Effect of Chlorophyll Hybrid Nanopigments from Broccoli Waste on Thermomechanical and Colour Behaviour of Polyester-Based Bionanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Broccoli Dye and Pomegranate Peels Biomordant Extraction
2.3. Synthesis of Chlorophyll Hybrid Nanopigments (CDNPs)
2.4. Bionanocomposites Preparation
2.5. Bionanocomposite Characterization
2.6. Statistical Analysis
3. Results
3.1. Chlorophyll Hybrid Nanopigments (CDNPs)
Thermal Stability of CDNPs
3.2. Bionanocomposites Characterization
3.2.1. Evaluation of Colour Parameters
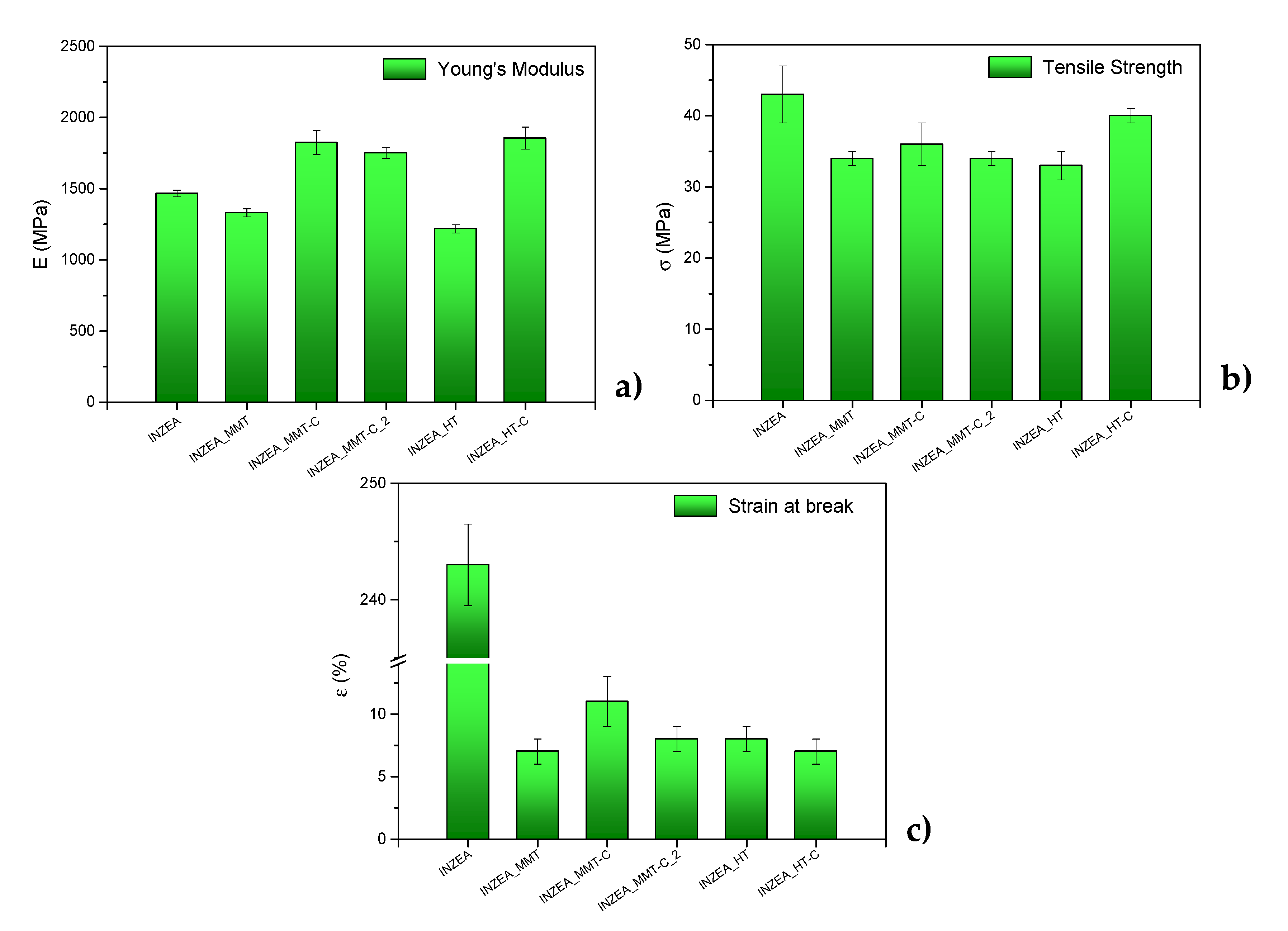
3.2.2. Tensile Properties
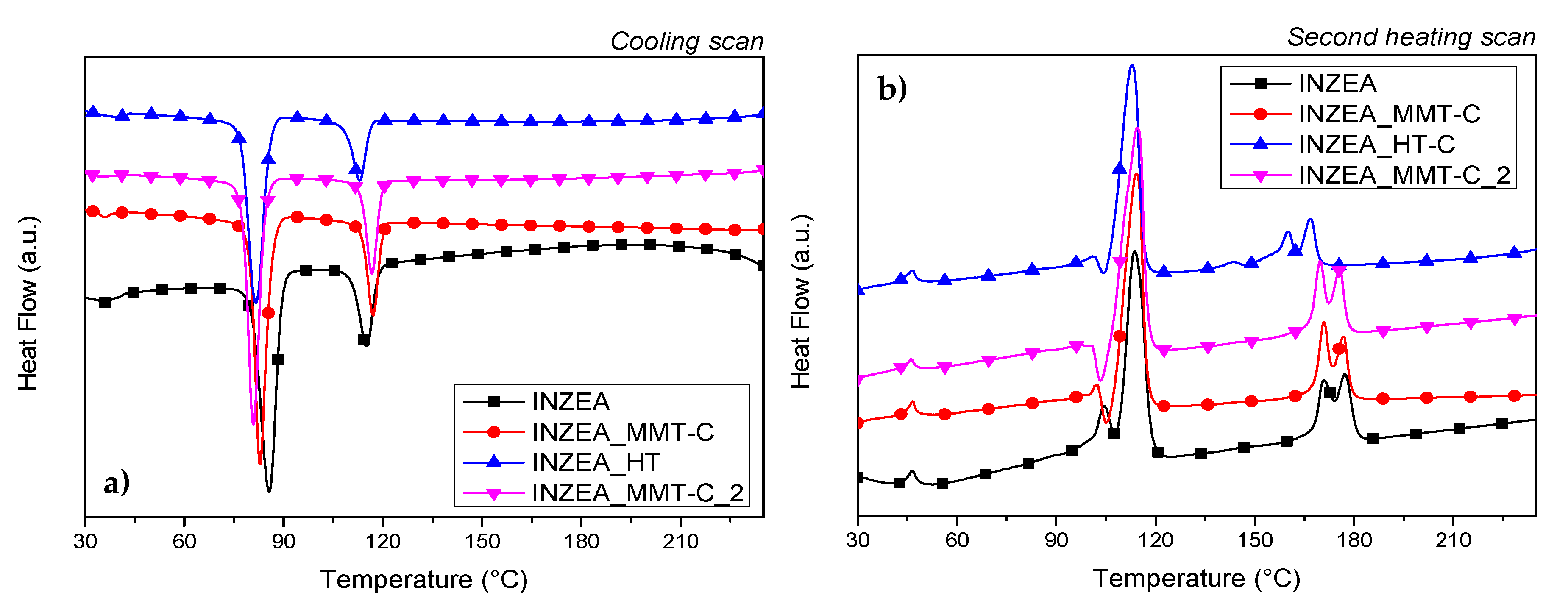
3.2.3. DMTA Tests
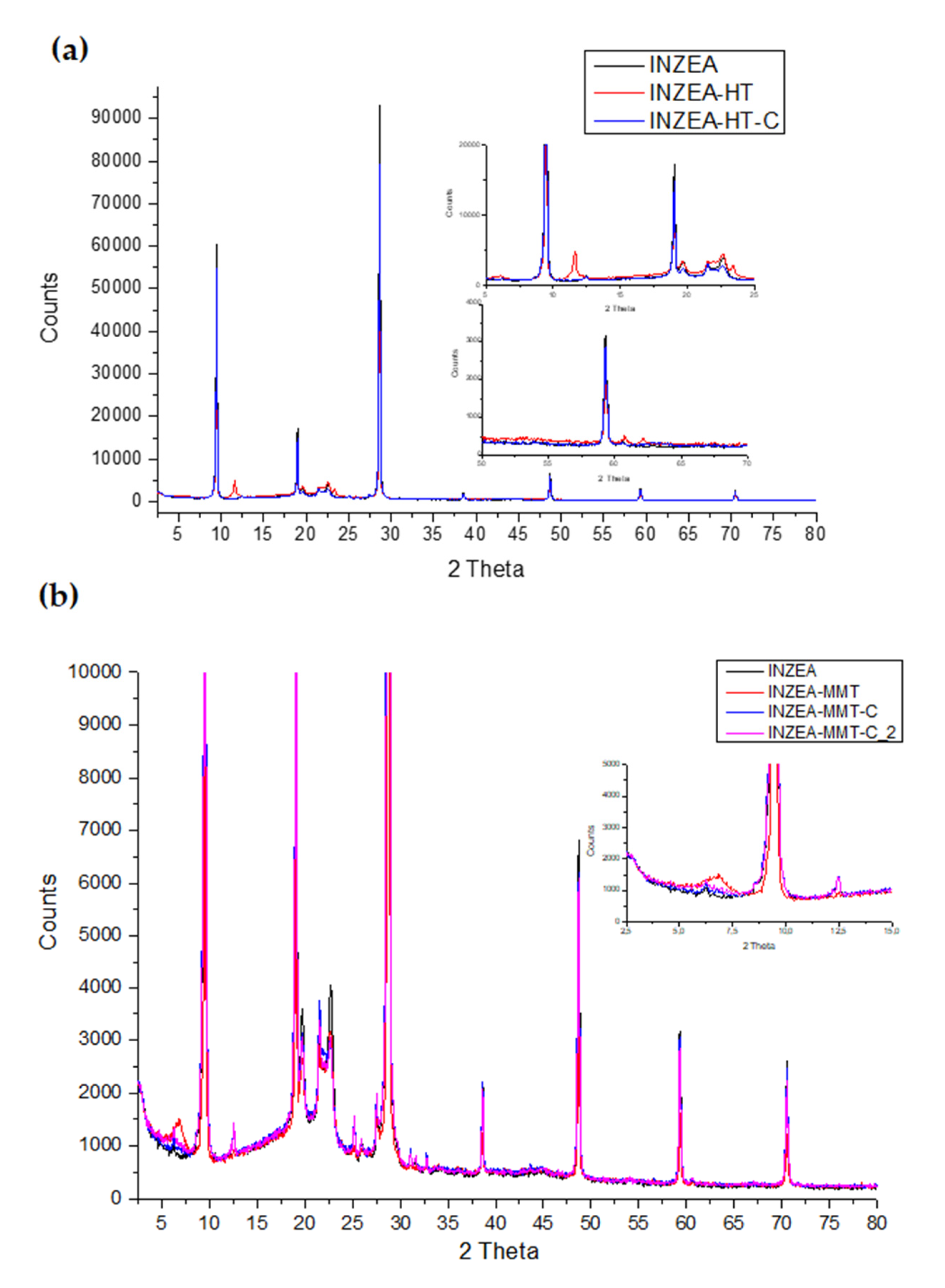
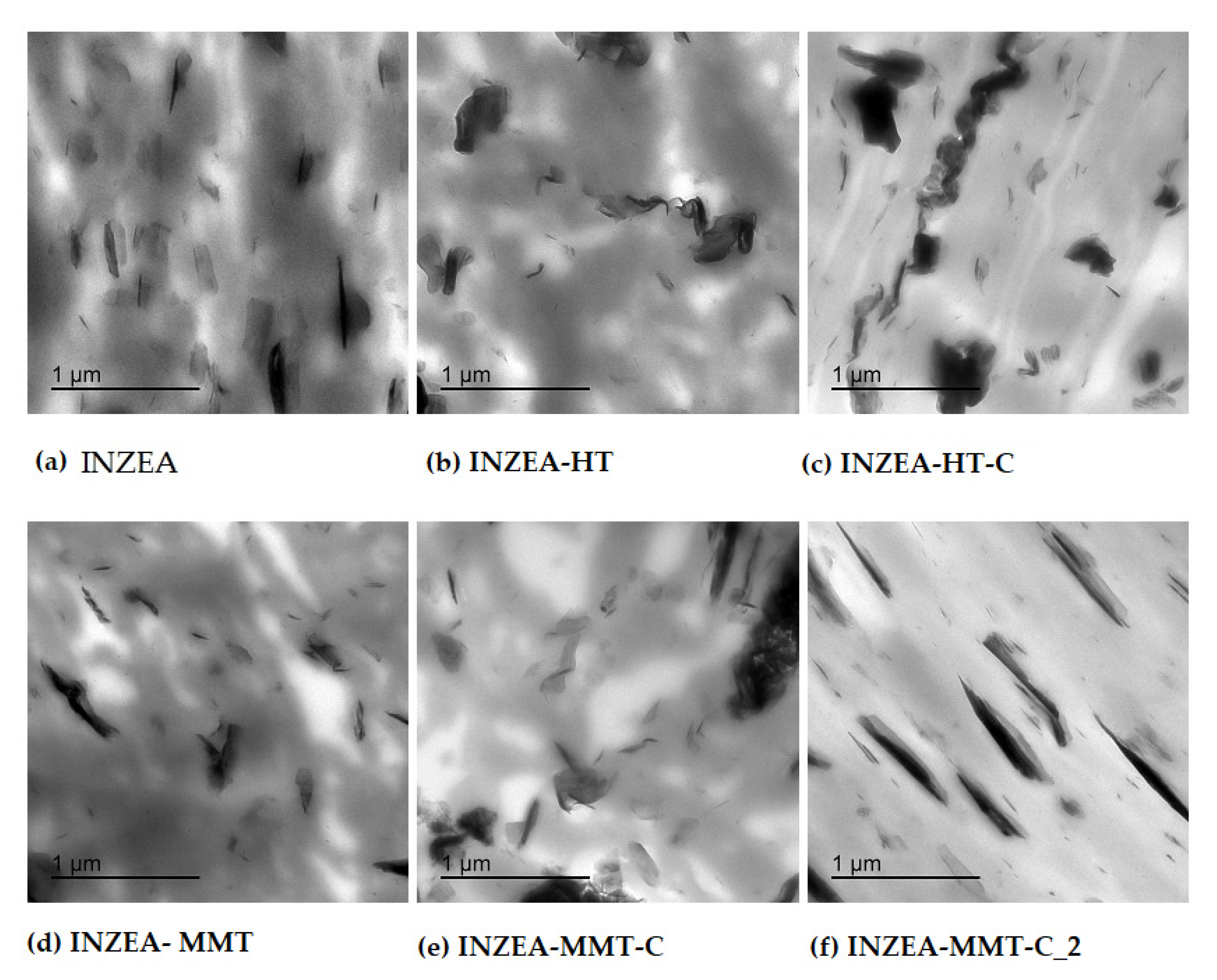
3.2.4. Structural and Morphological Properties
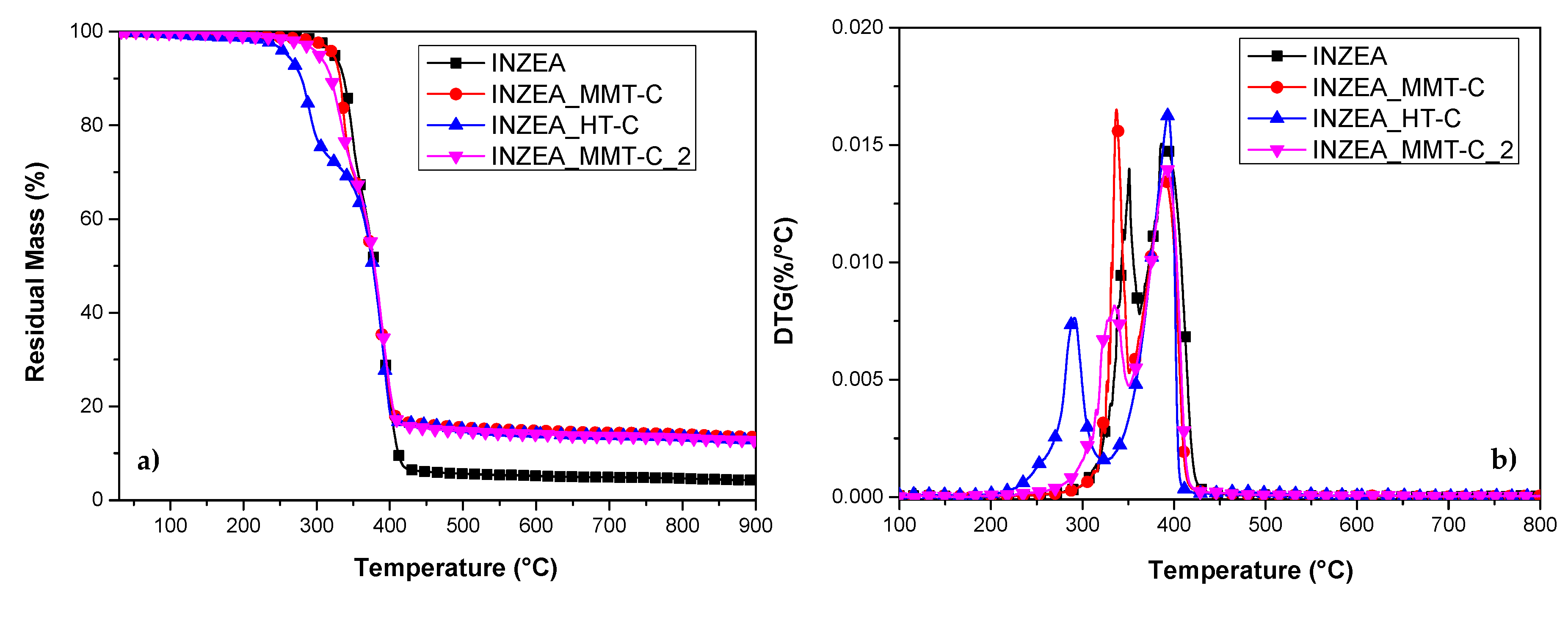
3.2.5. Thermal stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ebrahimi, I.; Parvinzadeh Gashti, M. Extraction of polyphenolic dyes from henna, pomegranate rind, and Pterocarya fraxinifolia for nylon 6 dyeing. Color. Technol. 2016, 132, 162–176. [Google Scholar] [CrossRef]
- Shalini, S.; Prasanna, S.; Mallick, T.K.; Senthilarasu, S. Review on natural dye sensitized solar cells: Operation, materials and methods. Renew. Sustain. Energy Rev. 2015, 51, 1306–1325. [Google Scholar] [CrossRef]
- Heinonen, J.; Farahmandazad, H.; Vuorinen, A.; Kallio, H.; Yang, B.; Sainio, T. Extraction and purification of anthocyanins from purple-fleshed potato. Food Bioprod. Process. 2016, 99, 136–146. [Google Scholar] [CrossRef]
- Keppler, K.; Humpf, H.-U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Monteiro, F.; Passos, C.P.; Silva, A.M.S.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Blanching impact on pigments, glucosinolates, and phenolics of dehydrated broccoli by-products. Food Res. Int. 2020, 132, 109055. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of industrial broccoli discards (Brassica oleracea var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Cardoso, S.M.; Wessel, D.F.; Coimbra, M.A. Microwave assisted dehydration of broccoli by-products and simultaneous extraction of bioactive compounds. Food Chem. 2018, 246, 386–393. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Viqueira, V.; Ramos, M.; Luzi, F.; Dominici, F.; Torre, L.; Jiménez, A.; Puglia, D.; Garrigós, M.C. Effect of Lemon Waste Natural Dye and Essential Oil Loaded into Laminar Nanoclays on Thermomechanical and Color Properties of Polyester Based Bionanocomposites. Polymers 2020, 12, 1451. [Google Scholar] [CrossRef]
