Synthesis, Characterization and Cytotoxicity Studies of Aminated Microcrystalline Cellulose Derivatives against Melanoma and Breast Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Tosylation of Cellulose
2.2.2. Subsitution of CTOS with Amines to Synthesize 6-Deoxy-Aminocellulose
2.3. Measurement
3. Results
3.1. Tosylation
3.2. Synthesis of Microcrystalline Cellulose Amine Derivatives
3.3. Biological Evaluation
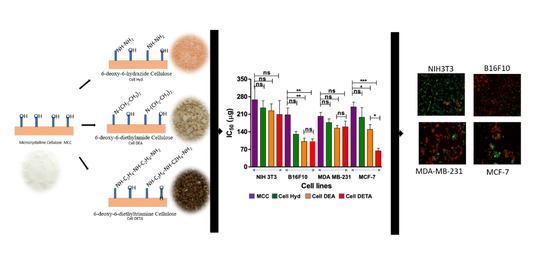
3.3.1. Cytotoxicity
3.3.2. Live Dead Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moku, B.; Ravindar, L.; Rakesh, K.; Qin, H.-L. The significance of N-methylpicolinamides in the development of anticancer therapeutics: Synthesis and structure-activity relationship (SAR) studies. Bioorg. Chem. 2019, 86, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Ohkoshi, E.; Li, L.-H.; Yang, L.; Lee, K.-H. Design, synthesis and cytotoxic activity of novel spin-labeled rotenone derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- El-Saied, H.; Basta, A.; Barsoum, B.N.; Elberry, M.M. Cellulose membranes for reverse osmosis Part I. RO cellulose acetate membranes including a composite with polypropylene. Desalination 2003, 159, 171–181. [Google Scholar] [CrossRef]
- Heinze, T.; Pfeifer, A.; Koschella, A.; Schaller, J.; Meister, F. Solvent-free synthesis of 6-deoxy-6-(ω-aminoalkyl) amino cellulose. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef]
- Bochek, A.M. Effect of Hydrogen Bonding on Cellulose Solubility in Aqueous and Nonaqueous Solvents. Russ. J. Appl. Chem. 2003, 76, 1711–1719. [Google Scholar] [CrossRef]
- Liesiene, J.; Kazlauske, J. Functionalization of cellulose: Synthesis of water-soluble cationic cellulose derivatives. Cell. Chem. Technol. 2013, 47, 515–525. [Google Scholar]
- Malmström, E.; Carlmark, A. Controlled grafting of cellulose fibres—An outlook beyond paper and cardboard. Polym. Chem. 2012, 3, 1702–1713. [Google Scholar] [CrossRef]
- Heinze, T.; Rahn, K. Cellulose-p-toluenesulfonates: A valuable intermediate in cellulose chemistry. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 1997. [Google Scholar]
- Petzold-Welcke, K.; Michaelis, N.; Heinze, T. Unconventional cellulose products through nucleophilic displacement reactions. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2009. [Google Scholar]
- Koschella, A.; Heinze, T. Novel regioselectively 6-functionalized cationic cellulose polyelectrolytes prepared via cellulose sulfonates. Macromol. Biosci. 2001, 1, 178–184. [Google Scholar] [CrossRef]
- Liebert, T.; Hänsch, C.; Heinze, T. Click Chemistry with Polysaccharides. Macromol. Rapid Commun. 2006, 27, 208–213. [Google Scholar] [CrossRef]
- Rafieian, F.; Mousavi, M.; Yu, Q.; Jonoobi, M.; Mousavi, M. Amine functionalization of microcrystalline cellulose assisted by (3-chloropropyl)triethoxysilane. Int. J. Biol. Macromol. 2019, 130, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Siebert, M.; Berlin, P.; Koschella, A. Biofunctional Materials Based on Amino Cellulose Derivatives—A Nanobiotechnological Concept. Macromol. Biosci. 2015, 16, 10–42. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Fukutome, A.; Sannami, Y.; Kamitakahara, H.; Takano, T. Preparation and Evaluation of the Oxidation Ability of Hematin-Appended 6-Amino-6-Deoxycellulose. J. Wood Chem. Technol. 2014, 34, 262–272. [Google Scholar] [CrossRef]
