Atmospheric Pressure Plasma Polymerized 2-Ethyl-2-oxazoline Based Thin Films for Biomedical Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Deposition and Discharge Diagnostics
2.3. Surface Characterization
2.4. Characterization of Mechanical Properties
2.5. Antibacterial Tests
2.6. Cytocompatibility Test
3. Results
3.1. Discharge Diagnostics
3.2. Film Thickness and Film Stability in Water
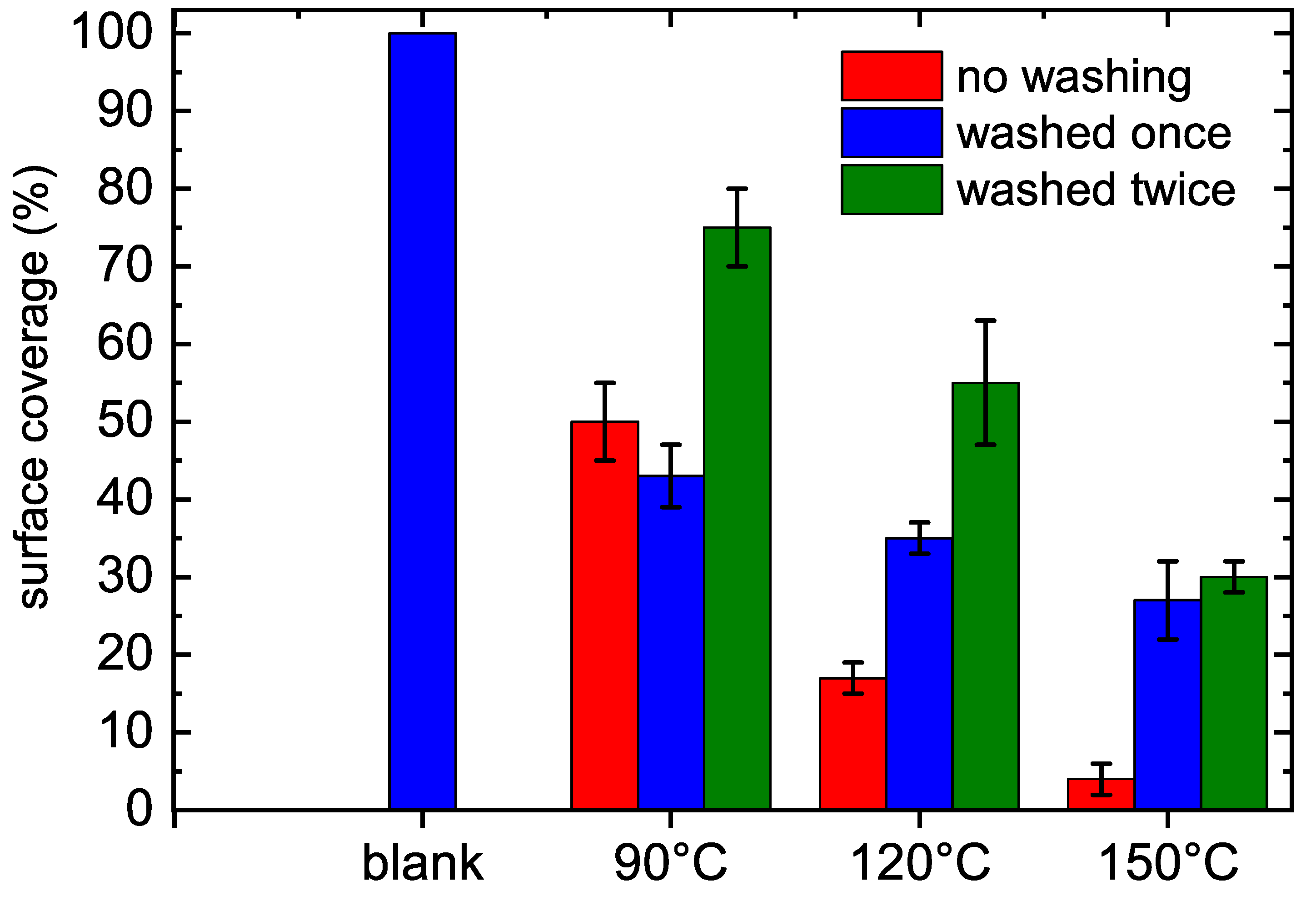
3.3. Antibacterial Properties
3.4. Cytocompatibility Results
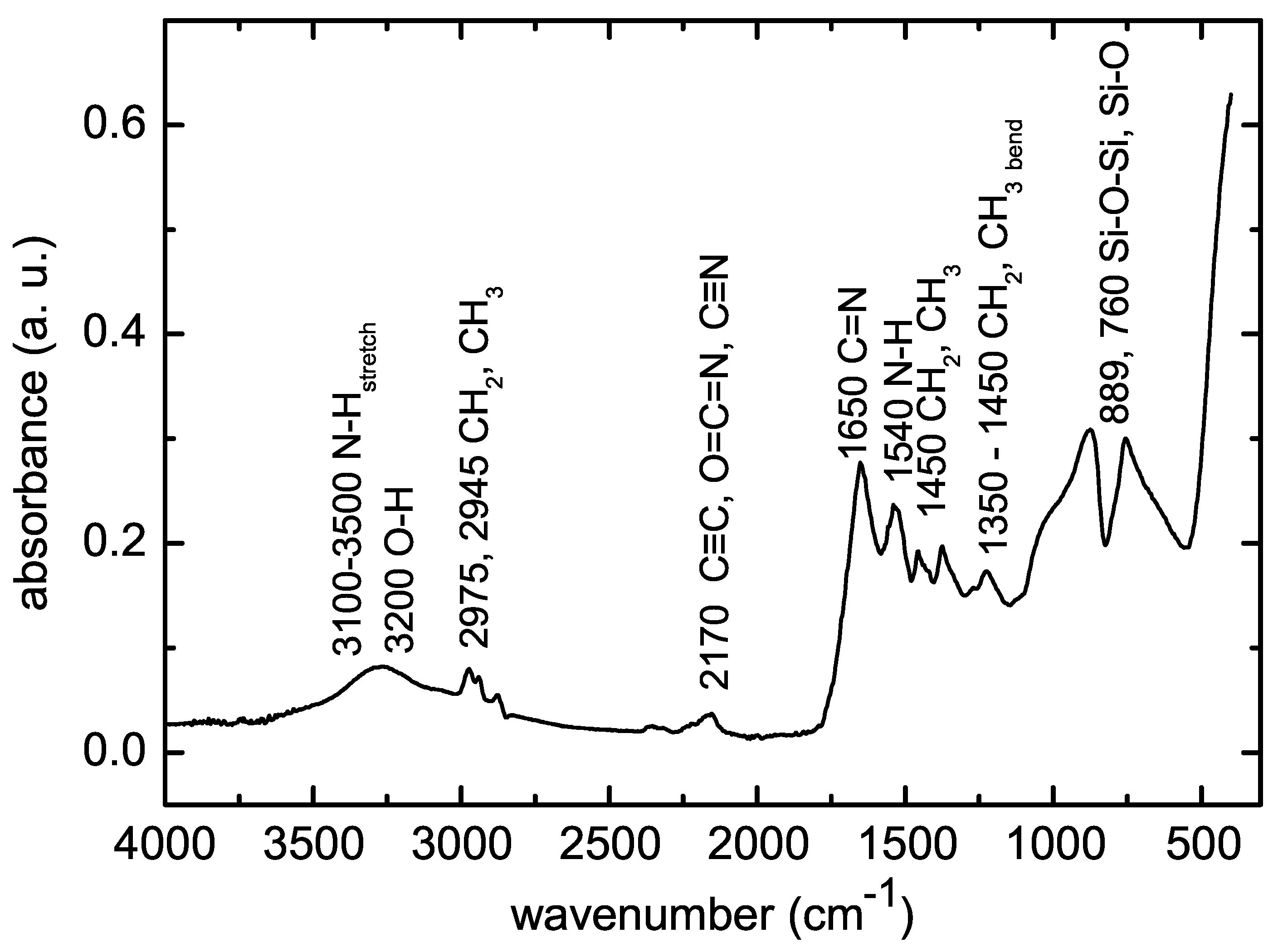
3.5. Surface Characterization
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trachsel, L.; Romio, M.; Ramakrishna, S.N.; Benetti, E.M. Fabrication of Biopassive Surfaces Using Poly(2-alkyl-2-oxazoline)s: Recent Progresses and Applications. Adv. Mater. Interfaces 2020, 7, 2000943. [Google Scholar] [CrossRef]
- Woodle, M.C.; Engbers, C.M.; Zalipsky, S. New Amphipatic Polymer Lipid Conjugates Forming Long-Circulating Reticuloendothelial System-Evading Liposomes. Bioconj. Chem. 1994, 5, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Zalipsky, S.; Hansen, C.B.; Oaks, J.M.; Allen, T.M. Evaluation of Blood Clearance Rates and Biodistribution of Poly(2-oxazoline)-grafted Liposomes. J. Pharm. Sci. 1996, 85, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Goddard, P.; Hutchinson, L.E.; Brown, J.; Brookman, L.J. Soluble Polymeric Carriers for Drug Delivery. Part 2. Preparation and in Vivo Behaviour of N-acylethylenimine Copolymers. J. Control. Release 1989, 10, 5–16. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Tong, Q.; Yan, M. Evaluation of Photochemically Immobilized Poly(2-ethyl-2-oxazoline) Thin Films as Protein-Resistant Surfaces. ACS Appl. Mater. Interfaces 2011, 3, 3463–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pidhatika, B.; Rodenstein, M.; Chen, Y.; Rakhmatullina, E.; Mühlebach, A.; Acikgöz, C.; Textor, M.; Konradi, R. Comparative