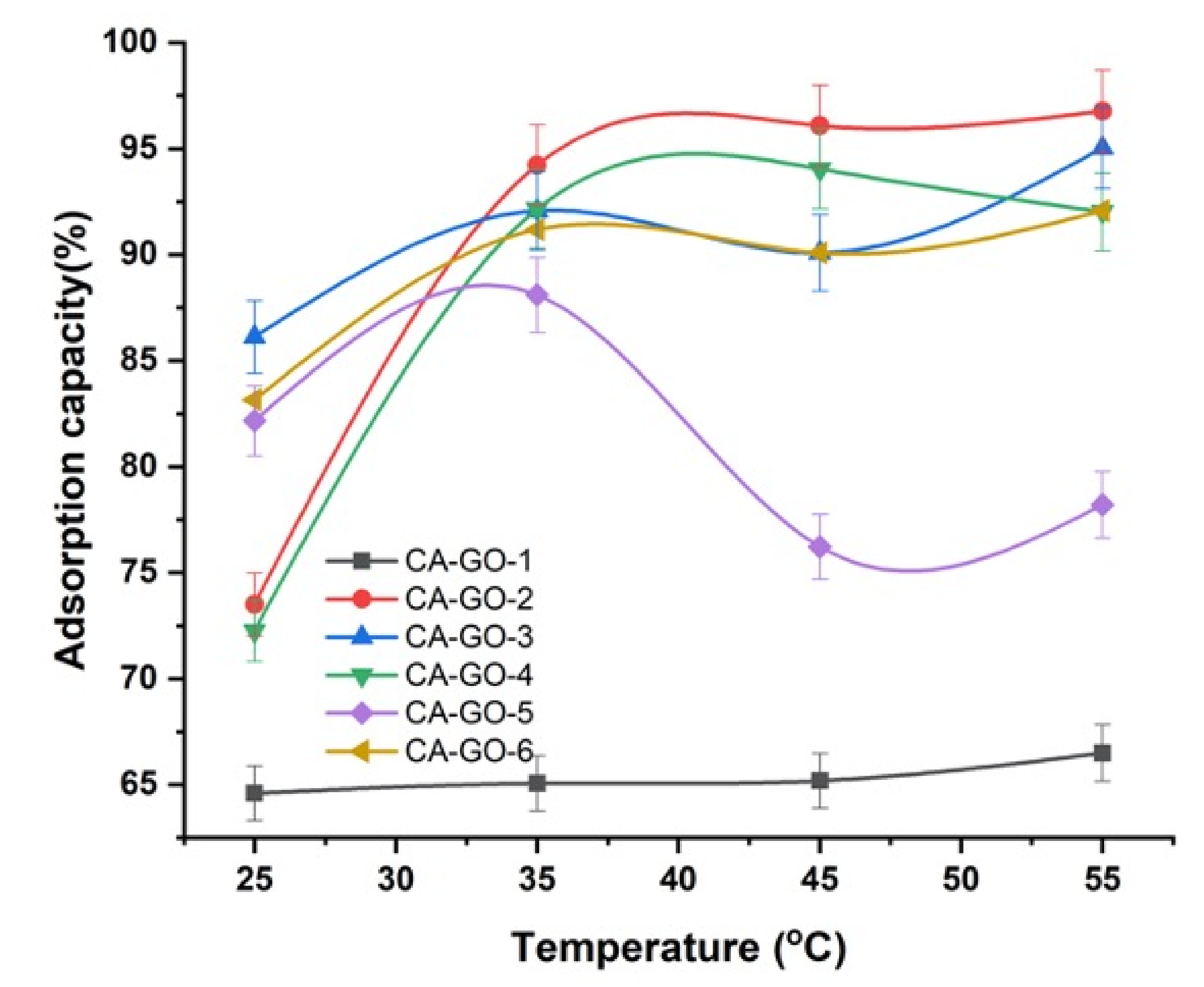
3.3.2. Effect of Temperature
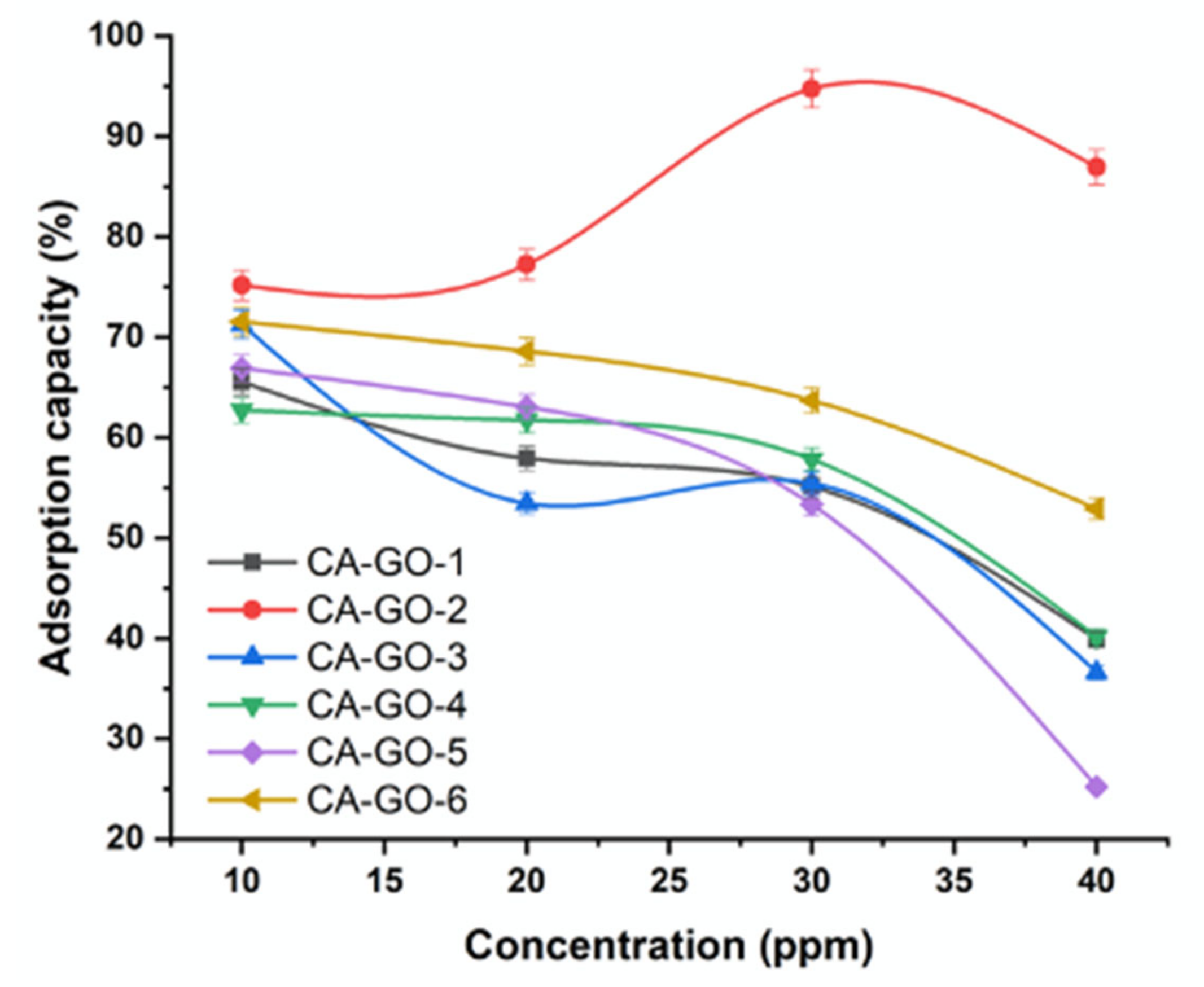
3.3.3. Effect of Ni2+ Concentration
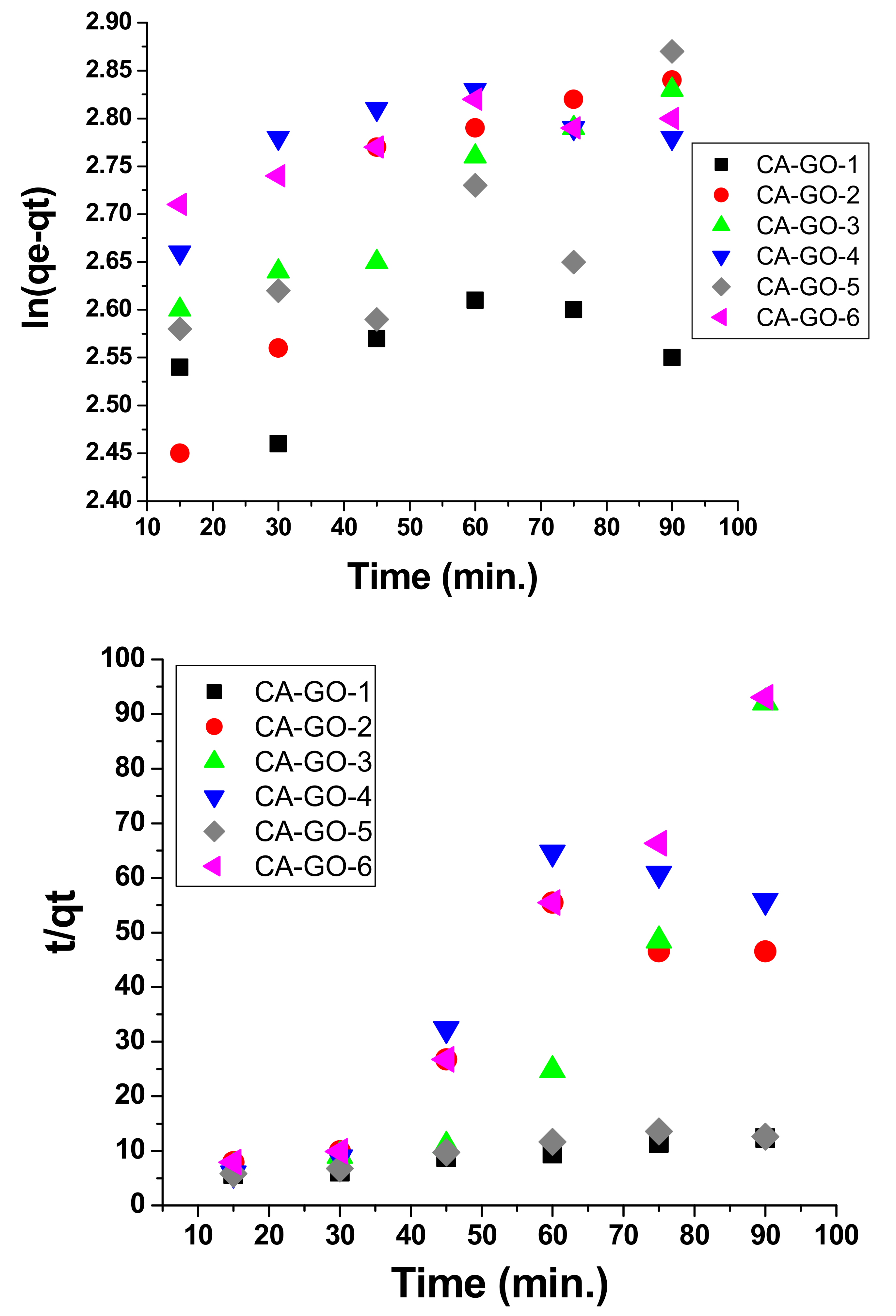
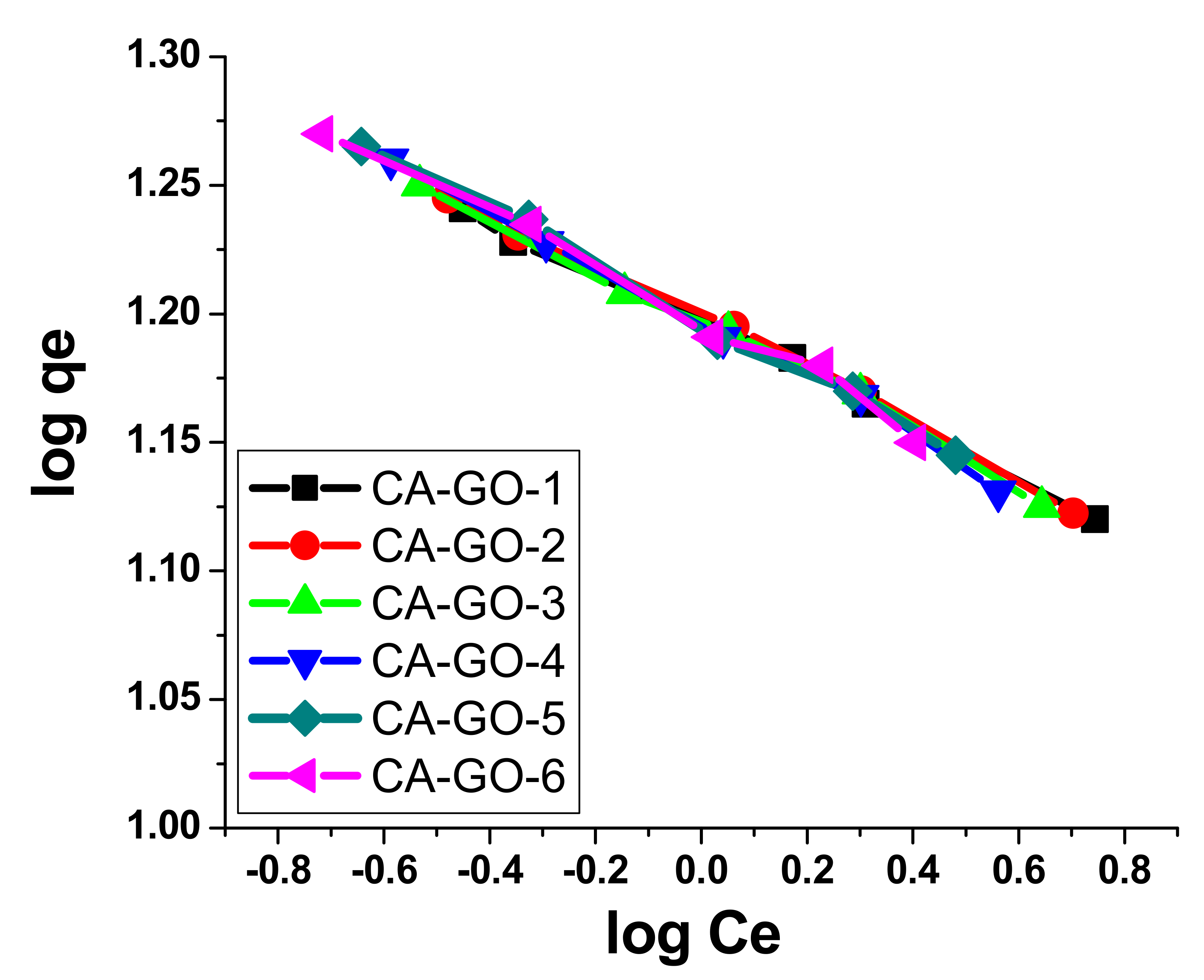
3.4. Kinetic Models and Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hebeish, A.; El-Naggar, M.E.; Tawfik, S.; Zaghloul, S.; Sharaf, S. Hyperbranched polymer–silver nanohybrid induce super antibacterial activity and high performance to cotton fabric. Cellulose 2019, 26, 3543–3555. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Shaarawy, S.; Shafie, A.E.; Hebeish, A. Development of antimicrobial medical cotton fabrics using synthesized nanoemulsion of reactive cyclodextrin hosted coconut oil inclusion complex. Fibers Polym. 2017, 18, 1486–1495. [Google Scholar] [CrossRef]
- Khattab, T.A.; Dacrory, S.; Abou-Yousef, H.; Kamel, S. Smart microfibrillated cellulose as swab sponge-like aerogel for real-time colorimetric naked-eye sweat monitoring. Talanta 2019, 205, 120166. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.M.; Lotfy, V.F.; Mwafy, E.A.; Basta, A.H. Influence of coating by Cu and Ag nanoparticles via pulsed laser deposition technique on optical, electrical and mechanical properties of cellulose paper. J. Mol. Struct. 2020, 1203, 127472. [Google Scholar] [CrossRef]
- Mwafy, E.A.; Hasanin, M.S.; Mostafa, A.M. Cadmium oxide/TEMPO-oxidized cellulose nanocomposites produced by pulsed laser ablation in liquid environment: Synthesis, characterization, and antimicrobial activity. Opt. Laser Technol. 2019, 120, 105744. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; El-Naggar, M.E.; Alotaiby, S.; El-Hamshary, H.; Moydeen, M.; Al-Deyab, S. Green Electrospining of Hydroxypropyl Cellulose Nanofibres for Drug Delivery Applications. J. Nanosci. Nanotechnol. 2018, 18, 805–814. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Y. Integrative process for sugarcane bagasse biorefinery to co-produce xylooligosaccharides and gluconic acid. Bioresour. Technol. 2019, 282, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, L.; Zhao, N.; Qin, L.; Wang, Y.; Xing, F. The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption. Ind. Crops Prod. 2019, 128, 290–297. [Google Scholar] [CrossRef]
- Khattab, T.A.; Mowafi, S.; El-Sayed, H. Development of mechanically durable hydrophobic lanolin/silicone rubber coating on viscose fibers. Cellulose 2019, 26, 9361–9371. [Google Scholar] [CrossRef]
- El-Nahrawy, A.M.; Abou Hammad, A.B.; Khattab, T.A.; Haroun, A.; Kamel, S. Development of electrically conductive nanocomposites from cellulose nanowhiskers, polypyrrole and silver nanoparticles assisted with Nickel (III) oxide nanoparticles. React. Funct. Polym. 2020, 149, 104533. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Candido, R.G.; Gonçalves, A.R. Synthesis of cellulose acetate and carboxymethylcellulose from sugarcane straw. Carbohydr. Polym. 2016, 152, 679–686. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Khattab, T.A.; Aldalbahi, A.; Hatshan, M.R.; El-Naggar, M.E. Facile development of microporous cellulose acetate xerogel immobilized with hydrazone probe for real time vapochromic detection of toxic ammonia. J. Environ. Chem. Eng. 2020, 8, 104573. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Jiao, Y.; Yang, H.; Li, Y.; Zhang, J.; Gao, P. The graphene/lanthanum oxide nanocomposites as electrode materials of supercapacitors. J. Power Sources. 2019, 419, 99–105. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, K.; Ji, Y.; Liu, G.; Jin, W.; Xu, N. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, Q.; Huang, G.; Fan, Q. Removal of heavy metals in aquatic environment by graphene oxide composites: A review. Environ. Sci. Pollut. Res. 2020, 27, 190–209. [Google Scholar] [CrossRef]
- Ahmad, H.; Fan, M.; Hui, D. Graphene oxide incorporated functional materials: A review. Compos. Part B Eng. 2018, 145, 270–280. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L. 2D materials for gas sensing applications: A review on graphene oxide, MoS2, WS2 and phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. Rsc Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Agarwal, S.; Zaman, T.; Murat Tuzcu, E.; Kapadia, S.R. Heavy metals and cardiovascular disease: Results from the National Health and Nutrition Examination Survey (NHANES) 1999-2006. Angiology 2011, 62, 422–429. [Google Scholar] [CrossRef]
- Tsiridis, V.; Petala, M.; Samaras, P.; Hadjispyrou, S.; Sakellaropoulos, G.; Kungolos, A. Interactive toxic effects of heavy metals and humic acids on Vibrio fischeri. Ecotoxicol. Environ. Saf. 2006, 63, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic. Biol. Med. 2010, 49, 1328–1341. [Google Scholar] [CrossRef] [PubMed]