- Dhafina, W.A.; Daud, M.Z.; Salleh, H. The sensitization effect of anthocyanin and chlorophyll dyes on optical and photovoltaic properties of zinc oxide based dye-sensitized solar cells. Optik 2019, 207, 163808. [Google Scholar] [CrossRef]
- Nan, H.; Shen, H.-P.; Wang, G.; Xie, S.-D.; Yang, G.-J.; Lin, H. Studies on the optical and photoelectric properties of anthocyanin and chlorophyll as natural co-sensitizers in dye sensitized solar cell. Opt. Mater. 2017, 73, 172–178. [Google Scholar] [CrossRef]
- Steet, J.A.; Tong, C.H. Degradation kinetics of green color and chlorophylls in peas by colorimetry and HPLC. J. Food Sci. 1996, 61, 924–928. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, L.; Li, J.; Oliveira, H.; Yang, N.; Jin, W.; Zhu, Z.; Li, S.; He, J. Microencapsulation of anthocyanins extracted from grape skin by emulsification/internal gelation followed by spray/freeze-drying techniques: Characterization, stability and bioaccessibility. LWT 2020, 123, 109097. [Google Scholar] [CrossRef]
- Hsiao, C.J.; Lin, J.F.; Wen, H.Y.; Lin, Y.M.; Yang, C.H.; Huang, K.S.; Shaw, J.F. Enhancement of the stability of chlorophyll using chlorophyll-encapsulated polycaprolactone microparticles based on droplet microfluidics. Food Chem. 2020, 306, 125300. [Google Scholar] [CrossRef] [PubMed]
- Bertuoli, P.T.; Piazza, D.; Scienza, L.C.; Zattera, A.J. Preparation and characterization of montmorillonite modified with 3-aminopropyltriethoxysilane. Appl. Clay Sci. 2014, 87, 46–51. [Google Scholar] [CrossRef]
- Beltrán, M.I.; Benavente, V.; Marchante, V.; Dema, H.; Marcilla, A. Characterisation of montmorillonites simultaneously modified with an organic dye and an ammonium salt at different dye/salt ratios. Properties of these modified montmorillonites EVA nanocomposites. Appl. Clay Sci. 2014, 97, 43–52. [Google Scholar] [CrossRef]
