Build-To-Specification Vanillin and Phloroglucinol Derived Biobased Epoxy-Amine Vitrimers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
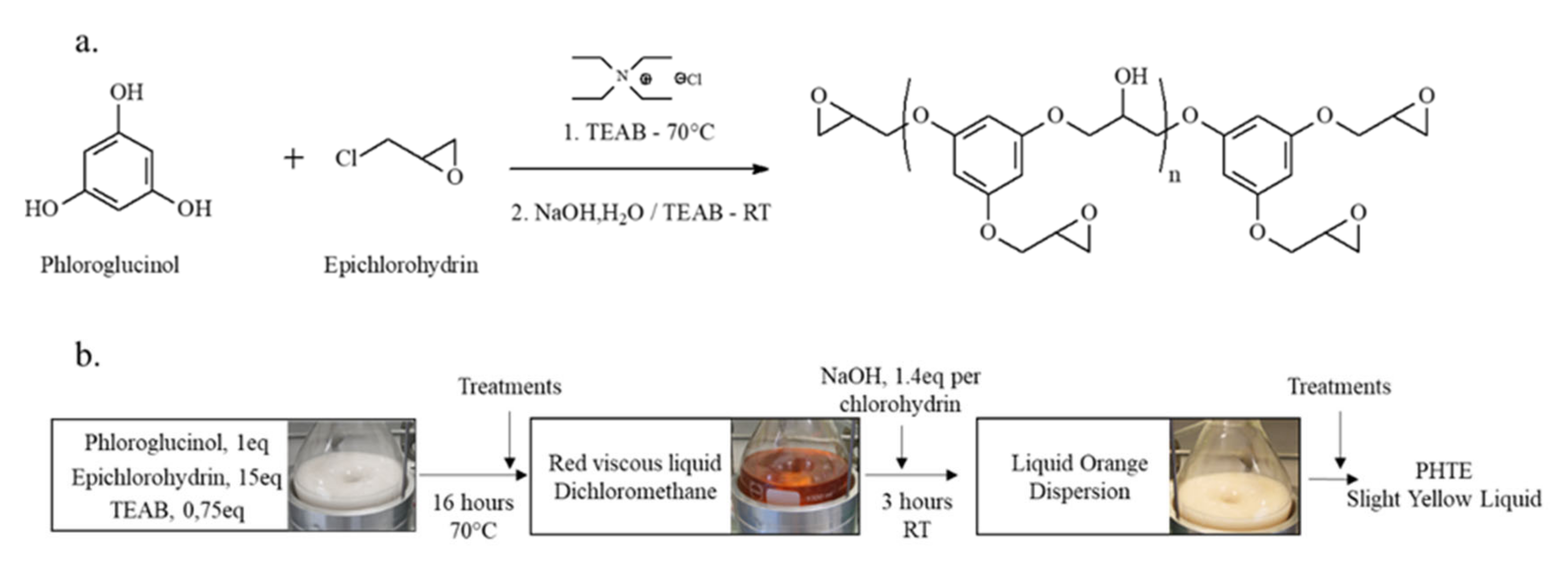
2.3. Synthesis of Biobased Epoxy Resins and Vitrimers
2.3.1. Synthesis of DGEVA
2.3.2. Synthesis of PHTE
2.3.3. Synthesis of Epoxy-Vitrimers
3. Results and Discussion
3.1. Thermal and Mechanical Characterization of the Pristine Material
3.1.1. Thermal Properties
3.1.2. Mechanical Properties
3.1.3. Crosslink Density
3.1.4. Dynamic Properties
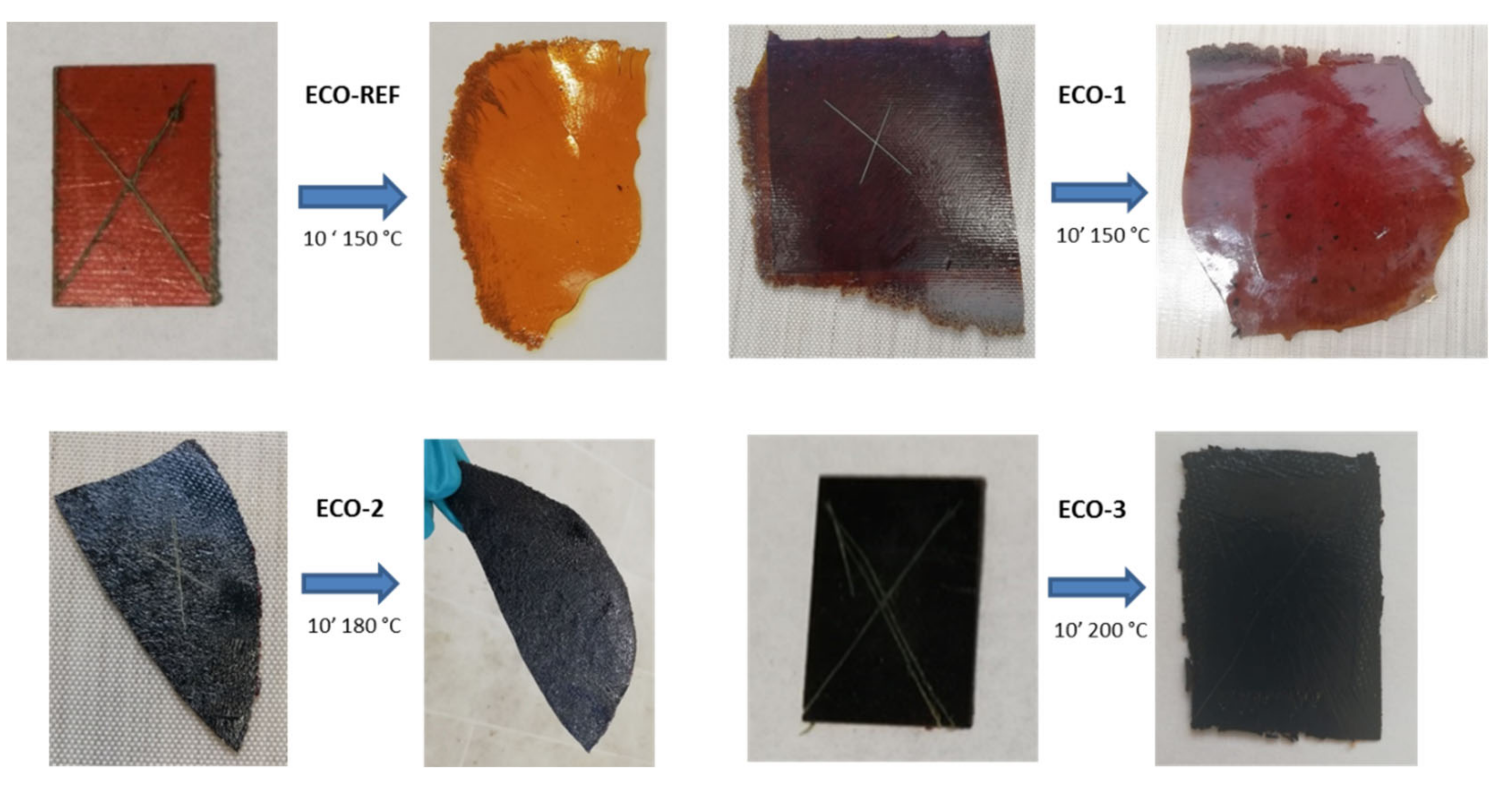
- Reprocessability
- Repairability
- Reciclability
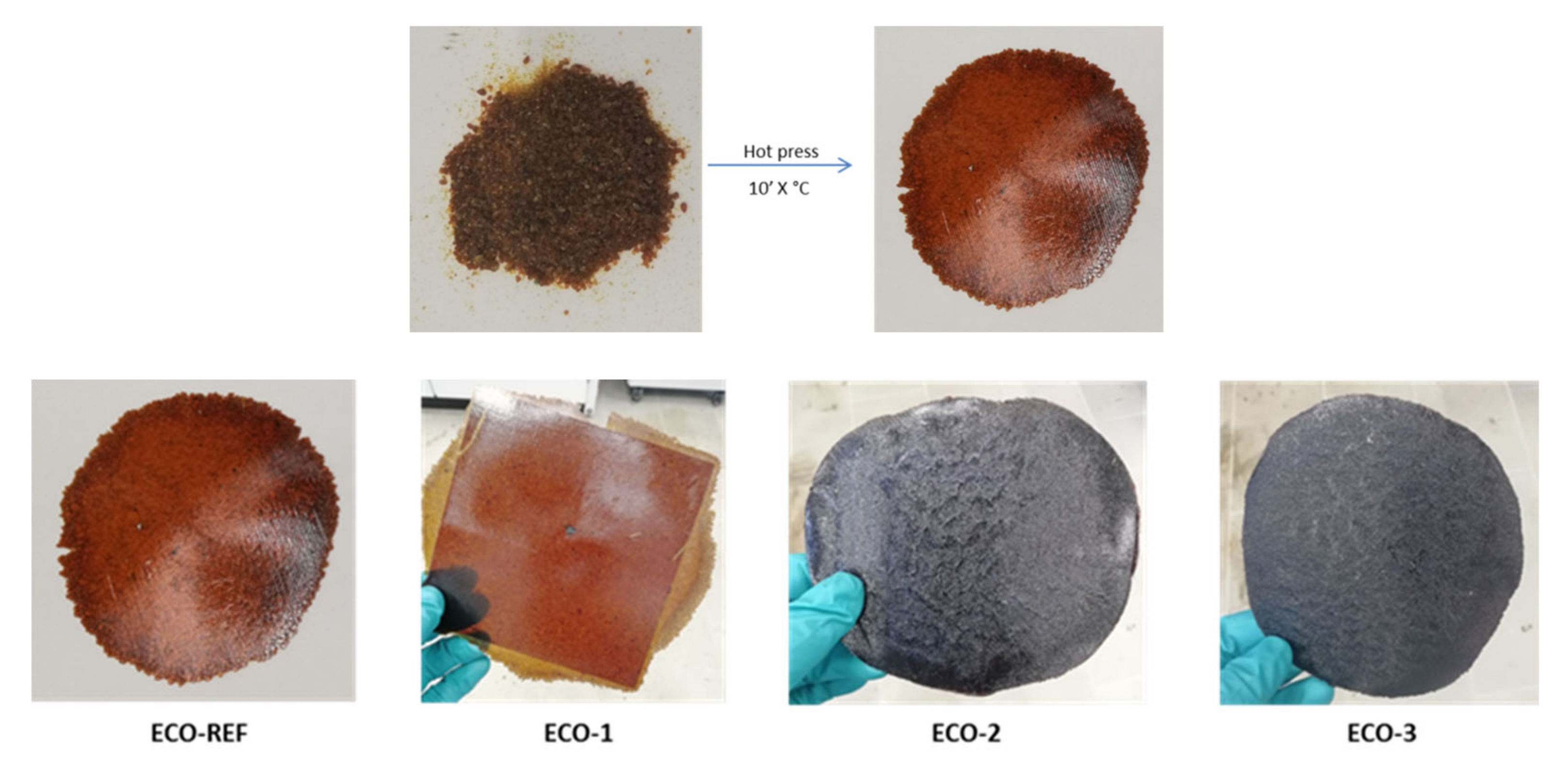
3.2. Thermal and Mechanical Characterization of the Recycled Material
Thermomechanical Properties Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vozzola, E.; Overcash, M.; Twomey, J.; Griffing, E.; Asmatulu, E. Thermoset composite recycling—Driving forces, development, and evolution of new opportunities. J. Compos. Mater. 2017, 52, 1033–1043. [Google Scholar]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [PubMed] [Green Version]
- Addicoat, M.A.; Tsotsalas, M. Covalently linked organic networks. Front. Mater. 2015, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Alabiso, W. The Impact of Vitrimers on the Industry of the Future: Chemistry, Properties and Sustainable Forward-Looking Applications. Polymers 2020, 12, 1660. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M. Implantation of Recyclability and Healability into Cross-Linked Commercial Polymers by Applying the Vitrimer Concept. Polymers 2020, 12, 1322. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmed, N.; Rabnawaz, M. Covalent Adaptable Network and Self-Healing Materials: Current Trends and Future Prospects in Sustainability. Polymers 2020, 12, 2027. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Feng, Y.; He, H.; Yang, Z. Preparation and Characteristics of Biocomposites Based on Steam Exploded Sisal Fiber Modified with Amphiphatic Epoxidized Soybean Oil Resin. Materials 2018, 11, 1731. [Google Scholar] [CrossRef] [Green Version]
- Garcia-garcia, D.; Id, R.B. Manufacturing and Characterization of Composite Fibreboards with Posidonia oceanica Wastes with an Environmentally-Friendly Binder from Epoxy Resin. Materials 2018, 11, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.P. Biobased thermosetting epoxy: Present and future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin, a key-intermediate of biobased polymers. Eur. Polym. J. 2015, 68, 488–502. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkita, T. Fully biobased epoxy resin systems composed of a vanillin-derived epoxy resin and renewable phenolic hardeners. Eur. Polym. J. 2017, 92, 165–173. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Hamidi, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naebe, M. A renewable bio-based epoxy resin with improved mechanical performance that can compete with DGEBA. RSC Adv. 2017, 7, 8694–8701. [Google Scholar] [CrossRef] [Green Version]
- Frost, J.W. Biosynthesis of Philoroglucinol and Preparation of 1,3-dihydroxybenzene Therefrom. U.S. Patent 8,329,445B2, 27 April 2006. [Google Scholar]
- Noè, C.; Malburet, S.; Bouvet-Marchand, A.; Graillot, A.; Loubat, C.; Sangermano, M. Cationic photopolymerization of bio-renewable epoxidized monomers. Prog. Org. Coat. 2019, 133, 131–138. [Google Scholar] [CrossRef]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: From Old Chemistry to Modern Day Innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef] [PubMed]
- Ciarella, S.; Wouter, G. Ellenbroek. Swap-Driven Self-Adhesion and Healing of Vitrimers. Coatings 2019, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- De Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- De Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Correction: Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horiz. 2020, 7, 2460–2461. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]







| Sample Name | DGEVA/PHTE Ratio (wt) | DGEVA | PHTE | SUPERSAP CLR | 4-AFD | Curing Cycle |
|---|---|---|---|---|---|---|
| ECO-1 | 100/0 | 30 g, 0.22 epoxy eq. | - | - | 16.14 g, 0.26 amino eq. | 1 h 150 °C |
| ECO-2 | 60/40 | 18 g, 0.13 epoxy eq. | 12 g, 0.08 epoxy eq. | - | 15.52 g, 0.25 amino eq. | 1 h 150 °C |
| ECO-3 | 40/60 | 12 g, 0.09 epoxy eq. | 18 g, 0.12 epoxy eq. | - | 15.52 g, 0.25 amino eq. | 1 h 160 °C |
| ECO-4 | 0/100 | - | 30 g, 0.20 epoxy eq. | - | 14.90 g, 0.24 amino eq. | 1 h 150 °C + 30′ 180 °C |