- Schaumlöffel, D. Nickel species: Analysis and toxic effects. J. Trace Elem. Med. Biol. 2012, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cai, S.; Mo, C.; Chu, B.; Peng, L.; Yang, F. Toxic effects of heavy metals and their accumulation in vegetables grown in a saline soil. Ecotoxicol. Environ. Saf. 2010, 73, 84–88. [Google Scholar] [CrossRef]
- Trombetta, D.; Mondello, M.R.; Cimino, F.; Cristani, M.; Pergolizzi, S.; Saija, A. Toxic effect of nickel in an in vitro model of human oral epithelium. Toxicol. Lett. 2005, 159, 219–225. [Google Scholar] [CrossRef]
- Seregin, I.; Kozhevnikova, A.D. Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- Yuliyati, Y.B.; Solihudin, S.; Noviyanti, A.R. Effect of Sodium Periodate on the Adsorption Capacity of Silica-Lignin from Rice Husk on Chromium (VI). J. Kim. Sains Dan Apl. 2019, 22, 242–249. [Google Scholar] [CrossRef]
- Chhatre, A.J.; Marathe, K.V. Dynamic analysis and optimization of surfactant dosage in micellar enhanced ultrafiltration of nickel from aqueous streams. Sep. Sci. Technol. 2006, 41, 2755–2770. [Google Scholar] [CrossRef]
- Ansari, M.I.; Malik, A. Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with industrial wastewater. Bioresour. Technol. 2007, 98, 3149–3153. [Google Scholar] [CrossRef]
- Ren, Y.; Yan, N.; Wen, Q.; Fan, Z.; Wei, T.; Zhang, M.; Ma, J. Graphene/δ-MnO2 composite as adsorbent for the removal of nickel ions from wastewater. Chem. Eng. J. 2011, 175, 1–7. [Google Scholar] [CrossRef]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Onundi, Y.B.; Mamun, A.A.; Al Khatib, M.F.; Ahmed, Y.M. Adsorption of copper, nickel and lead ions from synthetic semiconductor industrial wastewater by palm shell activated carbon. Int. J. Environ. Sci. Technol. 