- Kohno, Y.; Asai, S.; Shibata, M.; Fukuhara, C.; Maeda, Y.; Tomita, Y.; Kobayashi, K. Improved photostability of hydrophobic natural dye incorporated in organo-modified hydrotalcite. J. Phys. Chem. Solids 2014, 75, 945–950. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Tao, Q.; He, H.; Zhu, J.; Yuan, P.; Zhu, R. Silylation of montmorillonite surfaces: Dependence on solvent nature. J. Colloid Interface Sci. 2013, 391, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Micó-Vicent, B.; Jordán, J.; Perales, E.; Martínez-Verdú, F.M.; Cases, F. Finding the Additives Incorporation Moment in Hybrid Natural Pigments Synthesis to Improve Bioresin Properties. Coatings 2019, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Rather, L.J.; Shabbir, M.; Bukhari, M.N.; Shahid, M.; Khan, M.A.; Mohammad, F. Ecological dyeing of Woolen yarn with Adhatoda vasica natural dye in the presence of biomordants as an alternative copartner to metal mordants. J. Environ. Chem. Eng. 2016, 4, 3041–3049. [Google Scholar] [CrossRef]
- Wu, S.; Cui, H.; Wang, C.; Hao, F.; Liu, P.; Xiong, W. In situ self-assembled preparation of the hybrid nanopigment from raw sepiolite with excellent stability and optical performance. Appl. Clay Sci. 2018, 163, 1–9. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Jordán, J.; Martínez-Verdú, F.; Balart, R. A combination of three surface modifiers for the optimal generation and application of natural hybrid nanopigments in a biodegradable resin. J. Mater. Sci. 2017, 52, 889–898. [Google Scholar] [CrossRef]
- Fournier, F.; de Viguerie, L.; Balme, S.; Janot, J.-M.; Walter, P.; Jaber, M. Physico-chemical characterization of lake pigments based on montmorillonite and carminic acid. Appl. Clay Sci. 2016, 130, 12–17. [Google Scholar] [CrossRef]
- Armaǧan, B.; Özdemir, O.; Turan, M.; Celik, M.S. The removal of reactive azo dyes by natural and modified zeolites. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2003, 78, 725–732. [Google Scholar] [CrossRef]
- Raha, S.; Quazi, N.; Ivanov, I.; Bhattacharya, S. Dye/Clay intercalated nanopigments using commercially available non-ionic dye. Dye. Pigment. 2012, 93, 1512–1518. [Google Scholar] [CrossRef]
- Sommer Márquez, A.E.; Lerner, D.A.; Fetter, G.; Bosch, P.; Tichit, D.; Palomares, E. Preparation of layered double hydroxide/chlorophyll a hybrid nano-antennae: A key step. Dalt. Trans. 2014, 43, 10521. [Google Scholar] [CrossRef] [PubMed]