Stability Studies of Poly(2-methyl-2-oxazoline) and Poly(ethyleneglycol) Brush Coatings. Biointerphases 2012, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, K. Nanoengineered Plasma Polymer Films for Biomaterial Applications. Plasma Chem. Plasma Process. 2014, 34, 545–558. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization—A Review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Bhatt, S.; Pulpytel, J.; Mirshahi, M.; Arefi-Khonsari, F. Cell Resistant Peptidomimetic Poly (2-ethyl-2-oxazoline) Coatings Developed by Low Pressure Inductively Excited Pulsed Plasma Polymerization for Biomedical Purpose. Plasma Process. Polym. 2015, 12, 519–532. [Google Scholar] [CrossRef]
- Ramiasa, M.; Cavallaro, A.; Mierczynska, A.; Christo, S.; Gleadle, J.; Hayball, J.D.; Vasilev, K. Plasma Polymerised PolyOxazoline Thin Films for Biomedical Applications. Chem. Commun. 2015, 51, 4279–4282. [Google Scholar] [CrossRef]
- Macgregor-Ramiasa, M.N.; Cavallaro, A.A.; Vasilev, K. Properties and Reactivity of Polyoxazoline Plasma Polymer Films. J. Mater. Chem. B 2015, 3, 6327–6337. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, A.A.; Macgregor-Ramiasa, M.N.; Vasilev, K. Antibiofouling Properties of Plasma-Deposited Oxazoline-Based Thin Films. ACS Appl. Mater. Interfaces 2016, 8, 6354–6362. [Google Scholar] [CrossRef] [PubMed]
- Zanini, S.; Zoia, L.; Dell’Orto, E.C.; Natalello, A.; Villa, A.M.; Della Pergola, R.; Riccardi, C. Plasma Polymerized 2-ethyl-2-oxazoline: Chemical Characterization and Study of the Reactivity towards Different Chemical Groups. Mater. Des. 2016, 108, 791–800. [Google Scholar] [CrossRef]
- Mori, Y.; Yoshii, K.; Kakiuchi, H.; Yasutake, K. Atmospheric Pressure Plasma Chemical Vapor Deposition System for High-rate Deposition of Functional Materials. Rev. Sci. Instrum. 2000, 71, 3173. [Google Scholar] [CrossRef]
