Size-Controlled Transformation of Cu2O into Zero Valent Copper within the Matrix of Anion Exchangers via Green Chemical Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
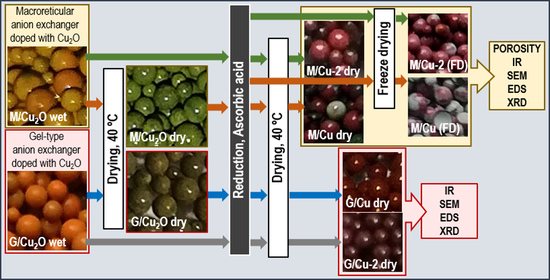
2.2. Synthesis of An/Cu2O
2.3. Synthesis of An/Cu0
2.4. Characterization
3. Results and Discussion
3.1. Optimization of ZVC Content within the Matrix of Anion Exchangers

3.2. Characterization of the Form of Inorganic Deposit
3.2.1. XRD Analysis
3.2.2. FTIR Analysis
3.2.3. Scanning Electron Microscopy Studies
3.2.4. Studies of Porosity
3.3. Operational Control of Physical Form and Copper Deposit Crystallinity of Hybrid Anion Exchangers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- Deka, P.; Borah, B.J.; Saikia, H.; Bharali, P. Cu-based nanoparticles as emerging environmental catalysts. Chem. Rec. 2019, 19, 462–473. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Palza, H.; Nuñez, M.; Bastías, R.; Delgado, K. In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int. J. Antimicrob. Agents 2018, 51, 912–917. [Google Scholar] [CrossRef]
- Ojha, N.K.; Zyryanov, G.V.; Majee, A.; Charushin, V.N.; Chupakhin, O.N.; Santra, S. Copper nanoparticles as inexpensive and efficient catalyst: A valuable contribution in organic synthesis. Coord. Chem. Rev. 2017, 353, 1–57. [Google Scholar] [CrossRef]
- Ali, Z.I.; Ghazy, O.A.; Meligi, G.; Saleh, H.H.; Bekhit, M. Copper nanoparticles: Synthesis, characterization and its application as catalyst for p-nitrophenol reduction. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1195–1205. [Google Scholar] [CrossRef]
- Sharma, P.; Pant, S.; Poonia, P.; Kumari, S.; Dave, V.; Sharma, S. Green synthesis of colloidal copper nanoparticles capped with Tinospora cordifolia and its application in catalytic degradation in textile dye: An ecologically sound approach. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2463–2472. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Y.; Xing, D.; Ren, Z.; Ye, L. Enhanced abatement of organic contaminants by zero-valent copper and sulfite. Environ. Chem. Lett. 2020, 18, 237–241. [Google Scholar] [CrossRef]
- Mott, D.; Galkowski, J.; Wang, L.; Luo, J.; Zhong, C.-J. Synthesis of size-controlled and shaped copper nanoparticles. Langmuir 2007, 23, 5740–5745. [Google Scholar] [CrossRef] [PubMed]
- Biçer, M.; Şişman, İ. Controlled synthesis of copper nano/microstructures using ascorbic acid in aqueous CTAB solution. Powder Technol. 2010, 198, 279–284. [Google Scholar] [CrossRef]
- AL-Thabaiti, S.A.; Obaid, A.Y.; Khan, Z.; Bashir, O.; Hussain, S. Cu nanoparticles: Synthesis, crystallographic characterization, and stability. Colloid Polym. Sci. 2015, 293, 2543–2554. [Google Scholar] [CrossRef]
- Dang, T.M.D.; Le, T.T.T.; Fribourg-Blanc, E.; Dang, M.C. The influence of solvents and surfactants on the preparation of copper nanoparticles by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2. [Google Scholar] [CrossRef]
- Andal, V.; Buvaneswari, G. Effect of reducing agents in the conversion of Cu2O nanocolloid to Cu nanocolloid. Eng. Sci. Technol. Int. J. 2017, 20, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Wang, Y.; Xue, Q.; Wu, X. Synthesis of highly stable dispersions of nanosized copper particles using L-ascorbic acid. Green Chem. 2011, 13, 900–904. [Google Scholar] [CrossRef]
- Jardón-Maximino, N.; Pérez-Alvarez, M.; Sierra-Ávila, R.; Ávila-Orta, C.A.; Jiménez-Regalado, E.; Bello, A.M.; González-Morones, P.; Cadenas-Pliego, G. Oxidation of copper nanoparticles protected with different coatings and stored under ambient conditions. J. Nanomater. 2018, 2018, 9512768. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.S.; Ibrahim, N.A.; Shameli, K.; Zainuddin, N.; Yunus, W.M.Z.W. Copper nanoparticles mediated by chitosan: Synthesis and characterization via chemical methods. Molecules 2012, 17, 14928–14936. [Google Scholar] [CrossRef] [Green Version]