- Campanholi, K.D.S.S.; Braga, G.; Da Silva, J.B.; Da Rocha, N.L.; De Francisco, L.M.B.; De Oliveira, É.L.; Bruschi, M.L.; De Castro-Hoshino, L.V.; Sato, F.; Hioka, N.; et al. Biomedical Platform Development of a Chlorophyll-Based Extract for Topic Photodynamic Therapy: Mechanical and Spectroscopic Properties. Langmuir 2018, 34, 8230–8244. [Google Scholar] [CrossRef]
- Arof, A.K.; Ping, T.L. Chlorophyll as Photosensitizer in Dye-Sensitized Solar Cells. In Chlorophyll; InTech: London, UK, 2017. [Google Scholar]
- Ahmed, J.K. Effect of Chlorophyll and Anthocyanin on the Secondary Bonds of Poly Vinyl Chloride (PVC). Int. J. Mater. Sci. Appl. 2015, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Chandrappa, H.; Bhajantri, R.F.; Prarthana, N. Simple fabrication of PVA-ATE (Amaranthus tricolor leaves extract) polymer biocomposites: An efficient UV-Shielding material for organisms in terrestrial and aquatic ecosystems. Opt. Mater. 2020, 109, 110204. [Google Scholar]
- Chavoshizadeh, S.; Pirsa, S.; Mohtarami, F. Conducting/smart color film based on wheat gluten/chlorophyll/polypyrrole nanocomposite. Food Packag. Shelf Life 2020, 24, 100501. [Google Scholar] [CrossRef]
- Chavoshizadeh, S.; Pirsa, S.; Mohtarami, F. Sesame oil oxidation control by active and smart packaging system using wheat gluten/chlorophyll film to increase shelf life and detecting expiration date. Eur. J. Lipid Sci. Technol. 2020, 122, 1900385. [Google Scholar] [CrossRef]
- Marchante, V.; Marcilla, A.; Benavente, V.; Martínez-Verdú, F.M.; Beltrán, M.I. Linear low-density polyethylene colored with a nanoclay-based pigment: Morphology and mechanical, thermal, and colorimetric properties. J. Appl. Polym. Sci. 2013, 129, 2716–2726. [Google Scholar] [CrossRef]
- Marchante, V.; Benavente, V.; Marcilla, A.; Martínez-Verdú, F.M.; Beltrán, M.I. Ethylene vinyl acetate/nanoclay-based pigment composites: Morphology, rheology, and mechanical, thermal, and colorimetric properties. J. Appl. Polym. Sci. 2013, 130, 2987–2994. [Google Scholar] [CrossRef]
- Mahmoodi, A.; Ebrahimi, M.; Khosravi, A.; Eivaz Mohammadloo, H. A hybrid dye-clay nano-pigment: Synthesis, characterization and application in organic coatings. Dye. Pigment. 2017, 147, 234–240. [Google Scholar] [CrossRef]
- León-Cabezas, M.A.; Martínez-García, A.; Varela-Gandía, F.J. Innovative functionalized monofilaments for 3D printing using fused deposition modeling for the toy industry. Procedia Manuf. 2017, 13, 738–745. [Google Scholar] [CrossRef]