2010, 7, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, A.; Minocha, A.K. Biosorption optimization of nickel removal from water using Punica granatum peel waste. Colloid. Surface. B 2010, 76, 544–548. [Google Scholar] [CrossRef]
- Mousavi, H.Z.; Seyedi, S.R. Nettle ash as a low cost adsorbent for the removal of nickel and cadmium from wastewater. Int. J. Environ. Sci. Technol. 2011, 8, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Kadirvelu, K.; Senthilkumar, P.; Thamaraiselvi, K.; Subburam, V. Activated carbon prepared from biomass as adsorbent: Elimination of Ni (II) from aqueous solution. Bioresour. Technol. 2002, 81, 87–90. [Google Scholar] [CrossRef]
- Katal, R.; Hasani, E.; Farnam, M.; Baei, M.S.; Ghayyem, M.A. Charcoal ash as an adsorbent for Ni (II) adsorption and its application for wastewater treatment. J. Chem. Eng. Data 2012, 57, 374–383. [Google Scholar] [CrossRef]
- De Angelis, G.; Medeghini, L.; Conte, A.M.; Mignardi, S. Recycling of eggshell waste into low-cost adsorbent for Ni removal from wastewater. J. Clean. Prod. 2017, 164, 1497–1506. [Google Scholar] [CrossRef]
- Somanathan, T.; Prasad, K.; Ostrikov, K.K.; Saravanan, A.; Krishna, V.M. Graphene oxide synthesis from agro waste. Nanomaterials 2015, 5, 826–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candido, R.G.; Godoy, G.G.; Goncalves, A.R. Characterization and application of cellulose acetate synthesized from sugarcane bagasse. Carbohydr. Polym. 2017, 167, 280–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alyüz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.C.; Kan, C.-C.; Pingul-Ong, S.M.B.; de Luna, M.D.G. Utilization of groundwater treatment plant (GWTP) sludge for nickel removal from aqueous solutions: Isotherm and kinetic studies. J. Environ. Chem. Eng. 2017, 5, 5746–5753. [Google Scholar] [CrossRef]