- Biswas, P.; Bandyopadhyaya, R. Demonstration of complete bacterial-killing in water at a very high flow-rate: Use of a surface-enhanced copper nanoparticle with activated carbon as a hybrid. J. Chem. Technol. Biotechnol. 2018, 93, 508–517. [Google Scholar] [CrossRef]
- Olad, A.; Alipour, M.; Nosrati, R. The use of biodegradable polymers for the stabilization of copper nanoparticles synthesized by chemical reduction method. Bull. Mater. Sci. 2017, 40, 1013–1020. [Google Scholar] [CrossRef]
- Ilnicka, A.; Walczyk, M.; Lukaszewicz, J.P.; Janczak, K.; Malinowski, R. Antimicrobial carbon materials incorporating copper nano-crystallites and their PLA composites. J. Appl. Polym. Sci. 2016, 133, 43429. [Google Scholar] [CrossRef]
- Coneo Rodríguez, R.; Yate, L.; Coy, E.; Martínez-Villacorta, Á.M.; Bordoni, A.V.; Moya, S.; Angelomé, P.C. Copper nanoparticles synthesis in hybrid mesoporous thin films: Controlling oxidation state and catalytic performance through pore chemistry. Appl. Surf. Sci. 2019, 471, 862–868. [Google Scholar] [CrossRef]
- Ríos, P.L.; Povea, P.; Cerda-Cavieres, C.; Arroyo, J.L.; Morales-Verdejo, C.; Abarca, G.; Camarada, M.B. Novel in situ synthesis of copper nanoparticles supported on reduced graphene oxide and its application as a new catalyst for the decomposition of composite solid propellants. RSC Adv. 2019, 9, 8480–8489. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Guibal, E.; Quignard, F.; SenGupta, A.K. Polymer-supported metals and metal oxide nanoparticles: Synthesis, characterization, and applications. J. Nanoparticle Res. 2012, 14, 715. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.H.; Wu, J. Organic-inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: A review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.C.; Li, J.; Padungthon, S.; Sengupta, A.K. Nexus between polymer support and metal oxide nanoparticles in hybrid nanosorbent materials (HNMs) for sorption/desorption of target ligands. Front. Environ. Sci. Eng. 2015, 9, 929–938. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, L.; Pan, B.; Zhang, W.; Zhang, S.; Zhang, Q. Polymer-supported nanocomposites for environmental application: A review. Chem. Eng. J. 2011, 170, 381–394. [Google Scholar] [CrossRef]
- Sarkar, S.; Chatterjee, P.K.; Cumbal, L.H.; SenGupta, A.K. Hybrid ion exchanger supported nanocomposites: Sorption and sensing for environmental applications. Chem. Eng. J. 2011, 166, 923–931. [Google Scholar] [CrossRef]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2016, 2, 43–70. [Google Scholar] [CrossRef] [Green Version]
- Khalaj, M.; Kamali, M.; Khodaparast, Z.; Jahanshahi, A. Copper-based nanomaterials for environmental decontamination—An overview on technical and toxicological aspects. Ecotoxicol. Environ. Saf. 2018, 148, 813–824. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Uflyand, I.E. Preparation of metal-polymer nanocomposites by chemical reduction of metal ions: Functions of polymer matrices. J. Polym. Res. 2018, 25, 255. [Google Scholar] [CrossRef]
- Kravchenko, T.A.; Chayka, M.Y.; Bulavina, E.V.; Glotov, A.V.; Yaroslavtsev, A.B. Formation of nanosized copper clusters in ion-exchange matrix. Dokl. Phys. Chem. 2010, 433, 111–114. [Google Scholar] [CrossRef]
- Zolotukhina, E.V.; Kravchenko, T.A. Synthesis and kinetics of growth of metal nanoparticles inside ion-exchange polymers. Electrochim. Acta 2011, 56, 3597–3604. [Google Scholar] [CrossRef]
- Kravchenko, T.A.; Sakardina, E.A.; Kalinichev, A.I.; Zolotukhina, E.V. Stabilization of copper nanoparticles with volume- and surface-distribution inside ion-exchange matrices. Russ. J. Phys. Chem. A 2015, 89, 1648–1654. [Google Scholar] [CrossRef]
