Utilization of Nano-TiO2 as an Influential Additive for Complementing Separation Performance of a Hybrid PVDF-PVP Hollow Fiber: Boron Removal from Leachate
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.1.1. Dope Formulation
2.1.2. Spinning of Nano-Hybrid PVDF/PVP/DMAC/TiO2- Hollow Fiber Membrane
2.2. Membrane Characterization
2.2.1. Morphological Study
2.2.2. Energy Dispersive X-ray Spectroscopy (EDX) Analysis
2.2.3. Hydrophilic Analysis
2.2.4. Porosity Analysis
2.3. Evaluation of Membrane Performances
2.3.1. Permeability and Flux Performance
2.3.2. Rejection Performance (Re%)
2.3.3. Antifouling and Reusability Analysis
2.4. Zeta Potential Measurement
2.5. Analytical Method
3. Results and Discussion
3.1. Influence of TiO2 on Membrane Characteristics
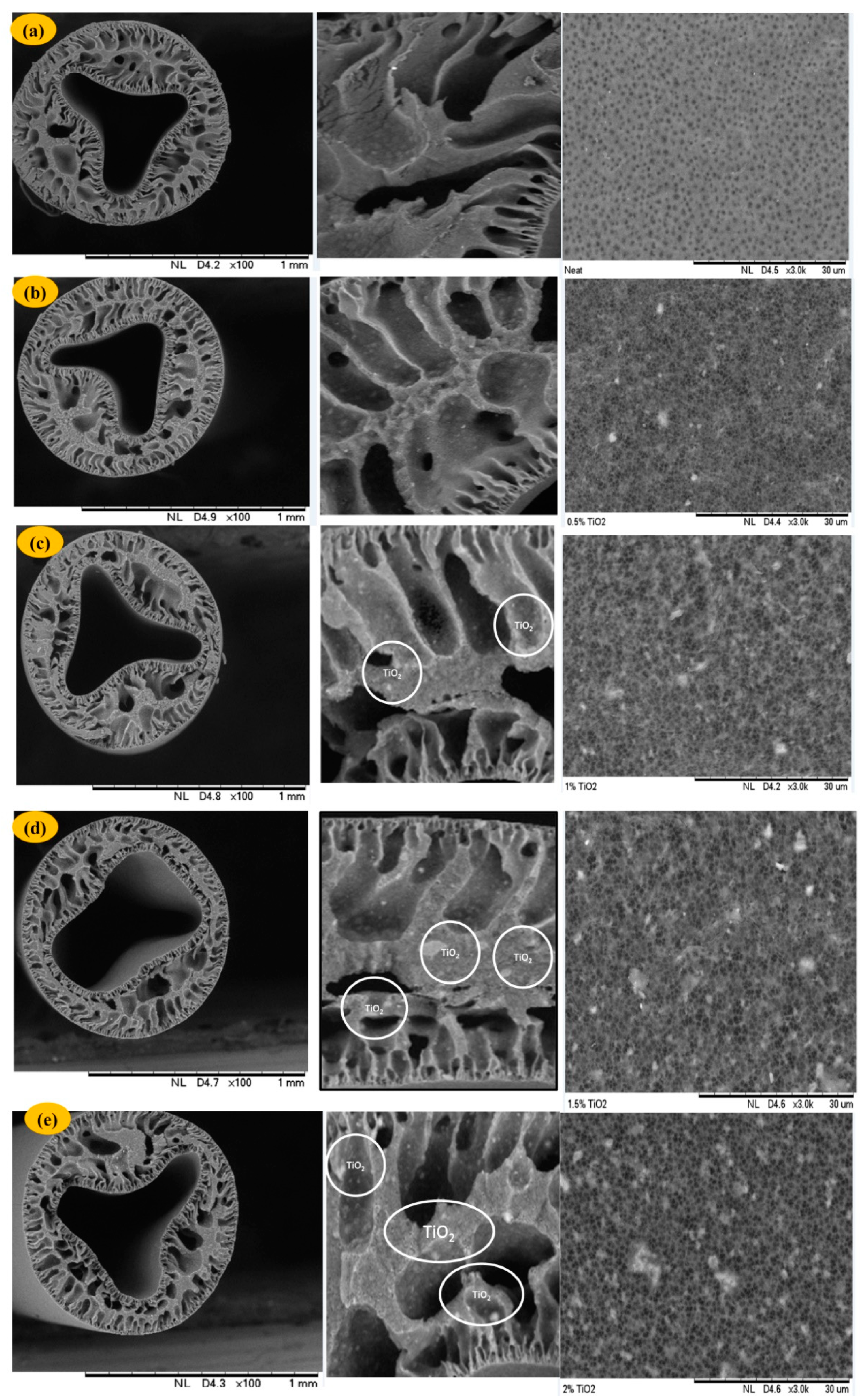
3.1.1. Structural Morphology
3.1.2. EDX Elemental Analysis
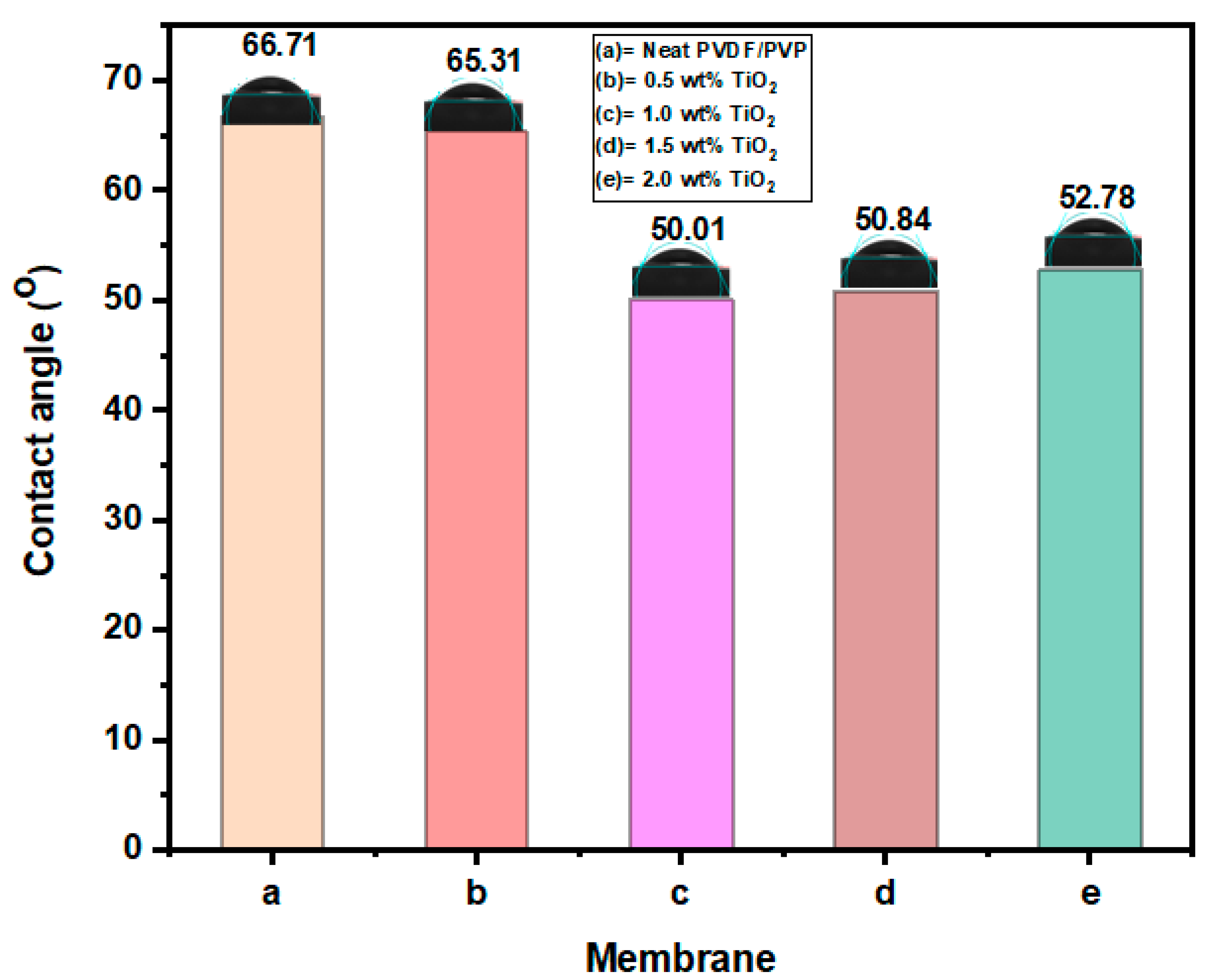
3.1.3. Hydrophilicity Analysis
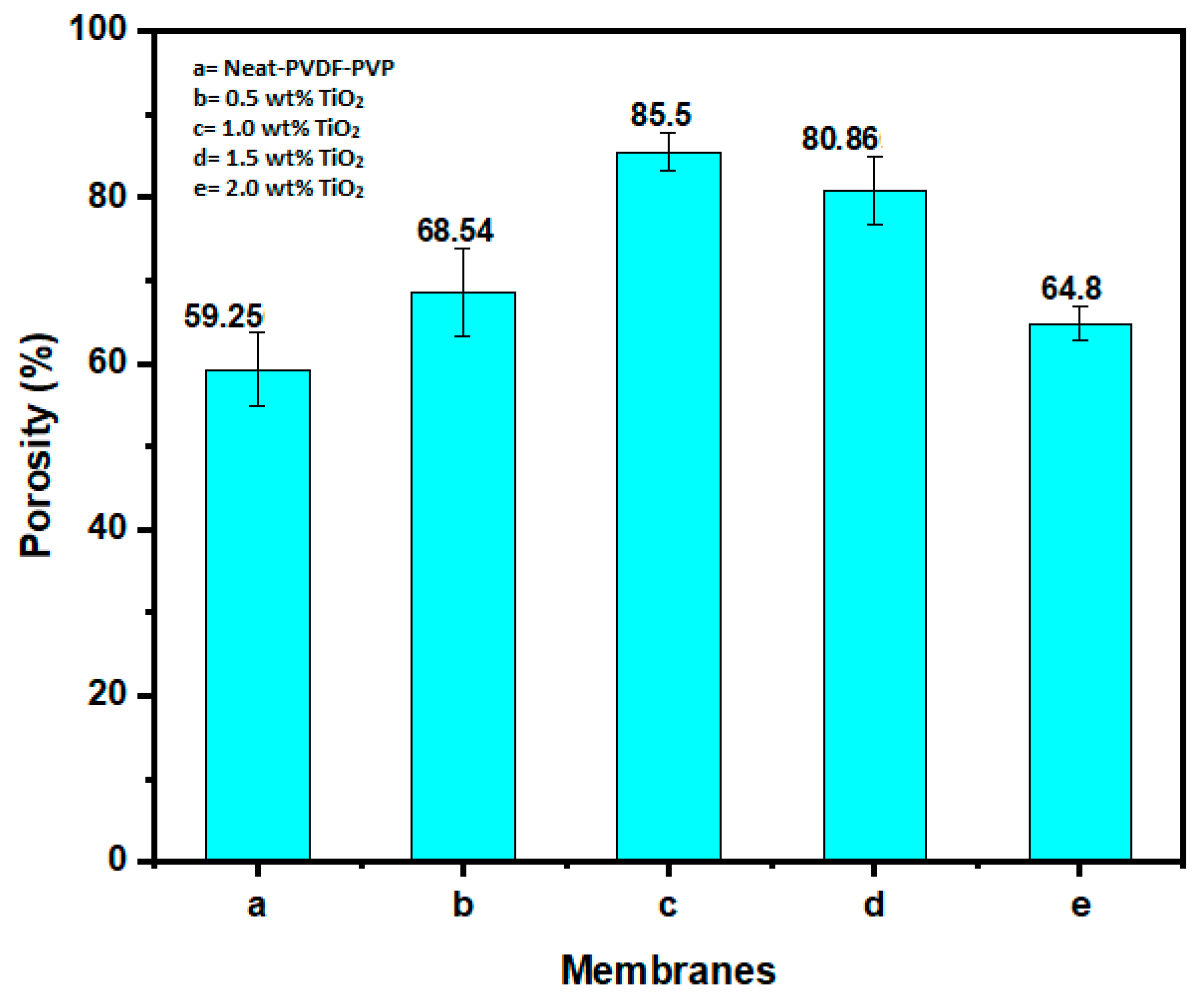
3.1.4. Membrane Porosity
3.2. Influence of TiO2 on Membrane Performance
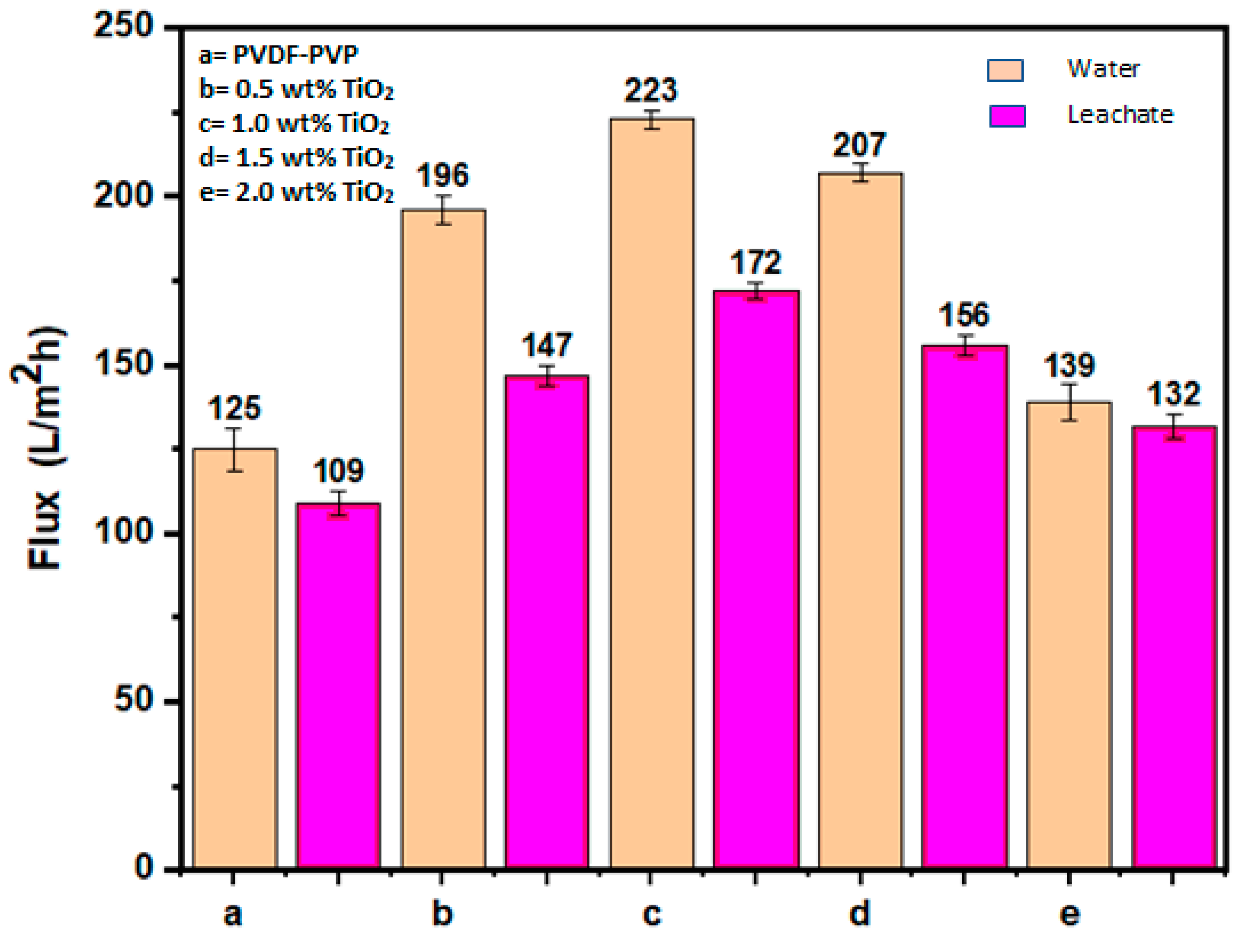
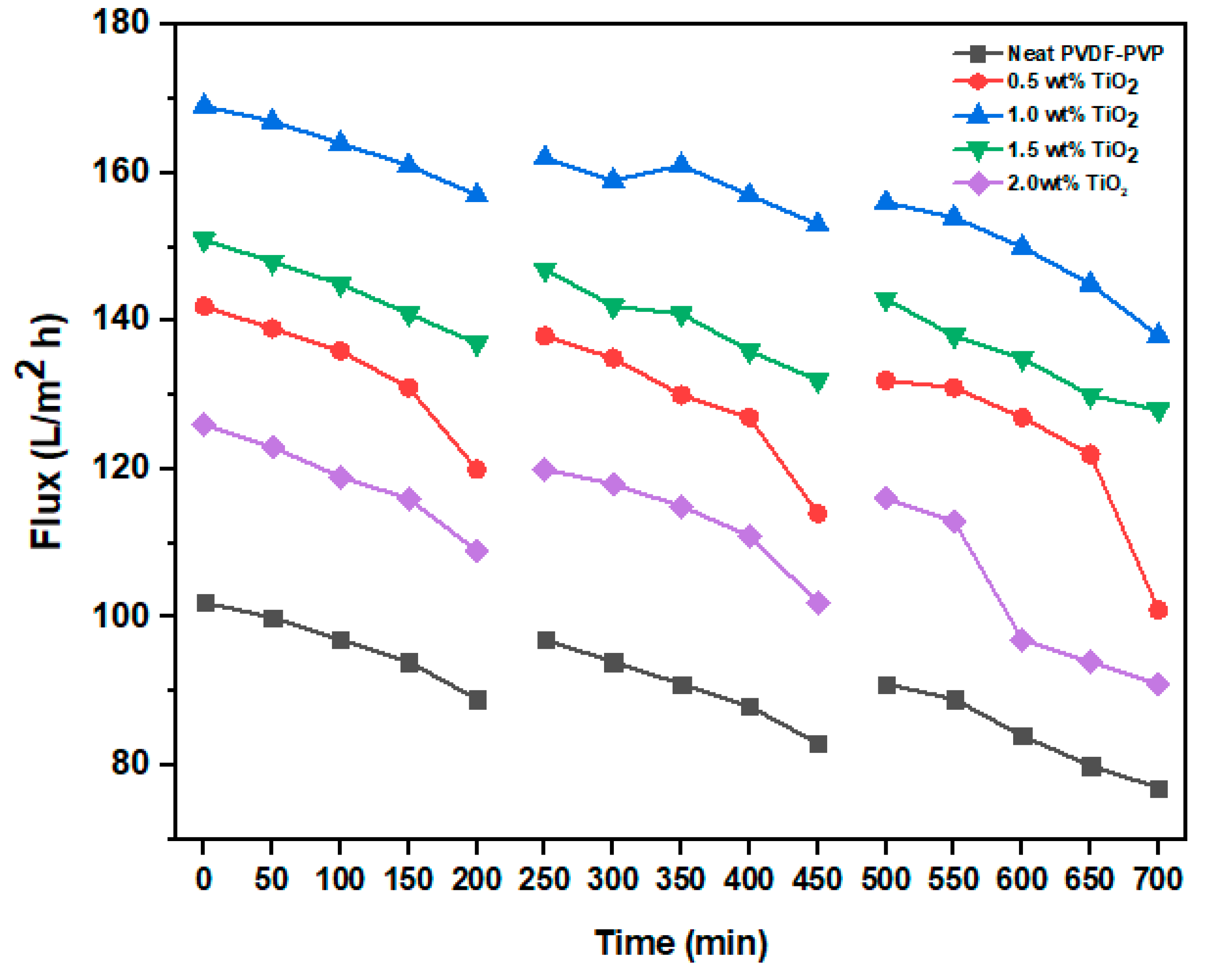
3.2.1. Flux
3.2.2. Boron Rejection
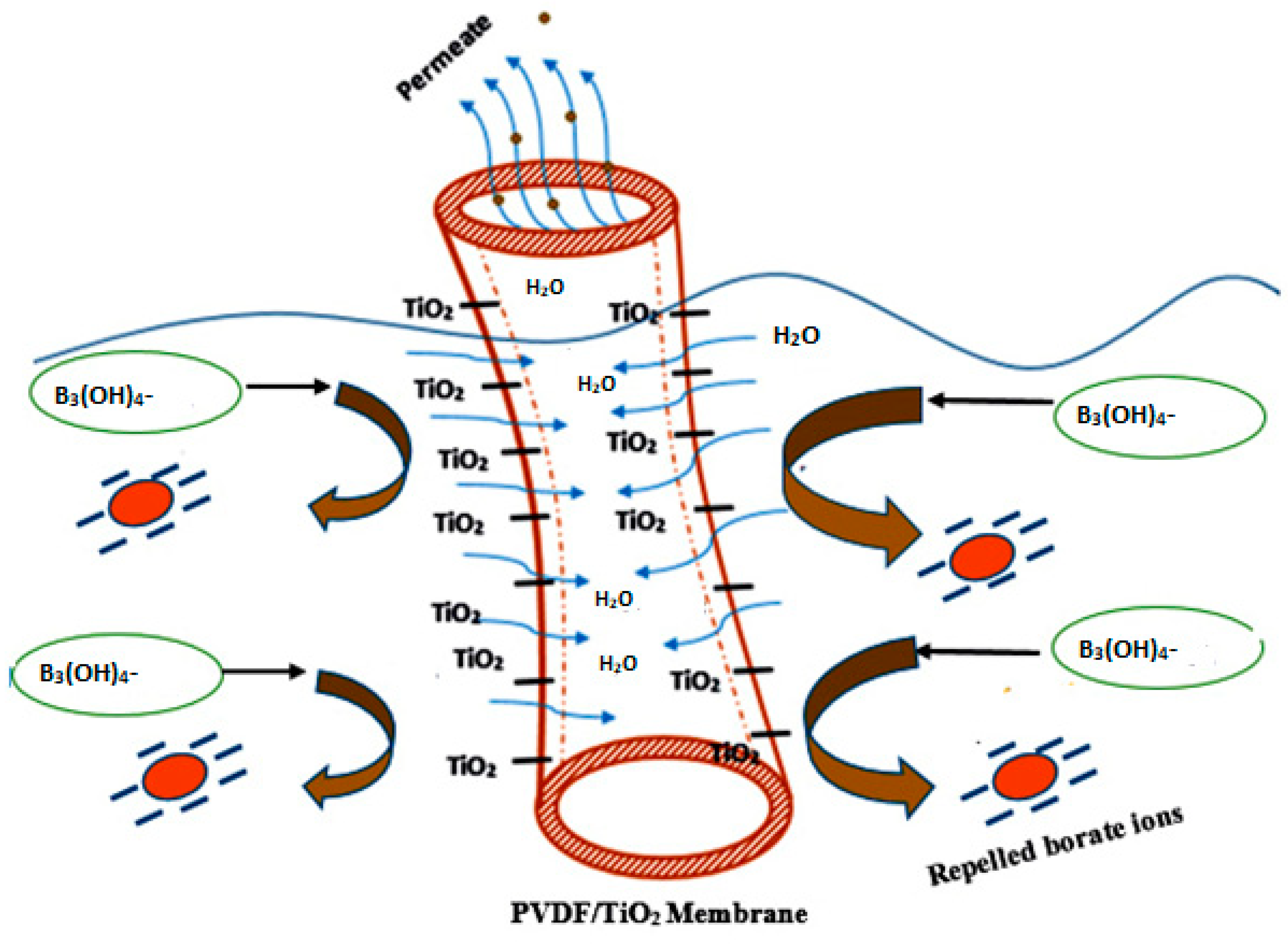
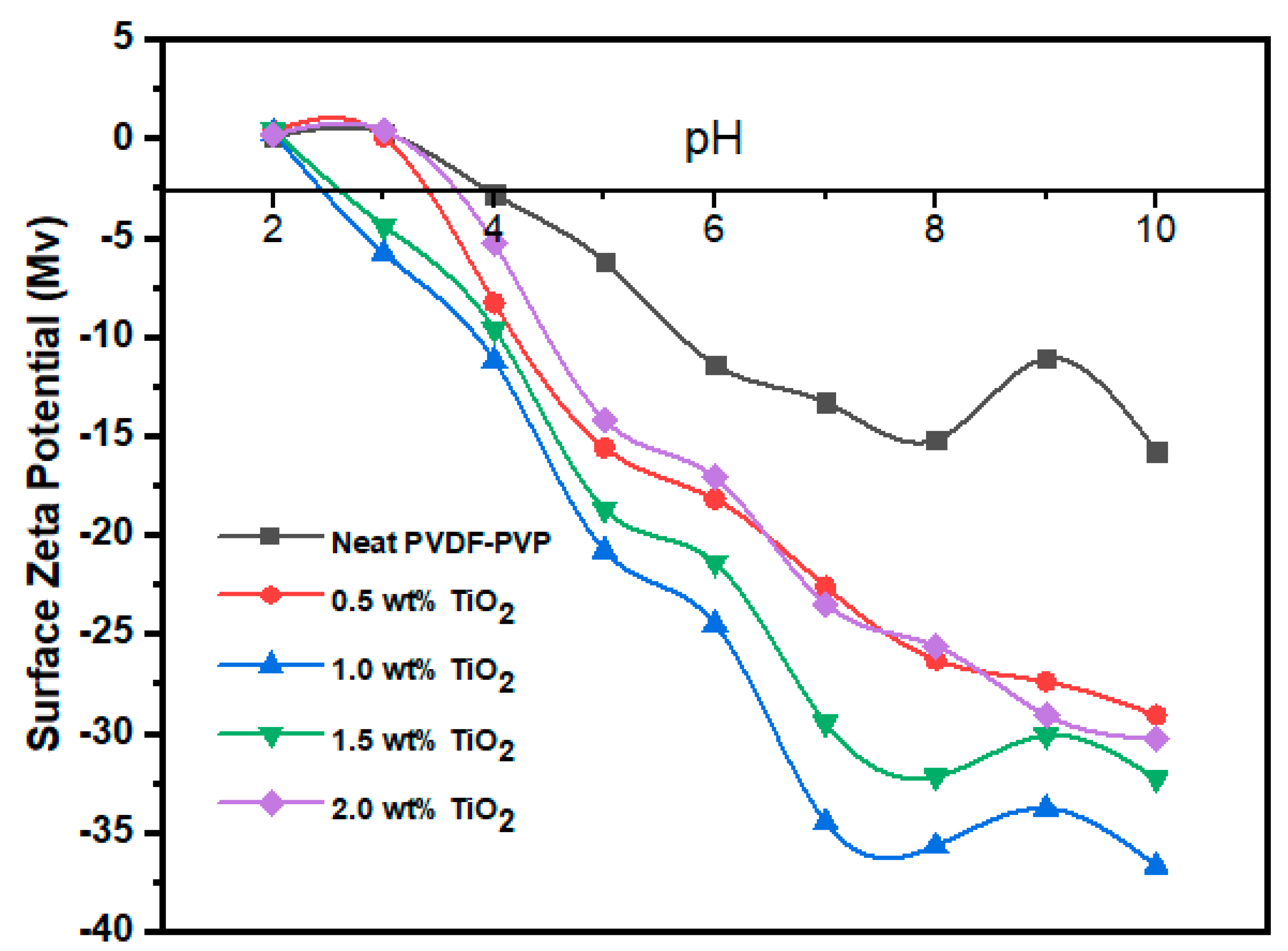
3.2.3. TiO2 Surface Charge
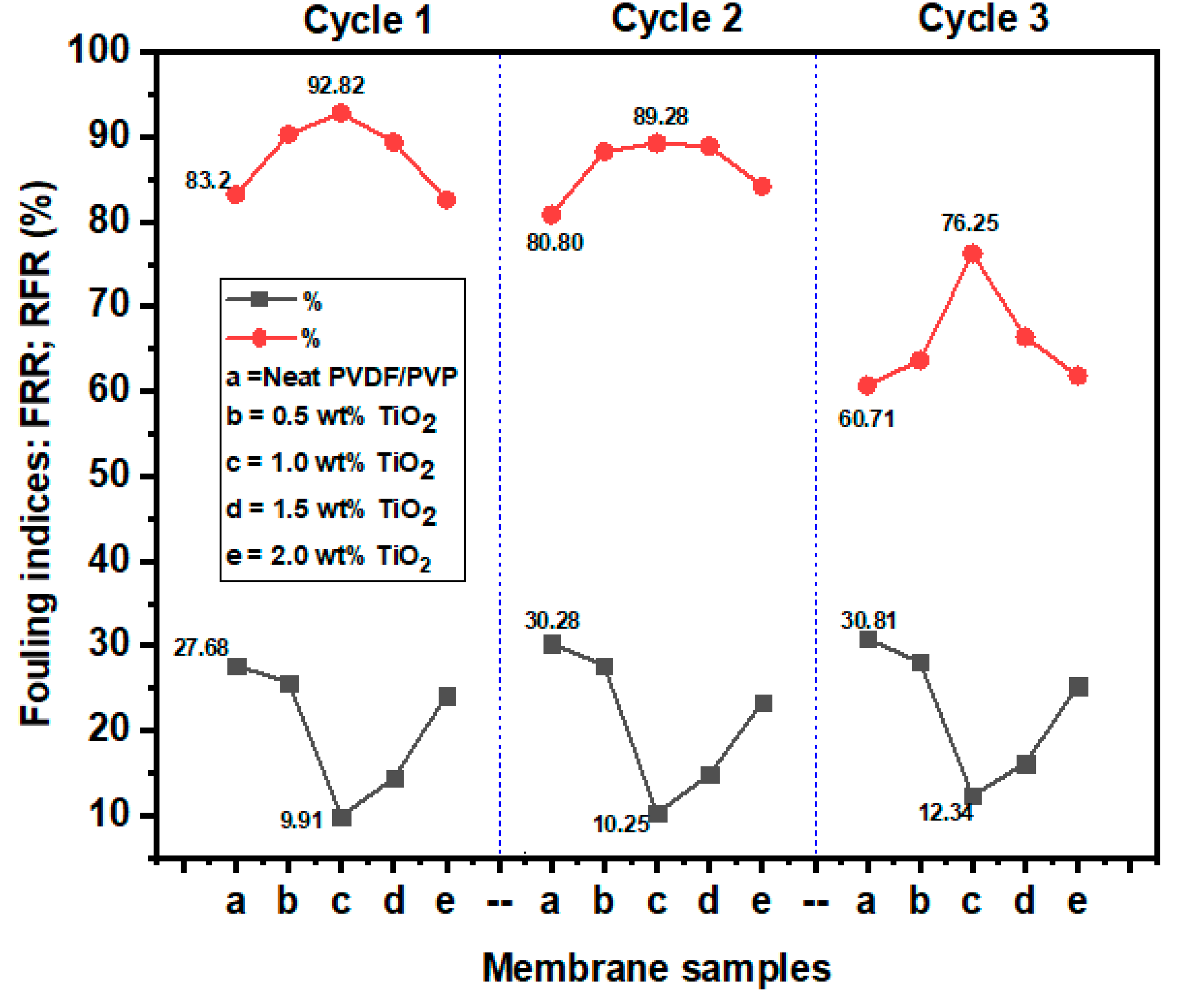
3.2.4. Membrane Fouling Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chianese, A.; Ranauro, R.; Verdone, N. Treatment of Landfill Leachate by Reverse Osmosis. Water Res. 1999, 33, 647–652. [Google Scholar] [CrossRef]
- Aziz, S.Q.; Aziz, H.A.; Yusoff, M.S.; Bashir, M.J.K.; Umar, M. Leachate characterization in semi-aerobic and anaerobic sanitary landfills: A comparative study. J. Environ. Manag. 2010, 91, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Hossini, H.; Rezaee, A.; Ayati, B.; Mahvi, A.H. Optimizing ammonia volatilization by air stripping from aquatic solutions using response surface methodology (RSM). Desalin. Water Treat. 2016, 57, 11765–11772. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Kheriji, J.; Hamrouni, B. Boron removal from brackish water by reverse osmosis and nanofiltration membranes: Application of Spiegler-Kedem model and optimization. Water Sci. Technol. Water Supply 2016, 16, 684–694. [Google Scholar] [CrossRef]