- Demircan, D.; Zhang, B. Facile synthesis of novel soluble cellulose-grafted hyperbranched polymers as potential natural antimicrobial materials. Carbohydr. Polym. 2017, 157, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Obst, M.; Heinze, T. Simple Synthesis of Reactive and Nanostructure Forming Hydrophobic Amino Cellulose Derivatives. Macromol. Mater. Eng. 2015, 301, 65–70. [Google Scholar] [CrossRef]
- Kollár, P.; Závalová, V.; Hošek, J.; Havelka, P.; Sopuch, T.; Karpisek, M.; Třetinová, D.; Suchý, P. Cytotoxicity and effects on inflammatory response of modified types of cellulose in macrophage-like THP-1 cells. Int. Immunopharmacol. 2011, 11, 997–1001. [Google Scholar] [CrossRef]
- Kalita, R.D.; Nath, Y.; Ochubiojo, M.E.; Buragohain, A.K. Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Colloids Surf. B Biointerfaces 2013, 108, 85–89. [Google Scholar] [CrossRef]
- Basta, A.H.; El-Saied, H.; El-Deftar, M.M.; El-Henawy, A.A.; El-Sheikh, H.H.; Abdel-Shakour, E.H.; Hasanin, M.S. Properties of modified carboxymethyl cellulose and its use as bioactive compound. Carbohydr. Polym. 2016, 153, 641–651. [Google Scholar] [CrossRef]
- Jimenez, A.S.; Jaramillo, F.; Hemraz, U.D.; Boluk, Y.; Ckless, K.; Sunasee, R. Effect of surface organic coatings of cellulose nanocrystals on the viability of mammalian cell lines. Nanotechnol. Sci. Appl. 2017, 10, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Her, S.; Keunen, R.; Zhang, S.; Allen, C.; Winnik, M.A. Functionalization of Cellulose Nanocrystals with PEG-Metal-Chelating Block Copolymers via Controlled Conjugation in Aqueous Media. ACS Omega 2016, 1, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Hemraz, U.D.; Campbell, K.A.; Burdick, J.S.; Ckless, K.; Boluk, Y.; Sunasee, R. Cationic Poly(2-aminoethylmethacrylate) and Poly(N-(2-aminoethylmethacrylamide) Modified Cellulose Nanocrystals: Synthesis, Characterization, and Cytotoxicity. Biomacromolecules 2014, 16, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, M.; Shojaei, S.; Safa, K.D.; Ghasemi, Z.; Salehi, R.; Yousefi, B.; Shafiei-Irannejad, V. Biocompatible magnetic tris(2-aminoethyl)amine functionalized nanocrystalline cellulose as a novel nanocarrier for anticancer drug delivery of methotrexate. New J. Chem. 2017, 41, 2160–2168. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; El-Sakhawy, M.; Brun, N.; Hesemann, P.; Kamel, S. New approach for immobilization of 3-aminopropyltrimethoxysilane and TiO2 nanoparticles into cellulose for BJ1 skin cells proliferation. Carbohydr. Polym. 2018, 199, 193–204. [Google Scholar] [CrossRef]
- EL-Sayed, N.S.; El-Ziaty, A.K.; El-Meligy, M.G.; Nagieb, Z.A. Syntheses of New Antimicrobial Cellulose Materials Based 2-((2-aminoethyl)amino)-4-aryl-6-indolylnicotinonitriles. Egypt. J. Chem. 2017, 60, 465–477. [Google Scholar]
- Schmidt, S.; Liebert, T.; Heinze, T. Synthesis of soluble cellulose tosylates in an eco-friendly medium. Green Chem. 2014, 16, 1941–1946. [Google Scholar] [CrossRef]
- Rahn, K.; Diamantoglou, M.; Klemm, D.; Berghmans, H.; Heinze, T. Homogeneous synthesis of cellulose p-toluenesulfonates in N,N-dimethylacetamide/LiCl solvent system. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1996, 238, 143–163. [Google Scholar] [CrossRef]
- Elchinger, P.-H.; Faugeras, P.-A.; Zerrouki, C.; Montplaisir, D.; Brouillette, F.; Zerrouki, R. Tosylcellulose synthesis in aqueous medium. Green Chem. 2012, 14, 3126–3131. [Google Scholar] [CrossRef]