- ColorFabb and Grafe collaborate to introduce colour on demand for 3D printing filaments. Addit. Polym. 2018, 2018, 4. [CrossRef]
- Lee, J.-Y.; An, J.; Chua, C.K. Fundamentals and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Mahmoodi, A.; Ghodrati, S.; Khorasani, M. High-strength, low-permeable, and light-protective nanocomposite films based on a hybrid nanopigment and biodegradable PLA for food packaging applications. ACS Omega 2019, 4, 14947–14954. [Google Scholar] [CrossRef] [Green Version]
- Usop, R.; Abidin, Z.H.Z.; Mazni, N.A.; Hadi, A.N.; Halim, N.A.; Taha, R.M.; Careem, M.A.; Majid, S.R.; Arof, A.K. The colour stability of natural dye coating films consisting of chlorophyll after exposed to UV-A. Pigment Resin Technol. 2016, 45, 149–157. [Google Scholar] [CrossRef]
- Kohno, Y.; Inagawa, M.; Ikoma, S.; Shibata, M.; Matsushima, R.; Fukuhara, C.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Stabilization of a hydrophobic natural dye by intercalation into organo-montmorillonite. Appl. Clay Sci. 2011, 54, 202–205. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, Y.; Iyi, N.; Bujdák, J.; Sasai, R.; Fujita, T. Effect of layer charge density on orientation and aggregation of a cationic laser dye incorporated in the interlayer space of montmorillonites. J. Colloid Interface Sci. 2004, 269, 22–25. [Google Scholar] [CrossRef]
- Bahrami, S.H.; Movassagh, B.; Amirshahi, S.H.; Arami, M. Removal of disperse blue 56 and disperse red 135 dyes from aqueous dispersions by modified montmorillonite nanoclay. Chem. Ind. Chem. Eng. Q 2017, 23, 21–29. [Google Scholar]
- Samide, A.; Tutunaru, B. Thermal behavior of the chlorophyll extract from a mixture of plants and seaweed. J. Therm. Anal. Calorim. 2017, 127, 597–604. [Google Scholar] [CrossRef]
- Esfahani, J.M.; Sabet, A.R.; Esfandeh, M. Assessment of nanocomposites based on unsaturated polyester resin/nanoclay under impact loading. Polym. Adv. Technol. 2012, 23, 817–824. [Google Scholar] [CrossRef]
- Zdiri, K.; Elamri, A.; Hamdaoui, M.; Harzallah, O.; Khenoussi, N.; Brendlé, J. Reinforcement of recycled PP polymers by nanoparticles incorporation. Green Chem. Lett. Rev. 2018, 11, 296–311. [Google Scholar] [CrossRef] [Green Version]