- Lotti, P.; Gatta, G.D.; Demitri, N.; Guastella, G.; Rizzato, S.; Ortenzi, M.A.; Magrini, F.; Comboni, D.; Guastoni, A.; Fernandez-Diaz, M.T. Crystal chemistry and temperature behavior of the natural hydrous borate colemanite, a mineral commodity of boron. Phys. Chem. Miner. 2018, 45, 405–422. [Google Scholar] [CrossRef]
- Guan, Z.; Lv, J.; Bai, P.; Guo, X. Boron removal from aqueous solutions by adsorption—A review. DES 2016, 383, 29–37. [Google Scholar] [CrossRef]
- Kluczka, J.; Korolewicz, T.; Zołotajkin, M.; Adamek, J. Boron removal from water and wastewater using new polystyrene-based resin grafted with glycidol. Water Resour. Ind. 2015, 11, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Patrick, H.; Hening, H. Does only a boron play a structural role in the growing tissues of higher plants. Plant Soil 1997, 196, 211–215. [Google Scholar]
- Fujita, Y.; Hata, T.; Nakamaru, M.; Iyo, T. A study of boron adsorption onto activated sludge. Bioresour. Technol. 2005, 96, 1350–1356. [Google Scholar] [CrossRef]
- Department of Environment Malaysia, M.N.R. Environmental Requirements; Federal Government Administrative Centre 62574: Putrajaya, Malaysia, 2010.
- Xu, Y.; Jiang, J. Technologies for Boron Removal. Ind. Eng. Chem. Res. 2008, 47, 16–24. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Q.; Lyu, J.; Bai, P.; Guo, X. Boron removal and reclamation by magnetic magnetite (Fe3O4) nanoparticle: An adsorption and isotopic separation study. Sep. Purif. Technol. 2020, 231, 115930. [Google Scholar] [CrossRef]
- Foo, K.Y.; Lee, L.K.; Hameed, B.H. Preparation of banana frond activated carbon by microwave induced activation for the removal of boron and total iron from landfill leachate. Chem. Eng. J. 2013, 223, 604–610. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, C.Y.; Quill, A.K.; Simon, B.J.; Shettle, K.B. Mechanisms of Boron Removal with Electrocoagulation. Environ. Chem. 2006, 350–354. [Google Scholar] [CrossRef]