- Jacukowicz-Sobala, I.; Kociołek-Balawejder, E.; Stanisławska, E.; Dworniczek, E.; Seniuk, A. Antimicrobial activity of anion exchangers containing cupric compounds against Enterococcus faecalis. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 576, 103–109. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Jacukowicz-Sobala, I.; Baszczuk, A.; Jasiorski, M. Deposition of spherical and bracelet-like Cu2O nanoparticles within the matrix of anion exchangers via reduction of tetrachlorocuprate anions. J. Environ. Chem. Eng. 2020, 8, 103722. [Google Scholar] [CrossRef]
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S.; Imran, M. A green method for the synthesis of copper nanoparticles using l-ascorbic acid. Rev. Mater. 2014, 19, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Wu, S. Preparation of fine copper powder using ascorbic acid as reducing agent and its application in MLCC. Mater. Lett. 2007, 61, 1125–1129. [Google Scholar] [CrossRef]
- Liu, Q.; Yasunami, T.; Kuruda, K.; Okido, M. Preparation of Cu nanoparticles with ascorbic acid by aqueous solution reduction method. Trans. Nonferrous Met. Soc. China 2012, 22, 2198–2203. [Google Scholar] [CrossRef]
- Ramos, A.R.; Tapia, A.K.G.; Piñol, C.M.N.; Lantican, N.B.; del Mundo, M.L.F.; Manalo, R.D.; Herrera, M.U. Effects of reaction temperatures and reactant concentrations on the antimicrobial characteristics of copper precipitates synthesized using L-ascorbic acid as reducing agent. J. Sci. Adv. Mater. Devices 2019, 4, 66–71. [Google Scholar] [CrossRef]
- Feng, Z.; Wei, W.; Wang, L.; Hong, R. Cross-linked PS-DVB/Fe3O4 microspheres with quaternary ammonium groups and application in removal of nitrate from water. RSC Adv. 2015, 5, 96911–96917. [Google Scholar] [CrossRef]
- Shephard, A.B.; Nichols, S.C.; Braithwaite, A. Moisture induced solid phase degradation of L-ascorbic acid part 3, structural characterisation of the degradation products. Talanta 1999, 48, 607–622. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kim, K.; Shin, I.-S.; Shin, K.S. Antioxidative metallic copper nanoparticles prepared by modified polyol method and their catalytic activities. J. Nanoparticle Res. 2020, 22, 8. [Google Scholar] [CrossRef]
- Kawamura, G.; Alvarez, S.; Stewart, I.E.; Catenacci, M.; Chen, Z.; Ha, Y.C. Production of oxidation-resistant Cu-based nanoparticles by wire explosion. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Chen, C.S.; Chen, C.C.; Lai, T.W.; Wu, J.H.; Chen, C.H.; Lee, J.F. Water adsorption and dissociation on Cu nanoparticles. J. Phys. Chem. C 2011, 115, 12891–12900. [Google Scholar] [CrossRef]
- Traboulsi, A.; Dupuy, N.; Rebufa, C.; Sergent, M.; Labed, V. Investigation of gamma radiation effect on the anion exchange resin Amberlite IRA-400 in hydroxide form by Fourier transformed infrared and 13C nuclear magnetic resonance spectroscopies. Anal. Chim. Acta 2012, 717, 110–121. [Google Scholar] [CrossRef]
- Ramanarayanan, T.A.; Alonzo, J. Oxidation of copper and reduction of Cu2O in an environmental scanning electron microscope at 800 °C. Oxid. Met. 1985, 24, 17–27. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Ciechanowska, A. Iron(III) (hydr)oxide loaded anion exchange hybrid polymers obtained via tetrachloroferrate ionic form—Synthesis optimization and characterization. J. Environ. Chem. Eng. 2017, 5, 3354–3361. [Google Scholar] [CrossRef]
- Dzyazko, Y.; Volfkovich, Y.; Perlova, O.; Ponomaryova, L.; Perlova, N.; Kolomiets, E. Effect of porosity on ion transport through polymers and polymer-based composites containing inorganic nanoparticles (Review). In Nanophotonics, Nanooptics, Nanobiotechnology, and Their Applications; Fesenko, O., Yatsenko, L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 235–253. [Google Scholar]