- Darie, R.N.; Pâslaru, E.; Sdrobis, A.; Pricope, G.M.; Hitruc, G.E.; Poiatǎ, A.; Baklavaridis, A.; Vasile, C. Effect of nanoclay hydrophilicity on the poly(lactic acid)/clay nanocomposites properties. Ind. Eng. Chem. Res. 2014, 53, 7877–7890. [Google Scholar] [CrossRef]
- Świetlicki, M.; Chocyk, D.; Klepka, T.; Prószyński, A.; Kwaśniewska, A.; Borc, J.; Gładyszewski, G. The structure and mechanical properties of the surface layer of polypropylene polymers with talc additions. Materials. 2020, 13, 698. [Google Scholar] [CrossRef] [Green Version]
- Pi-Puig, T.; Animas-Torices, D.Y.; Solé, J. Mineralogical and geochemical characterization of talc from two mexican ore deposits (oaxaca and puebla) and nine talcs marketed in mexico: Evaluation of its cosmetic uses. Minerals 2020, 10, 388. [Google Scholar] [CrossRef]
- Wang, K.; Jiao, T.; Wang, Y.; Li, M.; Li, Q.; Shen, C. The microstructures of extrusion cast biodegradable poly(butylene succinate) films investigated by X-ray diffraction. Mater. Lett. 2013, 92, 334–337. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Zhang, S.; Ma, R.; Wang, Y.; Cao, W.; Liu, C.; Shen, C. Crystallization behavior of poly(lactic acid) with a self-assembly aryl amide nucleating agent probed by real-time infrared spectroscopy and X-ray diffraction. Polym. Test. 2017, 64, 12–19. [Google Scholar] [CrossRef]
- Heraldy, E.; Nugrahaningtyas, K.D. X-RAY diffraction and fourier transform infrared study of Ca-Mg-Al hydrotalcite from artificial brine water with synthesis hydrothermal treatments. IOP Conf. Ser. Mater. Sci. Eng. Pap. 2018, 333. [Google Scholar]
- Bascialla, G.; Regazzoni, A.E. Immobilization of anionic dyes by intercalation into hydrotalcite. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 328, 34–39. [Google Scholar] [CrossRef]
- Abdallah, W.; Mirzadeh, A.; Tan, V.; Kamal, M.R. Influence of nanoparticle pretreatment on the thermal, rheological and mechanical properties of PLA-PBSA nanocomposites incorporating cellulose nanocrystals or montmorillonite. Nanomaterials 2019, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Dias, G.; Prado, M.; Le Roux, C.; Poirier, M.; Micoud, P.; Ligabue, R.; Martin, F.; Einloft, S. Synthetic talc as catalyst and filler for waterborne polyurethane-based nanocomposite synthesis. Polym. Bull. 2020, 77, 975–987. [Google Scholar] [CrossRef]