- Gherardi, N.; Gouda, G.; Gat, E.; Ricard, A.; Massines, F. Transition from Glow Silent Discharge to Micro-discharges in Nitrogen Gas. Plasma Sources Sci. Technol. 2000, 9, 340–346. [Google Scholar] [CrossRef]
- Gherardi, N.; Martin, S.; Massines, F. A New Approach to SiO2 Deposit using a N2–SiH4–N2O Glow Dielectric Barrier-Controlled Discharge at Atmospheric Pressure. J. Phys. D Appl. Phys. 2000, 33, L104–L108. [Google Scholar] [CrossRef]
- Trunec, D.; Navratil, Z.; Stahel, P.; Zajickova, L.; Bursikova, V.; Cech, J. Deposition of Thin Organosilicon Polymer Films in Atmospheric Pressure Glow Discharge. J. Phys. D Appl. Phys. 2004, 37, 2112–2120. [Google Scholar] [CrossRef]
- Trunec, D.; Zajickova, L.; Bursikova, V.; Studnicka, F.; Stahel, P.; Prysiazhnyi, V.; Perina, V.; Houdkova, J.; Navratil, Z.; Franta, D. Deposition of Hard Thin Films from HMDSO in Atmospheric Pressure Dielectric Barrier Discharge. J. Phys. D Appl. Phys. 2010, 43, 225403. [Google Scholar] [CrossRef]
- Yokoyama, T.; Kogoma, M.; Moriwaki, T.; Okazaki, S. The Mechanism of the Stabilisation of Glow Plasma at Atmospheric Pressure. J. Phys. D Appl. Phys. 1990, 23, 1125–1128. [Google Scholar] [CrossRef]
- Trunec, D.; Brablec, A.; Buchta, J. Atmospheric Pressure Glow Discharge in Neon. J. Phys. D Appl. Phys. 2001, 34, 1697–1699. [Google Scholar] [CrossRef]
- Al-Bataineh, S.A.; Cavallaro, A.A.; Michelmore, A.; Macgregor, M.N.; Whittle, J.D.; Vasilev, K. Deposition of 2-oxazoline-based Plasma Polymer Coatings using Atmospheric Pressure Helium Plasma Jet. Plasma Process. Polym. 2019, 16, e1900104. [Google Scholar] [CrossRef]
- Van Guyse, J.F.R.; Cools, P.; Egghe, T.; Asadian, M.; Vergaelen, M.; Rigole, P.; Yan, W.; Benetti, E.M.; Jerca, V.; Declercq, H.; et al. Influence of the Aliphatic Side Chain on the Near Atmospheric Pressure Plasma Polymerization of 2-Alkyl-2-oxazolines for Biomedical Applications. ACS Appl. Mater. Interfaces 2019, 11, 31356–31366. [Google Scholar] [CrossRef] [PubMed]