- Argun, M.E. Use of clinoptilolite for the removal of nickel ions from water: Kinetics and thermodynamics. J. Hazard. Mater. 2008, 150, 587–595. [Google Scholar] [CrossRef]
- Puleo, A.C.; Paul, D.; Kelley, S.S. The effect of degree of acetylation on gas sorption and transport behavior in cellulose acetate. J. Memb. Sci. 1989, 47, 301–332. [Google Scholar] [CrossRef]



| Sample | Yield (%) |
|---|---|
| CA-GO-0 | 46 |
| CA-GO-1 | 42 |
| CA-GO-2 | 48 |
| CA-GO-3 | 53 |
| CA-GO-4 | 57 |
| CA-GO-5 | 62 |
| CA-GO-6 | 34 |
| Model | Parameter | CA-GO Adsorbent | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Pseudo first order | qexp. | 13.453 | 17.155 | 17.402 | 17.544 | 15.008 | 17.327 |
| qcalc. | 12.094 | 15.034 | 15.124 | 16.263 | 13.454 | 14.826 | |
| K1 | 0.363 | 47 × 10−4 | 32 × 10−3 | 17 × 10−3 | 57 × 10−4 | 18 × 10−3 | |
| R2 | 0.635 | 0.64 | 0.996 | 0.774 | 0.736 | 0.816 | |
| Pseudo second order | qcalc. | 4.092 | 0.63 | 0.883 | 0.808 | 3.495 | 0.727 |
| K2 | 0.759 | 44 × 10−2 | 43 × 10−2 | 13 × 10−2 | 3.248 | 62 × 10−2 | |
| R2 | 0.902 | 0.943 | 0.889 | 0.795 | 0.906 | 0.982 | |
| Model | Parameter | CA-GO Adsorbent | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Langmuir | qm (mg g−1) | 3.278 | 12.725 | 13.986 | 15.452 | 12.718 | 14.541 |
| R2 | 0.902 | 0.998 | 0.998 | 0.998 | 0.999 | 0.997 | |
| Freundlich | Kf (mg(1-1/n) g-1 L1/n) | 6.813 | 3.33 | 3.335 | 3.336 | 3.667 | 3.665 |