- Costa, A.L.; Gomes, A.C.; Pillinger, M.; Gonçalves, I.S.; de Melo, J.S.S. An indigo carmine-based hybrid nanocomposite with supramolecular control of dye aggregation and photobehavior. Chem. Eur. J. 2015, 21, 12069–12078. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; He, Y.; Qu, J. Structure and properties of polylactide/poly(butylene succinate)/organically modified montmorillonite nanocomposites with high-efficiency intercalation and exfoliation effect manufactured via volume pulsating elongation flow. Polymer 2019, 180, 121656. [Google Scholar] [CrossRef]
- Tanna, V.A.; Enokida, J.S.; Coughlin, E.B.; Winter, H.H. Functionalized polybutadiene for clay-polymer nanocomposite fabrication. Macromolecules 2019, 52, 6135–6141. [Google Scholar] [CrossRef]
- Delpouve, N.; Saiter-Fourcin, A.; Coiai, S.; Cicogna, F.; Spiniello, R.; Oberhauser, W.; Legnaioli, S.; Ishak, R.; Passaglia, E. Effects of organo-LDH dispersion on thermal stability, crystallinity and mechanical features of PLA. Polymer 2020, 208, 122952. [Google Scholar] [CrossRef]
- Coiai, S.; Cicogna, F.; de Santi, A.; Pérez Amaro, L.; Spiniello, R.; Signori, F.; Fiori, S.; Oberhauser, W.; Passaglia, E. MMT and LDH organo-modification with surfactants tailored for PLA nanocomposites. Express Polym. Lett. 2017, 11, 163–175. [Google Scholar] [CrossRef]
- Ramos, M.; Dominici, F.; Luzi, F.; Jimenez, A.; Garrigós, M.; Torre, L.; Puglia, D. Effect of almond shell waste on physicochemical properties of polyester-based biocomposites. Polymers 2020, 12, 835. [Google Scholar] [CrossRef] [Green Version]
- Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part II: An overview on thermal decomposition of polycondensation polymers. Thermochim. Acta 2011, 523, 25–45. [Google Scholar] [CrossRef]
- Mohapatra, A.K.; Mohanty, S.; Nayak, S.K. Study of thermo-mechanical and morphological behaviour of biodegradable PLA/PBAT/layered silicate blend nanocomposites. J. Polym. Environ. 2014, 22, 398–408. [Google Scholar] [CrossRef]






| Experiment | Nanoclay | Surfactant (wt%) * | Biomordant (wt%) | Silane (wt%) | Adsorbed Dye (Ads, %) |
|---|---|---|---|---|---|
| 1 | HT | 5 | 0 | 5 | 98.13 |
| 2 | HT | 0 | 0 | 0 | 98.44 |
| 3 | HT | 5 | 1 | 0 | 97.97 |
| 4 | HT | 0 | 1 | 5 | 98.12 |
| 5 | MMT | 5 | 0 | 0 | 97.83 |
| 6 | MMT | 0 | 0 | 5 | 97.52 |