- McCormick, C.L.; Dawsey, T.R.; Newman, J. Competitive formation of cellulose p-toluenesulfonate and chlorodeoxycellulose during homogeneous reaction of p-toluenesulfonyl chloride with cellulose in N,N-dimethylacetamide-lithium chloride. Carbohydr. Res. 1990, 208, 183–191. [Google Scholar] [CrossRef]
- Beroual, M.; Boumaza, L.; Mehelli, O.; Trache, D.; Tarchoun, A.F.; Khimeche, K. Physicochemical Properties and Thermal Stability of Microcrystalline Cellulose Isolated from Esparto Grass Using Different Delignification Approaches. J. Polym. Environ. 2020, 1–13. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; El-Aziz, M.A.; Kamel, S.; Turky, G. Synthesis and characterization of polyaniline/tosylcellulose stearate composites as promising semiconducting materials. Synth. Met. 2018, 236, 44–53. [Google Scholar] [CrossRef]
- Furuhata, K.-I.; Ikeda, H. Ionic cellulose derivatives: Synthesis of sodium 6-deoxycellulose-6-sulfonate with high degree of substitution. React. Funct. Polym. 1999, 42, 103–109. [Google Scholar] [CrossRef]
- Klemm, D.; Schmauder, H.; Heinze, T. Cellulose. Polym. News 2002, 27, 84–89. [Google Scholar]
- Pfeifer, A.; Gericke, M.; Heinze, T. Synthesis and characterization of novel water-soluble 6-deoxy-6-(2-amino-2-(hydroxymethyl)propane-1,3-diol)cellulose derivatives. Adv. Ind. Eng. Polym. Res. 2020, 3, 77–82. [Google Scholar] [CrossRef]
- Zieger, M.; Wurlitzer, M.; Wiegand, C.; Reddersen, K.; Finger, S.; Elsner, P.; Laudeley, P.; Liebert, T.F.; Heinze, T.; Hipler, U.-C. 6-Deoxy-6-aminoethyleneamino cellulose: Synthesis and study of hemocompatibility. J. Biomater. Sci. Polym. Ed. 2015, 26, 931–946. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Wang, Y.; Huang, Y.-H.; Bian, J.; Li, M.-F.; Peng, F.; Sun, R.-C. Benzoxazine enhanced amino cellulose-based composite films: Preparation, proposed mechanism, and improved performance. Carbohydr. Polym. 2019, 222, 115008. [Google Scholar] [CrossRef]
- Pretsch, P.D.E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Guan, Y.; Chen, J.; Qi, X.; Chen, G.; Peng, F.; Sun, R. Fabrication of Biopolymer Hydrogel Containing Ag Nanoparticles for Antibacterial Property. Ind. Eng. Chem. Res. 2015, 54, 7393–7400. [Google Scholar] [CrossRef]
- Harada, K.; Morishita, H. The Proton NMR Spectra of Dimethyl, Methylethyl, and Diethyl Ketone and Dimethyl, and Diethyl Amine. Spectrosc. Lett. 1977, 10, 49–55. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, Z.; Lin, X.; Ren, Z.; Li, B.; Zhang, Y. Preparation and characterization of microcrystalline cellulose (MCC) from tea waste. Carbohydr. Polym. 2018, 184, 164–170. [Google Scholar] [CrossRef]
- Gaspar, D.; Fernandes, S.N.; De Oliveira, A.G.; Fernandes, J.G.; Grey, P.; Pontes, R.V.; Pereira, L.; Martins, R.; Godinho, M.H.; Fortunato, E. Nanocrystalline cellulose applied simultaneously as the gate dielectric and the substrate in flexible field effect transistors. Nanotechnolgy 2014, 25, 094008. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Uniyal, V.; Naithani, S. Polymorphic transformation of cellulose I to cellulose II by alkali pretreatment and urea as an additive. Carbohydr. Polym. 2013, 94, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Katahira, R.; Himmel, M.E.; Johnson, D.K. Effects of alkaline or liquid-ammonia treatment on crystalline cellulose: Changes in crystalline structure and effects on enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezerra, R.D.S.; Leal, R.C.; Da Silva, M.S.; Morais, A.I.S.; Marques, T.H.C.; Osajima, J.A.; Meneguin, A.B.; Barud, H.S.; Filho, E.C.D.S. Direct Modification of Microcrystalline Cellulose with Ethylenediamine for Use as Adsorbent for Removal Amitriptyline Drug from Environment. Molecules 2017, 22, 2039. [Google Scholar] [CrossRef] [Green Version]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Krumm, B. New insensitive nitrogen-rich energetic polymers based on amino-functionalized cellulose and microcrystalline cellulose: Synthesis and characterization. Fuel 2020, 277, 118258. [Google Scholar] [CrossRef]