| ECO-REF | - | - | - | 30 g, 0.16 epoxy eq. | 11.80 g, 0.19 amino eq | 1 h 150 °C |
| Ref. | TgDSC (°C) | TgDMA (°C) | Td5% (°C) | Stress a (MPa) | Strain a (%) | E’ (30 °C) (GPa) | E’ (at Tg + 30 °C) (MPa) | νXL b·103 (mol·cm−3) |
|---|---|---|---|---|---|---|---|---|
| ECO-1 | 105 | 103 | 259 | 86 ± 1 | 7 ± 1 | 6 | 17 | 1.7 |
| ECO-2 | 135 | 137 | 251 | 105 ± 2 | 7 ± 0.6 | 9 | 28 | 2.6 |
| ECO-3 | 157 | 160 | 257 | 94 ± 4 | 5 ± 0.4 | 7 | 41 | 3.6 |
| ECO-4 | 194 | 197 | 239 | 92 ± 6 | 5 ± 0.3 | 11 | 83 | 6.7 |
| ECO-REF | 107 | 108 | 252 | 66 ± 10 | 4 ± 1 | 7 | 16 | 1.6 |
| Ref. | Relaxation Time (s) | |
|---|---|---|
| Tg + 20 °C | Tg + 50 °C | |
| ECO-1 | It does not relax (at 120 °C) | 100 s (at 150 °C) |
| ECO-2 | It does not relax (at 150 °C) | 163 s (at 180 °C) |
| ECO-3 | 215 s (at 170 °C) | 35 s (at 200 °C) |
| ECO-4 | 359 s (at 210 °C–too high T) | Too high T (240 °C) |
| ECO-REF | It does not relax (at 120 °C) | 212 s (at 150 °C) |
| Ref. | Initial TgDSC (°C) | TgDSC after 20 min at 200 °C (°C) | TgDSC after 20 min at 230 °C (°C) |
|---|---|---|---|
| ECO-1 | 105 | 97 | 95 |
| ECO-2 | 134 | 131 | 129 |
| ECO-3 | 156 | 152 | 137 |
| ECO-4 | 194 | 177 | 162 |
| ECO-REF | 107 | 102 | 92 |
| Ref. | Initial TgDSC (°C) | Recycled Tg DSC (°C) | Initial TgDMA (°C) | Recycled TgDMA (°C) | Initial Td5% (°C) | Recycled Td5% (°C) |
|---|---|---|---|---|---|---|
| ECO-1 | 105 | 106 | 103 | 103 | 259 | 255 |
| ECO-2 | 135 | 149 | 137 | 146 | 251 | 255 |
| ECO-3 | 157 | 165 | 160 | 169 | 257 | 255 |
| ECO-REF | 107 | 104 | 108 | 107 | 252 | 249 |
