Switching from Electron to Hole Transport in Solution-Processed Organic Blend Field-Effect Transistors
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Role of Rotation Speed
3.2. Impact of Solution Temperature
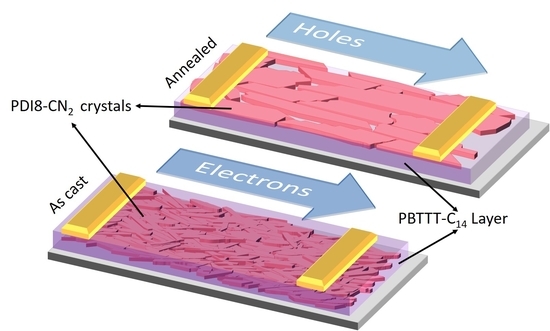
3.3. Effect of Thermal Annealing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yi, H.T.; Payne, M.M.; Anthony, J.E.; Podzorov, V. Ultra-flexible solution-processed organic field-effect transistors. Nat. Commun. 2012, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Diao, Y.; Tee, B.C.K.; Giri, G.; Xu, J.; Kim, D.H.; Becerril, H.A.; Stoltenberg, R.M.; Lee, T.H.; Xue, G.; Mannsfeld, S.C.B.; et al. Solution coating of large-area organic semiconductor thin films with aligned single-crystalline domains. Nat. Mater. 2013, 12, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.Y.; Subramanian, V. All inkjet-printed, fully self-aligned transistors for low-cost circuit applications. Org. Electron. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Zhou, K.; Dong, H.; Zhang, H.L.; Hu, W. High performance n-type and ambipolar small organic semiconductors for organic thin film transistors. Phys. Chem. Chem. Phys. 2014, 16, 22448–22457. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hu, W. The Emergence of Organic Single-Crystal Electronics. Angew. Chemie-Int. Ed. 2020, 59, 1408–1428. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Xu, X.; You, P.; Wang, N.; Wang, J.; Miao, Q.; Yan, F. Efficiency enhancement of organic photovoltaics by introducing high-mobility curved small-molecule semiconductors as additives. J. Mater. Chem. A 2019, 7, 12740–12750. [Google Scholar] [CrossRef]
- Dai, F.; Liu, X.; Yang, T.; Qian, J.; Li, Y.; Gao, Y.; Xiong, P.; Ou, H.; Wu, J.; Kanehara, M.; et al. Fabrication of two-dimensional crystalline organic films by tilted spin coating for high-performance organic field-effect transistors. ACS Appl. Mater. Interfaces 2019, 11, 7226–7234. [Google Scholar] [CrossRef]
- Trefz, D.; Gross, Y.M.; Dingler, C.; Tkachov, R.; Hamidi-Sakr, A.; Kiriy, A.; McNeill, C.R.; Brinkmann, M.; Ludwigs, S. Tuning Orientational Order of Highly Aggregating P(NDI2OD-T2) by Solvent Vapor Annealing and Blade Coating. Macromolecules 2019, 52, 43–54. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, J.; Ai, C.; Wen, D.; Bai, X. Enhancement of memory margins in the polymer composite of [6,6]-phenyl-C 61 -butyric acid methyl ester and polystyrene. Phys. Chem. Chem. Phys. 2016, 18, 30808–30814. [Google Scholar] [CrossRef]
- Ghittorelli, M.; Lenz, T.; Sharifi Dehsari, H.; Zhao, D.; Asadi, K.; Blom, P.W.M.; Kovács-Vajna, Z.M.; De Leeuw, D.M.; Torricelli, F. Quantum tunnelling and charge accumulation in organic ferroelectric memory diodes. Nat. Commun. 2017, 8, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Zhang, S.; Xu, Z.; Chen, X.; Li, L. Solution-Processed Organic Complementary Inverters Based on TIPS-Pentacene and PDI8-CN2. IEEE Trans. Electron Devices 2015, 62, 4220–4224. [Google Scholar] [CrossRef]