- Savas, A. Electrocoagulation of synthetically prepared waters containing high concentration of NOM using iron cast electrodes. J. Hazard. Mater. 2007, 139, 373–380. [Google Scholar] [CrossRef]
- Yilmaz, A.E.; Boncukcuo, R.; Kocakerim, M.M. A quantitative comparison between electrocoagulation and chemical coagulation for boron removal from boron-containing solution. J. Hazard. Mater. 2007, 149, 475–481. [Google Scholar] [CrossRef]
- Figueras, M.J.; Borrego, J.J. New Perspectives in Monitoring Drinking Water Microbial Quality. Int. J. Environ. Res. Public Health 2010, 7, 4179–4202. [Google Scholar] [CrossRef] [Green Version]
- Man, H.C.; Chin, W.H.; Zadeh, M.R.; Yusof, M.R.M. Adsorption potential of unmodified rice husk for boron removal. BioResources 2012, 7, 3810–3822. [Google Scholar]
- Environ, E.; Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.H.; Goh, P.S.; Ismail, A.F.; Ng, B.C.; Lai, G.S. Decolourization of aerobically treated palm oil mill effluent (AT-POME) using polyvinylidene fluoride (PVDF) ultrafiltration membrane incorporated with coupled zinc-iron oxide nanoparticles. Chem. Eng. J. 2017, 308, 359–369. [Google Scholar] [CrossRef]
- Zinadini, S.; Rostami, S.; Vatanpour, V.; Jalilian, E. Preparation of antibiofouling polyethersulfone mixed matrix NF membrane using photocatalytic activity of ZnO/MWCNTs nanocomposite. J. Membr. Sci. 2017, 529, 133–141. [Google Scholar] [CrossRef]
- Li, J.H.; Shao, X.S.; Zhou, Q.; Li, M.Z.; Zhang, Q.Q. The double effects of silver nanoparticles on the PVDF membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Mauter, M.S.; Okemgbo, K.C.; Osuji, C.O.; Elimelech, M.; Wang, Y.; Giannelis, E.P. Antifouling ultrafiltration membranes via post-fabrication grafting of biocidal nanomaterials. ACS Appl. Mater. Interfaces 2011, 3, 2861–2868. [Google Scholar] [CrossRef]
- Elango, M.; Deepa, M.; Subramanian, R.; Musthafa, A.M. Synthesis, characterization of polyindole/Ag-ZnO nanocomposites and its antibacterial activity. J. Alloys Compd. 2017, 696, 391–401. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ahmad, A.L.; Lim, J.K.; Ooi, B.S. Preparation and characterization of PVDF/TiO2 mixed matrix membrane via in situ colloidal precipitation method. DES 2012, 295, 61–69. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Lau, W.J.; Tan, Y.H.; Ng, B.C.; Ismail, A.F. Hydrophilic hollow fiber PVDF ultrafiltration membrane incorporated with titanate nanotubes for decolourization of aerobically-treated palm oil mill effluent. Chem. Eng. J. 2017, 316, 101–110. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.S.; Chae, S.R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef]
- Chae, H.R.; Lee, J.; Lee, C.H.; Kim, I.C.; Park, P.K. Graphene oxide-embedded thin-film composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance. J. Membr. Sci. 2015, 483, 128–135. [Google Scholar] [CrossRef]
- Abdulsalam, M.; Man, H.C.; Goh, P.S.; Yunos, K.F.; Abidin, Z.Z.; Isma, M.I.A.; Ismail, A.F. Permeability and Antifouling Augmentation of a Hybrid PVDF-PEG Membrane Using Nano-Magnesium Oxide as a Powerful Mediator for POME Decolorization. Polymer 2020, 12, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasrollahi, N.; Vatanpour, V.; Aber, S.; Mahmoodi, N.M. Preparation and characterization of a novel polyethersulfone (PES) ultrafiltration membrane modified with a CuO/ZnO nanocomposite to improve permeability and antifouling properties. Sep. Purif. Technol. 2018, 192, 369–382. [Google Scholar] [CrossRef]
- Yuan, Z.; Dan-Li, X. Porous PVDF/TPU blends asymmetric hollow fiber membranes prepared with the use of hydrophilic additive PVP (K30). Desalination 2008, 223, 438–447. [Google Scholar] [CrossRef]
- Xu, Z.; Chung, T.; Huang, Y.U. Effect of Polyvinylpyrrolidone Molecular Weights on Morphology, Oil/Water Separation, Mechanical and Thermal Properties of Polyetherimide/Polyvinylpyrrolidone Hollow Fiber Membranes. J. Appl. Polym. Sci. 1999, 74, 2220–2233. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.J.; Chou, H.H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ooi, B.S.; Ahmad, A.L. Study on PVDF-TiO2 mixed-matrix membrane behaviour towards humic acid adsorption. J. Water Process Eng. 2017, 15, 99–106. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Tereshchenko, A.; Karpicz, R.; Ratautaite, V.; Bubniene, U.; Maneikis, A.; Jagminas, A.; Ramanavicius, A. TiO2-x/TiO2-Structure Based ‘Self-Heated’ Sensor for the Determination of Some Reducing Gases. Sensors 2020, 20, 74. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, T.; Zhou, Y.; Meng, C.; Zhu, W.; Liu, L. TiO2-Based Nanoheterostructures for Promoting Gas Sensitivity Performance: Designs, Developments, and Prospects. Sensors 2017, 35, 1971. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, N.; Lee, Y.T. Preparation and characterization of PVDF/TiO2 organic-inorganic composite membranes for fouling resistance improvement. J. Membr. Sci. 2009, 345, 13–20. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Yu, Z.; Zhan, Y.; Ma, L.; Zhang, L. Preparation and characterization of a novel PVDF ultrafiltration membrane by blending with TiO2-HNTs nanocomposites. Appl. Surf. Sci. 2016, 371, 624–632. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.; Yu, Y.; Deng, B.; Li, J.; Jin, J. Sol—gel preparation of PAA-g-PVDF/TiO2 nanocomposite hollow fiber membranes with extremely high water flux and improved antifouling property. J. Membr. Sci. 2013, 432, 25–32. [Google Scholar] [CrossRef]
- Fulfillment, I.P.; Hu, W.; Supervisor, T. Fabrication of TiO2-Embedded PVDF Membranes and Their Application in Algae Membrane Bioreactor Systems. Master’s Thesis, University of Missouri, Columbia, MO, USA, 2013. [Google Scholar]
- Fan, L.; Shi, J.; Xi, Y. PVDF-Modified Nafion Membrane for Improved Performance of MFC. Membranes 2020, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, R.; Qian, X. Preparation and characterization of TiO2/g-C3N4/PVDF composite membrane with enhanced physical properties. Membranes 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, T.; Bao, C.; Zhang, J.; Yang, X. Performance and fouling study of asymmetric PVDF membrane applied in the concentration of organic fertilizer by direct contact membrane distillation (DCMD). Membranes 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Huang, S.; Zhang, Y.; Zhao, S. Comparing the antifouling effects of activated carbon and TiO2 in ultrafiltration membrane development. J. Colloid Interface Sci. 2018, 515, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Méricq, J.P.; Mendret, J.; Brosillon, S.; Faur, C. High performance PVDF-TiO2 membranes for water treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Hikku, G.S.; Jeyasubramanian, K.; Vignesh Kumar, S. Nanoporous MgO as self-cleaning and anti-bacterial pigment for alkyd based coating. J. Ind. Eng. Chem. 2017, 52, 168–178. [Google Scholar] [CrossRef]