| 7 | MMT | 0 | 1 | 0 | 95.66 |
| 8 | MMT | 5 | 1 | 5 | 96.62 |
| Factor | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| B (Surfactant) | 0.2387 | 1 | 0.2387 | 216.15 | 0.0432 |
| C (Mordant) | 2.8227 | 1 | 2.8227 | 2555.63 | 0.0126 |
| D (Silane) | 1.8126 | 1 | 1.8126 | 1641.11 | 0.0157 |
| AB (Nanoclay-Surfactant) | 0.8489 | 1 | 0.8489 | 768.59 | 0.0230 |
| AC (Nanoclay-Mordant) | 0.1624 | 1 | 0.1624 | 147.08 | 0.0524 |
| AD (Nanoclay-Silane) | 0.2549 | 1 | 0.2549 | 230.78 | 0.0418 |
| Total Error | 0.0011 | 1 | 0.0011 | ||
| Total (corr.) | 6.1414 | 7 | |||
| R2 (%): 99.98 | |||||
| Adj R2 (%): 99.87 | |||||
| Formulations | L* | a* | b* | Gloss | ∆E* |
|---|---|---|---|---|---|
| White Control | 99.47 ± 0.00 | −0.08 ± 0.01 | −0.08 ± 0.01 | 121 | - |
| INZEA | 81.66 ± 0.32 | 0.65 ± 0.05 | 5.11 ± 0.04 | 68 ± 3 | 18.56 ± 0.30 |
| INZEA_MMT | 79.50 ± 0.74 | 0.90 ± 0.07 | 8.83 ± 0.16 | 60 ± 5 | 21.89 ± 0.66 |
| INZEA_MMT-C | 74.22 ± 0.23 | 0.33 ± 0.04 | 12.00 ± 0.14 | 64 ± 1 | 28.00 ± 0.16 |
| INZEA_MMT-C_2 | 68.29 ± 0.05 | 0.13 ± 0.02 | 12.30 ± 0.03 | 70 ± 1 | 33.55 ± 0.04 |
| INZEA_HT | 86.22 ± 0.19 | 0.14 ± 0.04 | 4.22 ± 0.08 | 57 ± 1 | 13.93 ± 0.19 |
| INZEA_HT-C | 74.67 ± 0.24 | −0.07 ± 0.01 | 18.71 ± 0.15 | 65 ± 1 | 31.12 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micó-Vicent, B.; Ramos, M.; Luzi, F.; Dominici, F.; Viqueira, V.; Torre, L.; Jiménez, A.; Puglia, D.; Garrigós, M.C. Effect of Chlorophyll Hybrid Nanopigments from Broccoli Waste on Thermomechanical and Colour Behaviour of Polyester-Based Bionanocomposites. Polymers 2020, 12, 2508. https://doi.org/10.3390/polym12112508
Micó-Vicent B, Ramos M, Luzi F, Dominici F, Viqueira V, Torre L, Jiménez A, Puglia D, Garrigós MC. Effect of Chlorophyll Hybrid Nanopigments from Broccoli Waste on Thermomechanical and Colour Behaviour of Polyester-Based Bionanocomposites. Polymers. 2020; 12(11):2508. https://doi.org/10.3390/polym12112508
Chicago/Turabian StyleMicó-Vicent, Bàrbara, Marina Ramos, Francesca Luzi, Franco Dominici, Valentín Viqueira, Luigi Torre, Alfonso Jiménez, Debora Puglia, and María Carmen Garrigós. 2020. "Effect of Chlorophyll Hybrid Nanopigments from Broccoli Waste on Thermomechanical and Colour Behaviour of Polyester-Based Bionanocomposites" Polymers 12, no. 11: 2508. https://doi.org/10.3390/polym12112508