- Li, M.; Mangalore, D.K.; Zhao, J.; Carpenter, J.H.; Yan, H.; Ade, H.; Yan, H.; Müllen, K.; Blom, P.W.M.; Pisula, W.; et al. Integrated circuits based on conjugated polymer monolayer. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Khim, D.; Baeg, K.J.; Caironi, M.; Liu, C.; Xu, Y.; Kim, D.Y.; Noh, Y.Y. Control of ambipolar and unipolar transport in organic transistors by selective inkjet-printed chemical doping for high performance complementary circuits. Adv. Funct. Mater. 2014, 24, 6252–6261. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, X.; Zhou, L.; Mao, J.Y.; Han, S.T.; Zhou, Y. Recent Advances in Ambipolar Transistors for Functional Applications. Adv. Funct. Mater. 2019, 29, 1–65. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, X.; Huang, J.; Zhou, Y.; Zhang, W.; Pan, Y.; Yu, G. Multisubstituted Azaisoindigo-Based Polymers for High-Mobility Ambipolar Thin-Film Transistors and Inverters. ACS Appl. Mater. Interfaces 2019, 11, 34171–34177. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, B.; Bae, B.; Chai, J.; Lee, S.; Kim, C. Ambipolar thin-film transistors based on organic semiconductor blend. Synth. Met. 2019, 253, 40–47. [Google Scholar] [CrossRef]
- Dong, H.; Fu, X.; Liu, J.; Wang, Z.; Hu, W. 25th Anniversary Article: Key Points for High-Mobility Organic Field-Effect Transistors. Adv. Mater. 2013, 25, 6158–6183. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Noh, Y.Y. Contact engineering in organic field-effect transistors. Mater. Today 2015, 18, 79–96. [Google Scholar] [CrossRef]
- Kotadiya, N.B.; Lu, H.; Mondal, A.; Ie, Y.; Andrienko, D.; Blom, P.W.M.; Wetzelaer, G.J.A.H. Universal strategy for Ohmic hole injection into organic semiconductors with high ionization energies. Nat. Mater. 2018, 17, 329–334. [Google Scholar] [CrossRef]
- Zhang, K.; Kotadiya, N.B.; Wang, X.; Wetzelaer, G.J.A.H.; Marszalek, T.; Pisula, W.; Blom, P.W.M. Hole injection layers for improving charge carrier injection in organic field effect transistors. Adv. Electron. Mater. 2020, 6, 1901352. [Google Scholar] [CrossRef]
- Salzillo, T.; Campos, A.; Mas-Torrent, M. Solution-processed thin films of a charge transfer complex for ambipolar field-effect transistors. J. Mater. Chem. C 2019, 7, 10257–10263. [Google Scholar] [CrossRef]
- Wang, H.; Yan, D. Organic heterostructures in organic field-effect transistors. NPG Asia Mater. 2010, 2, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, H.; Zhang, B.; Xie, Z.; Wong, W.Y. Towards high-power-efficiency solution-processed OLEDs: Material and device perspectives. Mater. Sci. Eng. R Rep. 2020, 140, 100547. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Efficiency of bulk-heterojunction organic solar cells. Prog. Polym. Sci. 2013, 38, 1929–1940. [Google Scholar] [CrossRef] [Green Version]
- Lüssem, B.; Keum, C.M.; Kasemann, D.; Naab, B.; Bao, Z.; Leo, K. Doped Organic Transistors. Chem. Rev. 2016, 116, 13714–13751. [Google Scholar] [CrossRef]
- Chiba, T.; Kumagai, D.; Udagawa, K.; Watanabe, Y.; Kido, J. Dual mode OPV-OLED device with photovoltaic and light-emitting functionalities. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Nam, M.; Cha, M.; Lee, H.H.; Hur, K.; Lee, K.T.; Yoo, J.; Han, I.K.; Kwon, S.J.; Ko, D.H. Long-term efficient organic photovoltaics based on quaternary bulk heterojunctions. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Puniredd, S.R.; Kiersnowski, A.; Battagliarin, G.; Zaja̧czkowski, W.; Wong, W.W.H.; Kirby, N.; Müllen, K.; Pisula, W. Polythiophene-perylene diimide heterojunction field-effect transistors. J. Mater. Chem. C 2013, 1, 2433–2440. [Google Scholar] [CrossRef] [Green Version]