- Han, B.; Liang, S.; Wang, B.; Zheng, J.; Xie, X.; Xiao, K.; Wang, X.; Huang, X. Simultaneous determination of surface energy and roughness of dense membranes by a modified contact angle method. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 370–376. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, S.-Y.; Sohn, B.-H.; Park, T.H. Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem. J. Membr. Sci. 2003, 211, 157–165. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, J.; Wang, X.; Huo, X.; Xu, Y.; Li, Q.; Wang, L. Improving the hydrophilic and antifouling properties of polyvinylidene fluoride membrane by incorporation of novel nanohybrid GO@SiO2 particles. Chem. Eng. J. 2017, 314, 266–276. [Google Scholar] [CrossRef]
- Jayalakshmi, A.; Kim, I.; Kwon, Y. Suppression of gold nanoparticle agglomeration and its separation via nylon membranes. Chinese J. Chem. Eng. 2017, 25, 931–937. [Google Scholar] [CrossRef]
- Bae, T.H.; Tak, T.M. Effect of TiO2 nanoparticles on fouling mitigation of ultrafiltration membranes for activated sludge filtration. J. Membr. Sci. 2005, 249, 1–8. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, P.; Zheng, Q.; Li, J. The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J. Membr. Sci. 2007, 288, 231–238. [Google Scholar] [CrossRef]
- Lai, S.O. Antifouling Improvement of Polyethersulfone Membrane Incorporated with Negatively Charged Zinc—Iron Oxide for AT-POME Colour Removal. Arab. J. Sci. Eng. 2019, 44. [Google Scholar] [CrossRef]
- Taylor, P.; Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Matsuura, T.; Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; et al. Separation Science and Technology Effect of PVP Molecular Weights on the Properties of PVDF-TiO2 Composite Membrane for Oily Wastewater Treatment Process Effect of PVP Molecular Weights on the Properties of PVDF-TiO2 Composite Membrane for Oily Wastewater treatment Process. Membranes 2014, 37–41. [Google Scholar] [CrossRef]