- Stahel, P.; Mazankova, V.; Tomeckova, K.; Matouskova, P.; Brablec, A.; Prokes, L.; Jurmanova, P.; Bursikova, V.; Pribyl, R.; Lehocky, M.; et al. Atmospheric Pressure Plasma Polymerized Oxazoline-Based Thin Films–Antibacterial Properties and Cytocompatibility Performance. Polymers 2019, 11, 2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obrusnik, A.; Jelinek, P.; Zajickova, L. Modelling of the Gas Flow and Plasma Co-polymerization of Two Monomers in an Atmospheric-Pressure Dielectric Barrier Discharge. Surf. Coat. Technol. 2017, 314, 139–147. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise Review of Mechanisms of Bacterial Adhesion to Biomaterials and of Techniques Used in Estimating Bacteria–Material Interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Navratil, Z.; Bursikova, V.; Stahel, P.; Sira, M.; Zverina, P. On the Analysis of Surface Free Energy of DLC Coatings Deposited in Low Pressure RF Discharge. Czech J. Phys. (Suppl. C) 2004, 54, C877–C882. [Google Scholar] [CrossRef]
- Zlotnikov, I.; Zolotoyabko, E.; Fratzl, P. Nano-scale Modulus Mapping of Biological Composite Materials: Theory and Practice. Prog. Mater. Sci. 2017, 87, 292–320. [Google Scholar] [CrossRef]
- Lerouge, S.; Major, A.; Girault-Lauriault, P.-L.; Raymond, M.-A.; Laplante, P.; Soulez, G.; Mwale, F.; Wertheimer, M.R.; Hébert, M.-J. Nitrogen-Rich Coatings for Promoting Healing around Stent-Grafts after Endovascular Aneurysm Repair. Biomaterials 2007, 28, 1209–1217. [Google Scholar] [CrossRef]






| Sample | Thickness (m) | Thickness (m) | Loss (%) |
|---|---|---|---|
| as Deposited | after 48 h in Water | ||
| 20 C | 0.61 | 0 | 100 |
| 60 C | 0.60 | 0 | 100 |
| 90 C | 1.33 | 0.84 | 37 |
| 120 C | 2.14 | 1.26 | 41 |
| 150 C | 0.65 | 0.46 | 29 |
| 60 sccm | 1.15 | 0.75 | 35 |
| 100 sccm | 2.08 | 1.48 | 29 |