| R2 | 0.903 | 0.994 | 0.995 | 0.793 | 0.997 | 0.817 | |
| Parameter | CA-GO Adsorbent | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| ΔS (kJ mol−1) | 0.05 | 0.07 | 0.25 | 0.22 | 0.22 | 0.28 | |
| ΔH (kJ mol−1) | −11 × 103 | −13 × 103 | −62 × 103 | −55 × 103 | −55 × 103 | −65 × 103 | |
| ΔG (kJ mol−1) | 25 °C | −1.781 | −2.42 | −2.977 | −3.822 | −2.763 | −4.236 |
| 35 °C | −1.78 | −4.45 | −6.811 | −6.833 | −8.254 | −7.618 | |
| 45 °C | −0.972 | −2.611 | −8.66 | −8.222 | −8.581 | −8.094 | |
| 55 °C | −2.209 | −4.66 | −9.444 | −10.076 | −9.209 | −12.271 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldalbahi, A.; El-Naggar, M.; Khattab, T.; Abdelrahman, M.; Rahaman, M.; Alrehaili, A.; El-Newehy, M. Development of Green and Sustainable Cellulose Acetate/Graphene Oxide Nanocomposite Films as Efficient Adsorbents for Wastewater Treatment. Polymers 2020, 12, 2501. https://doi.org/10.3390/polym12112501
Aldalbahi A, El-Naggar M, Khattab T, Abdelrahman M, Rahaman M, Alrehaili A, El-Newehy M. Development of Green and Sustainable Cellulose Acetate/Graphene Oxide Nanocomposite Films as Efficient Adsorbents for Wastewater Treatment. Polymers. 2020; 12(11):2501. https://doi.org/10.3390/polym12112501
Chicago/Turabian StyleAldalbahi, Ali, Mehrez El-Naggar, Tawfik Khattab, Meram Abdelrahman, Mostafizur Rahaman, Abdulaziz Alrehaili, and Mohamed El-Newehy. 2020. "Development of Green and Sustainable Cellulose Acetate/Graphene Oxide Nanocomposite Films as Efficient Adsorbents for Wastewater Treatment" Polymers 12, no. 11: 2501. https://doi.org/10.3390/polym12112501