- Hunter, S.; Chen, J.; Anthopoulos, T.D. Microstructural Control of Charge Transport in Organic Blend Thin-Film Transistors. Adv. Funct. Mater. 2014, 24, 5969–5976. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Z.; Bi, S.; Chen, J.; Li, D. Conjugated Polymer Controlled Morphology and Charge Transport of Small-Molecule Organic Semiconductors. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Chou, L.H.; Na, Y.; Park, C.H.; Park, M.S.; Osaka, I.; Kim, F.S.; Liu, C.L. Semiconducting small molecule/polymer blends for organic transistors. Polymer 2020, 191, 122208. [Google Scholar] [CrossRef]
- Tsao, H.N.; Pisula, W.; Liu, Z.; Osikowicz, W.; Salaneck, W.R.; Müllen, K. From ambi- To unipolar behavior in discotic dye field-effect transistors. Adv. Mater. 2008, 20, 2715–2719. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.; Anthopoulos, T.D. Observation of unusual, highly conductive grain boundaries in high-mobility phase separated organic semiconducting blend films probed by lateral-transport conductive-AFM. Adv. Mater. 2013, 25, 4320–4326. [Google Scholar] [CrossRef] [PubMed]
- Reichenberger, M.; Kroh, D.; Matrone, G.M.M.; Schötz, K.; Pröller, S.; Filonik, O.; Thordardottir, M.E.; Herzig, E.M.; Bässler, H.; Stingelin, N.; et al. Controlling aggregate formation in conjugated polymers by spin-coating below the critical temperature of the disorder–order transition. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 532–542. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, I.; Heeney, M.; Bailey, C.; Genevicius, K.; MacDonald, I.; Shkunov, M.; Sparrowe, D.; Tierney, S.; Wagner, R.; Zhang, W.; et al. Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nat. Mater. 2006, 5, 328–333. [Google Scholar] [CrossRef]
- Snyder, C.R.; Kline, R.J.; Delongchamp, D.M.; Nieuwendaal, R.C.; Richter, L.J.; Heeney, M.; McCulloch, I. Classification of semiconducting polymeric mesophases to optimize device postprocessing. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1641–1653. [Google Scholar] [CrossRef]
- Delongchamp, D.M.; Kline, R.J.; Jung, Y.; Lin, E.K.; Fischer, D.A.; Gundlach, D.J.; Cotts, S.K.; Moad, A.J.; Richter, L.J.; Toney, M.F.; et al. Molecular basis of mesophase ordering in a thiophene-based copolymer. Macromolecules 2008, 41, 5709–5715. [Google Scholar] [CrossRef]
- Rivnay, J.; Jimison, L.H.; Northrup, J.E.; Toney, M.F.; Noriega, R.; Lu, S.; Marks, T.J.; Facchetti, A.; Salleo, A. Large modulation of carrier transport by grain-boundary molecular packing and microstructure in organic thin films. Nat. Mater. 2009, 8, 952–958. [Google Scholar] [CrossRef]
- Cochran, J.E.; Junk, M.J.N.; Glaudell, A.M.; Miller, P.L.; Cowart, J.S.; Toney, M.F.; Hawker, C.J.; Chmelka, B.F.; Chabinyc, M.L. Molecular interactions and ordering in electrically doped polymers: Blends of PBTTT and F4TCNQ. Macromolecules 2014, 47, 6836–6846. [Google Scholar] [CrossRef]