| 140 sccm | 0.68 | 0.32 | 53 |
| 200 sccm | 0.96 | 0.60 | 38 |
| 500 sccm | 1.21 | 1.06 | 22 |
| Sample | S. aureus CCM 2022 (CFU/cm) | E. coli CCM 4517 (CFU/cm) |
|---|---|---|
| substrate | ||
| 20 C | <1 | <1 |
| 60 C | <1 | <1 |
| 90 C | <1 | <1 |
| 120 C | <1 | |
| 150 C | <1 | |
| 60 sccm | <1 | <1 |
| 100 sccm | <1 | <1 |
| 140 sccm | 1.7 | |
| 200 sccm | <1 | <1 |
| 500 sccm | 1.7 | 3.3 |
| Sample | Contact Angle () | Surface Free Energy (mJ/m) | ||||
|---|---|---|---|---|---|---|
| CHI | glycerol | water | total | LW | AB | |
| substrate | 59.8 ± 1.2 | 35.5 ± 2.0 | 33.4 ± 2.3 | 52.6 ± 1.0 | 28.7 ± 0.7 | 23.9 ± 2.0 |
| 90 C | 37.8 ± 2.0 | 14.7 ± 2.9 | 24.1 ± 3.8 | 44.2 ± 7.0 | 40.7 ± 0.9 | 3.5 ± 3.0 |
| Sample | N | C | O | C-C | C-N | C=O | C-O | COO | N-C=O |
|---|---|---|---|---|---|---|---|---|---|
| 90 C | 21 | 66 | 13 | 24 | 30 | 6 | 21 | 4 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazánková, V.; Sťahel, P.; Matoušková, P.; Brablec, A.; Čech, J.; Prokeš, L.; Buršíková, V.; Stupavská, M.; Lehocký, M.; Ozaltin, K.; et al. Atmospheric Pressure Plasma Polymerized 2-Ethyl-2-oxazoline Based Thin Films for Biomedical Purposes. Polymers 2020, 12, 2679. https://doi.org/10.3390/polym12112679
Mazánková V, Sťahel P, Matoušková P, Brablec A, Čech J, Prokeš L, Buršíková V, Stupavská M, Lehocký M, Ozaltin K, et al. Atmospheric Pressure Plasma Polymerized 2-Ethyl-2-oxazoline Based Thin Films for Biomedical Purposes. Polymers. 2020; 12(11):2679. https://doi.org/10.3390/polym12112679
Chicago/Turabian StyleMazánková, Věra, Pavel Sťahel, Petra Matoušková, Antonín Brablec, Jan Čech, Lubomír Prokeš, Vilma Buršíková, Monika Stupavská, Marián Lehocký, Kadir Ozaltin, and et al. 2020. "Atmospheric Pressure Plasma Polymerized 2-Ethyl-2-oxazoline Based Thin Films for Biomedical Purposes" Polymers 12, no. 11: 2679. https://doi.org/10.3390/polym12112679