- Simone, S.; Galiano, F.; Faccini, M.; Boerrigter, M.E.; Chaumette, C.; Drioli, E.; Figoli, A. Preparation and Characterization of Polymeric-Hybrid PES/TiO2 Hollow Fiber Membranes for Potential Applications in Water Treatment. Fibers 2017, 5, 14. [Google Scholar] [CrossRef]
- Liu, C.; Lee, J.; Small, C.; Ma, J.; Elimelech, M. Comparison of organic fouling resistance of thin-film composite membranes modified by hydrophilic silica nanoparticles and zwitterionic polymer brushes. J. Membr. Sci. 2017, 544, 135–142. [Google Scholar] [CrossRef]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO2 hybrid membranes with different dosage of nano-TiO2. J. Membr. Sci. 2012, 389, 522–531. [Google Scholar] [CrossRef]
- Younas, H.; Bai, H.; Shao, J.; Han, Q.; Ling, Y.; He, Y. Super-hydrophilic and fouling resistant PVDF ultrafiltration membranes based on a facile prefabricated surface. J. Membr. Sci. 2017, 541, 529–540. [Google Scholar] [CrossRef]
- Henmi, M.; Fusaoka, Y.; Tomioka, H.; Kurihara, M. High performance RO membranes for desalination and wastewater reclamation and their operation results. Water Sci. Technol. 2010, 62, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.L.K.; Jensen, T.R.; Kucheryavskiy, S.V.; Simonsen, M.E. Investigation of surface energy, wettability and zeta potential of titanium dioxide/graphene oxide membranes. J. Photochem. Photobiol. A Chem. 2018, 366, 162–170. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Davoody, M.; Ismail, A.F. Super hydrophilic TiO2/HNT nanocomposites as a new approach for fabrication of high performance thin film nanocomposite membranes for FO application. Desalination 2015, 371, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Jamshidi Gohari, R.; Halakoo, E.; Nazri, N.A.M.; Lau, W.J.; Matsuura, T.; Ismail, A.F. Improving performance and antifouling capability of PES UF membranes via blending with highly hydrophilic hydrous manganese dioxide nanoparticles. Desalination 2014, 335, 87–95. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Astinchap, B.; Moradian, R. Enhancing antifouling capability of PES membrane via mixing with various types of polymer modified multi-walled carbon nanotube. J. Membr. Sci. 2013, 444, 184–191. [Google Scholar] [CrossRef]