- Filho, E.C.D.S.; Santana, S.A.A.; Melo, J.C.P.; Oliveira, F.J.V.E.; Airoldi, C. X-ray diffraction and thermogravimetry data of cellulose, chlorodeoxycellulose and aminodeoxycellulose. J. Therm. Anal. Calorim. 2009, 100, 315–321. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Chelouche, S.; Khimeche, K. New Insights on the Compatibility of Nitrocellulose with Aniline-Based Compounds. Propellants Explos. Pyrotech. 2019, 44, 970–979. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F. Differentiation of stabilized nitrocellulose during artificial aging: Spectroscopy methods coupled with principal component analysis. J. Chemom. 2019, 33, e3163. [Google Scholar] [CrossRef]
- Kunusa, W.R.; Isa, I.; Laliyo, L.A.R.L.; Iyabu, H. FTIR, XRD and SEM Analysis of Microcrystalline Cellulose (MCC) Fibers from Corncorbs in Alkaline Treatment. J. Physics: Conf. Ser. 2018, 1028. [Google Scholar] [CrossRef] [Green Version]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Derradji, M.; Bessa, W. Ecofriendly isolation and characterization of microcrystalline cellulose from giant reed using various acidic media. Cellulose 2019, 26, 7635–7651. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nima, J.; Divya, P.L. Synthesis, characterization and in vitro cytotoxicity analysis of a novel cellulose based drug carrier for the controlled delivery of 5-fluorouracil, an anticancer drug. Appl. Surf. Sci. 2015, 355, 64–73. [Google Scholar] [CrossRef]
- Jelkmann, M.; Menzel, C.; Baus, R.A.; Ausserhofer, P.; Baecker, D.; Gust, R.; Bernkop-Schnürch, A.; Ausserhofer, P. Chitosan: The One and Only? Aminated Cellulose as an Innovative Option for Primary Amino Groups Containing Polymers. Biomacromolecules 2018, 19, 4059–4067. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, S.P.; Zaman, M.; Mohammed, N.; Brinatti, C.; Batmaz, R.; Berry, R.; Loh, W.; Tam, K.C. Synthesis of amine functionalized cellulose nanocrystals: Optimization and characterization. Carbohydr. Res. 2015, 409, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Songsurang, K.; Siraleartmukul, K.; Muangsin, N. Mucoadhesive drug carrier based on functional-modified cellulose as poorly water-soluble drug delivery system. J. Microencapsul. 2015, 32, 450–459. [Google Scholar] [CrossRef]
- Fallah, Z.; Isfahani, H.N.; Tajbakhsh, M.; Tashakkorian, H.; Amouei, A. TiO2-grafted cellulose via click reaction: An efficient heavy metal ions bioadsorbent from aqueous solutions. Cellulose 2017, 25, 639–660. [Google Scholar] [CrossRef]
- Filho, E.C.D.S.; De Melo, J.C.; Airoldi, C. Preparation of ethylenediamine-anchored cellulose and determination of thermochemical data for the interaction between cations and basic centers at the solid/liquid interface. Carbohydr. Res. 2006, 341, 2842–2850. [Google Scholar] [CrossRef]