| Ref. | Initial E’ (30 °C) (GPa) | Recycled E’ (30 °C) (GPa) | Initial E’ (Tg + 30 °C) (MPa) | Recycled E’ (Tg + 30 °C) (MPa) | Initial νXL a·103 (mol·cm−3) | Recycled νXL a·103 (mol·cm−3) |
|---|---|---|---|---|---|---|
| ECO-1 | 6 | 4 | 17 | 20 | 1.7 | 2.0 |
| ECO-2 | 9 | 10 | 28 | 26 | 2.6 | 2.3 |
| ECO-3 | 7 | 4 | 41 | 54 | 3.6 | 4.6 |
| ECO-REF | 7 | 3 | 16 | 16 | 1.6 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genua, A.; Montes, S.; Azcune, I.; Rekondo, A.; Malburet, S.; Daydé-Cazals, B.; Graillot, A. Build-To-Specification Vanillin and Phloroglucinol Derived Biobased Epoxy-Amine Vitrimers. Polymers 2020, 12, 2645. https://doi.org/10.3390/polym12112645
Genua A, Montes S, Azcune I, Rekondo A, Malburet S, Daydé-Cazals B, Graillot A. Build-To-Specification Vanillin and Phloroglucinol Derived Biobased Epoxy-Amine Vitrimers. Polymers. 2020; 12(11):2645. https://doi.org/10.3390/polym12112645
Chicago/Turabian StyleGenua, Aratz, Sarah Montes, Itxaso Azcune, Alaitz Rekondo, Samuel Malburet, Bénédicte Daydé-Cazals, and Alain Graillot. 2020. "Build-To-Specification Vanillin and Phloroglucinol Derived Biobased Epoxy-Amine Vitrimers" Polymers 12, no. 11: 2645. https://doi.org/10.3390/polym12112645
APA StyleGenua, A., Montes, S., Azcune, I., Rekondo, A., Malburet, S., Daydé-Cazals, B., & Graillot, A. (2020). Build-To-Specification Vanillin and Phloroglucinol Derived Biobased Epoxy-Amine Vitrimers. Polymers, 12(11), 2645. https://doi.org/10.3390/polym12112645