- Kociołek-Balawejder, E.; Stanisławska, E.; Mucha, I. Freeze dried and thermally dried anion exchanger doped with iron(III) (hydr)oxide—Thermogravimetric studies. Thermochim. Acta 2019, 680, 178359. [Google Scholar] [CrossRef]
- Beirowski, B.J.; Gieseler, H. Stabilisation of nanoparticles during freeze drying: The difference to proteins. Eur. Pharm. Rev. 2011, 31, 1–11. [Google Scholar]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]











| Symbol of Solution | Reducing Agent Solution | Potential (mV) | pH |
|---|---|---|---|
| AA/2 | 1 M ascorbic acid | +113 | 2.12 |
| AA/6 | 1 M ascorbic acid in 1 M NaOH | −195 | 6.42 |
| Properties | Symbol of HIX | |
|---|---|---|
| M/An/Cu2O | G/An/Cu2O | |
| Polymeric carrier | Amberlite IRA 900Cl | Amberlite IRA 402OH |
| Physical form | Spherical beads | Spherical beads |
| Polymer matrix | Styrene/divinylbenzene copolymer | Styrene/divinylbenzene copolymer |
| Functional groups | Trimethylammonium | Trimethylammonium |
| Matrix structure | Macroreticular | Gel-like |
| Inorganic deposit | Cu2O | Cu2O |
| Cu content in HIX, wt% | 8.6 | 6.6 |
| Appearance of dispersed Cu2O deposit | Unsymmetrical bracelet-like clusters of a few to 10–20 adjoining spherical objects approximately 200 nm in diameter | Highly symmetrical, single, separate, ideally shaped spheres approximately 1.0 μm in diameter |
| Symbol of HIX | Solution | Temp. (°C) | Reaction Time (h) | Cu Content in An/Cu (wt%) |
|---|---|---|---|---|
| Method 1, dry Cu2O as a precursor of Cu | ||||
| M/An/Cu2O | AA/2 | 20 | 24 | 6.76 |
| 50 | 3 | 7.68 | ||
| AA/6 | 20 | 24 | 6.65 | |
| 50 | 3 | 6.83 | ||
| G/An/Cu2O | AA/2 | 20 | 24 | 4.53 |
| 50 | 3 | 4.50 | ||
| AA/6 | 20 | 24 | 5.35 | |
| 50 | 3 | 5.32 | ||
| Method 2, wet Cu2O as a precursor of Cu | ||||
| M/An/Cu2O | AA/2 | 50 | 0.5 | 6.90 |
| G/An/Cu2O | 5.10 | |||
| Assignment | Wavenumber cm−1 |
|---|---|
| O–H vibrations of hygroscopic water | 1650, 3100–3600 |
| C=C and C–H vibrations of benzene rings and aliphatic groups | 1020, 1111, 1418, 1478, 1512, 1613, 3020 |
| C–H, C–N, CCN vibrations in CH3–N | 707, 826, 859, 889, 926, 975, 1221, 1341, 1383, 2855, 2922 |
| Sample Code | SBET a (m2/g) | VT (Pore Volume) a (cm3/g) | Lm (Pore Diameter) a (nm) | Total Surface Area b (m2/g) | Total Intrusion Volume b (cm3/g) | Porosity b (%) | Bulk Density (g/cm3) |
|---|---|---|---|---|---|---|---|
| Amberlite IRA 900Cl * | |||||||
| M/An | 21.7 | 0.0544 | 5.0 | 60.95 | 0.3945 | 29.84 | 0.877 |
| M/An/FD | 27.8 | 0.0607 | 4.4 | 56.16 | 0.5195 | 37.17 | 0.716 |
| Product of Method 1 | |||||||
| M/An/Cu | 0.13 | 0.001 | Indeterminable | 16.3 | 0.0364 | 4.68 | 1.288 |
| M/An/Cu/FD | 20.8 | 0.040 | 7.7 | 46.2 | 0.443 | 37.3 | 0.842 |
| Product of Method 2 | |||||||
| M/An/Cu-2/FD | 18.4 | 0.037 | 7.9 | 43.5 | 0.390 | 34.6 | 0.886 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacukowicz-Sobala, I.; Stanisławska, E.; Baszczuk, A.; Jasiorski, M.; Kociołek-Balawejder, E. Size-Controlled Transformation of Cu2O into Zero Valent Copper within the Matrix of Anion Exchangers via Green Chemical Reduction. Polymers 2020, 12, 2629. https://doi.org/10.3390/polym12112629
Jacukowicz-Sobala I, Stanisławska E, Baszczuk A, Jasiorski M, Kociołek-Balawejder E. Size-Controlled Transformation of Cu2O into Zero Valent Copper within the Matrix of Anion Exchangers via Green Chemical Reduction. Polymers. 2020; 12(11):2629. https://doi.org/10.3390/polym12112629
Chicago/Turabian StyleJacukowicz-Sobala, Irena, Ewa Stanisławska, Agnieszka Baszczuk, Marek Jasiorski, and Elżbieta Kociołek-Balawejder. 2020. "Size-Controlled Transformation of Cu2O into Zero Valent Copper within the Matrix of Anion Exchangers via Green Chemical Reduction" Polymers 12, no. 11: 2629. https://doi.org/10.3390/polym12112629