- Finger, S.; Zieger, M.; Wiegand, C.; Liebert, T.F.; Heinze, T.; Elsner, P.; Hipler, U.-C. Biocompatibility and Antibacterial Effects of 6-Deoxy-6-Aminoethyleneamino Cellulose. J. Biosci. Med. 2018, 6, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Abercrombie, M.; Ambrose, E.J. The surface properties of cancer cells: A review. Cancer Res. 1962, 22 Pt 1, 525–548. [Google Scholar]
- Loos, C.; Syrovets, T.; Musyanovych, A.; Mailänder, V.; Landfester, K.; Nienhaus, G.U.; Simmet, T. Functionalized polystyrene nanoparticles as a platform for studying bio–nano interactions. Beilstein J. Nanotechnol. 2014, 5, 2403–2412. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Jiang, C.; Wu, L.; Bai, X.; Zhai, S. Cytotoxicity-Related Bioeffects Induced by Nanoparticles: The Role of Surface Chemistry. Front. Bioeng. Biotechnol. 2019, 7, 414. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wu, P.; Shen, X.-L.; Wei, X.-Y.; Jiang, Z.-H. Synthesis, cytotoxic activity and drug combination study of tertiary amine derivatives of 2′,4′-dihydroxyl-6′-methoxyl-3′,5′-dimethylchalcone. RSC Adv. 2017, 7, 48031–48038. [Google Scholar] [CrossRef] [Green Version]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
- Bortner, C.D.; Cidlowski, J.A. Apoptotic volume decrease and the incredible shrinking cell. Cell Death Differ. 2002, 9, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Mahdzir, M.A.; Yusoff, M.; Arshad, N.M.; Awang, K.; Nagoor, N.H. Cytotoxic Effects of Pinnatane A Extracted from Walsura pinnata (Meliaceae) on Human Liver Cancer Cells. Molecules 2018, 23, 2733. [Google Scholar] [CrossRef] [Green Version]







| Cell Lines | Carbon (Cal.) | Carbon (Found) | Hydrogen (Cal.) | Hydrogen (Found) | Nitrogen (Cal.) | Nitrogen (Found) | Sulphur (Cal.) | Sulphur (Found) |
|---|---|---|---|---|---|---|---|---|
| MCC | 44.31 | 40.85 | 6.51 | 6.34 | 0.15 | |||
| CTOS | 47.59 | 46.05 | 5.68 | 6.05 | 6.69 | 4.8 | ||
| Cell Hyd | 42.48 | 41.34 | 6.83 | 6.64 | 8.26 | 7.95 | 0.55 | |
| Cell DEA | 50.52 | 46.29 | 7.95 | 6.71 | 3.68 | 3.25 | 1.01 | |
| Cell DETA | 46.82 | 44.73 | 7.86 | 7.24 | 10.24 | 9.55 | 0.77 |
| Compounds | Zeta Potential ζ |
|---|---|
| MCC | −4.24 |
| Cell Hyd | +32.8 |
| Cell DEA | +13.9 |
| Cell DETA | +27 |
| Cell Lines | NIH3T3 | B16F10 | MDA-MB-231 | MCF-7 |
|---|---|---|---|---|
| MCC | 263 ± 13.5 | 207 ± 12.0 | 201.6 ± 11.8 | 238.9 ± 10.9 |
| Cell Hyd | 231.9 ±11.5 | 130.5 ± 11.2 | 176.5 ± 11.5 | 195.5 ± 10.8 |
| Cell DEA | 222.3 ± 11.3 | 103.64 ± 9.9 | 154.6 ± 7.4 | 148.8 ± 11.2 |
| Cell DETA | 202.6 ± 10.35 | 101.5 ± 6.8 | 158.5 ± 5.5 | 74.92 ± 9.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, F.; Iqbal, M. Synthesis, Characterization and Cytotoxicity Studies of Aminated Microcrystalline Cellulose Derivatives against Melanoma and Breast Cancer Cell Lines. Polymers 2020, 12, 2634. https://doi.org/10.3390/polym12112634
Nazir F, Iqbal M. Synthesis, Characterization and Cytotoxicity Studies of Aminated Microcrystalline Cellulose Derivatives against Melanoma and Breast Cancer Cell Lines. Polymers. 2020; 12(11):2634. https://doi.org/10.3390/polym12112634
Chicago/Turabian StyleNazir, Farzana, and Mudassir Iqbal. 2020. "Synthesis, Characterization and Cytotoxicity Studies of Aminated Microcrystalline Cellulose Derivatives against Melanoma and Breast Cancer Cell Lines" Polymers 12, no. 11: 2634. https://doi.org/10.3390/polym12112634
APA StyleNazir, F., & Iqbal, M. (2020). Synthesis, Characterization and Cytotoxicity Studies of Aminated Microcrystalline Cellulose Derivatives against Melanoma and Breast Cancer Cell Lines. Polymers, 12(11), 2634. https://doi.org/10.3390/polym12112634