- Jones, B.A.; Facchetti, A.; Wasielewski, M.R.; Marks, T.J. Tuning orbital energetics in arylene diimide semiconductors. Materials design for ambient stability of n-type charge transport. J. Am. Chem. Soc. 2007, 129, 15259–15278. [Google Scholar] [CrossRef]
- Kotadiya, N.B.; Mondal, A.; Blom, P.W.M.; Andrienko, D.; Wetzelaer, G.J.A.H. A window to trap-free charge transport in organic semiconducting thin films. Nat. Mater. 2019, 18, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Yim, K.-H.; Whiting, G.L.; Murphy, C.E.; Halls, J.J.M.; Burroughes, J.H.; Friend, R.H.; Kim-Seon, J. Controlling Electrical Properties of Conjugated Polymersvia a Solution-Based p-Type Doping. Adv. Mater. 2008, 20, 3319–3324. [Google Scholar] [CrossRef]
- Bardagot, O.; Kubik, P.; Marszalek, T.; Veyre, P.; Medjahed, A.A.; Sandroni, M.; Grévin, B.; Pouget, S.; Domschke, T.N.; Carella, A.; et al. Impact of Morphology on Charge Carrier Transport and Thermoelectric Properties of N-Type FBDOPV-Based Polymers. Adv. Funct. Mater. 2020, 30, 2000449. [Google Scholar] [CrossRef]
- Braga, D.; Horowitz, G. High-Performance organic field-effect transistors. Adv. Mater. 2009, 21, 1473–1486. [Google Scholar] [CrossRef]
- Horowitz, G. Organic thin film transistors: From theory to real devices. J. Mater. Res. 2004, 19, 1946–1962. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, J.; Guo, T.-F.; Shi, Y. The Control of Morphology and the Morphological Dependence of Device Electrical and Optical Properties in Polymer Electronics; Hotta, S., Ed.; Research Signpost: Kerala, India, 2003; Volume 661, ISBN 81-7736-083-3. [Google Scholar]
- Chlebosz, D.; Janasz, Ł.; Janus, K.; Gazińska, M.; Goldeman, W.; Ulanski, J.; Pisula, W.; Kiersnowski, A. Formation, growth and transformations of crystalline phases in solution-cast blends of poly(3-hexylthiopehene) and perylene dicarboximides. Dye. Pigment. 2017, 140, 491–499. [Google Scholar] [CrossRef]
- Li, L.H.; Kontsevoi, O.Y.; Rhim, S.H.; Freeman, A.J. Structural, electronic, and linear optical properties of organic photovoltaic PBTTT-C14 crystal. J. Chem. Phys. 2013, 138. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidyk, J.; Waliszewski, W.; Sleczkowski, P.; Kiersnowski, A.; Pisula, W.; Marszalek, T. Switching from Electron to Hole Transport in Solution-Processed Organic Blend Field-Effect Transistors. Polymers 2020, 12, 2662. https://doi.org/10.3390/polym12112662
Fidyk J, Waliszewski W, Sleczkowski P, Kiersnowski A, Pisula W, Marszalek T. Switching from Electron to Hole Transport in Solution-Processed Organic Blend Field-Effect Transistors. Polymers. 2020; 12(11):2662. https://doi.org/10.3390/polym12112662
Chicago/Turabian StyleFidyk, Julia, Witold Waliszewski, Piotr Sleczkowski, Adam Kiersnowski, Wojciech Pisula, and Tomasz Marszalek. 2020. "Switching from Electron to Hole Transport in Solution-Processed Organic Blend Field-Effect Transistors" Polymers 12, no. 11: 2662. https://doi.org/10.3390/polym12112662
APA StyleFidyk, J., Waliszewski, W., Sleczkowski, P., Kiersnowski, A., Pisula, W., & Marszalek, T. (2020). Switching from Electron to Hole Transport in Solution-Processed Organic Blend Field-Effect Transistors. Polymers, 12(11), 2662. https://doi.org/10.3390/polym12112662