| Composition | Polymer (PVDF) (wt%) | Solvent (DMAC) (wt%) | Additive PVP (wt%) | TiO2 (wt%) |
|---|---|---|---|---|
| Neat | 18.0 | 77.0 | 5.0 | 0.0 |
| Modified (0.5) | 18.0 | 76.5 | 5.0 | 0.5 |
| Modified (1.0) | 18.0 | 76.0 | 5.0 | 1.0 |
| Modified (1.5) | 18.0 | 75.5 | 5.0 | 1.5 |
| Modified (2.0) | 18.0 | 75.0 | 5.0 | 2.0 |
| Parameters | Condition |
|---|---|
| Spinnerets size | 1.15 mm OD/0.55 mm ID |
| Dope extrusion rate | 5 mL/min, 16.67 rpm |
| Bore fluid composition | water |
| Air gap | 5 cm |
| Internal/external coagulant | Water |
| Coagulant bath temperature | Room temperature (25 °C) |
| Collection drum speed | 9 rpm |
| Washing bath | Water |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, H.C.; Abba, M.U.; Abdulsalam, M.; Azis, R.S.; Idris, A.I.; Hamzah, M.H. Utilization of Nano-TiO2 as an Influential Additive for Complementing Separation Performance of a Hybrid PVDF-PVP Hollow Fiber: Boron Removal from Leachate. Polymers 2020, 12, 2511. https://doi.org/10.3390/polym12112511
Man HC, Abba MU, Abdulsalam M, Azis RS, Idris AI, Hamzah MH. Utilization of Nano-TiO2 as an Influential Additive for Complementing Separation Performance of a Hybrid PVDF-PVP Hollow Fiber: Boron Removal from Leachate. Polymers. 2020; 12(11):2511. https://doi.org/10.3390/polym12112511
Chicago/Turabian StyleMan, Hasfalina Che, Mohammed Umar Abba, Mohammed Abdulsalam, Raba’ah Syahidah Azis, Aida Isma Idris, and Muhammad Hazwan Hamzah. 2020. "Utilization of Nano-TiO2 as an Influential Additive for Complementing Separation Performance of a Hybrid PVDF-PVP Hollow Fiber: Boron Removal from Leachate" Polymers 12, no. 11: 2511. https://doi.org/10.3390/polym12112511
APA StyleMan, H. C., Abba, M. U., Abdulsalam, M., Azis, R. S., Idris, A. I., & Hamzah, M. H. (2020). Utilization of Nano-TiO2 as an Influential Additive for Complementing Separation Performance of a Hybrid PVDF-PVP Hollow Fiber: Boron Removal from Leachate. Polymers, 12(11), 2511. https://doi.org/10.3390/polym12112511





