Characteristics of the Non-Isothermal and Isothermal Crystallization for the β Polymorph in PVDF by Fast Scanning Calorimetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. X-ray Diffraction
2.4. Fourier Transform Infrared Spectroscopy
2.5. Differential Scanning Calorimetry (DSC)
2.6. Fast Scanning Chip Calorimetry (FSC)
3. Results and Discussion
3.1. Structural Characteristics
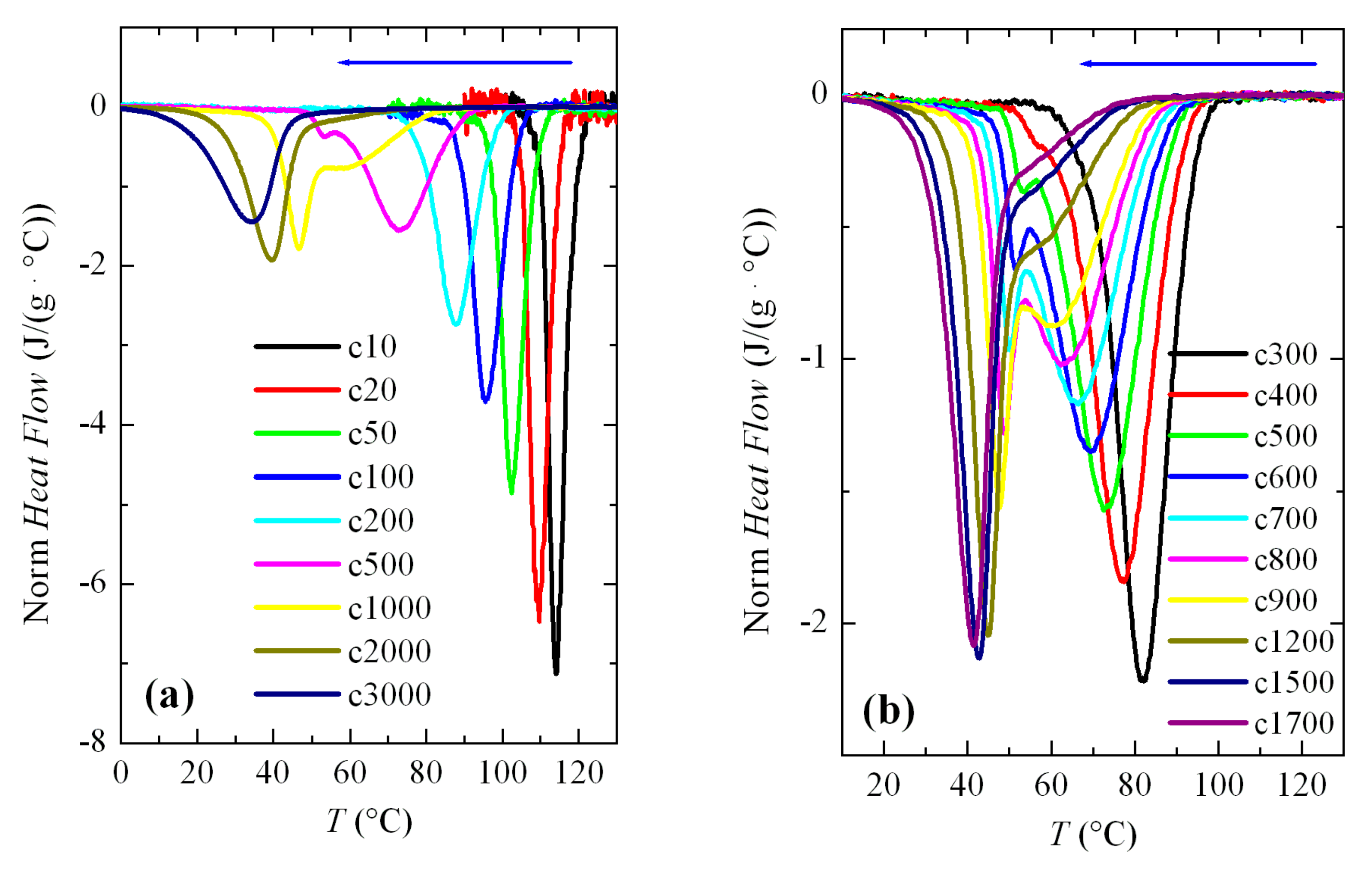
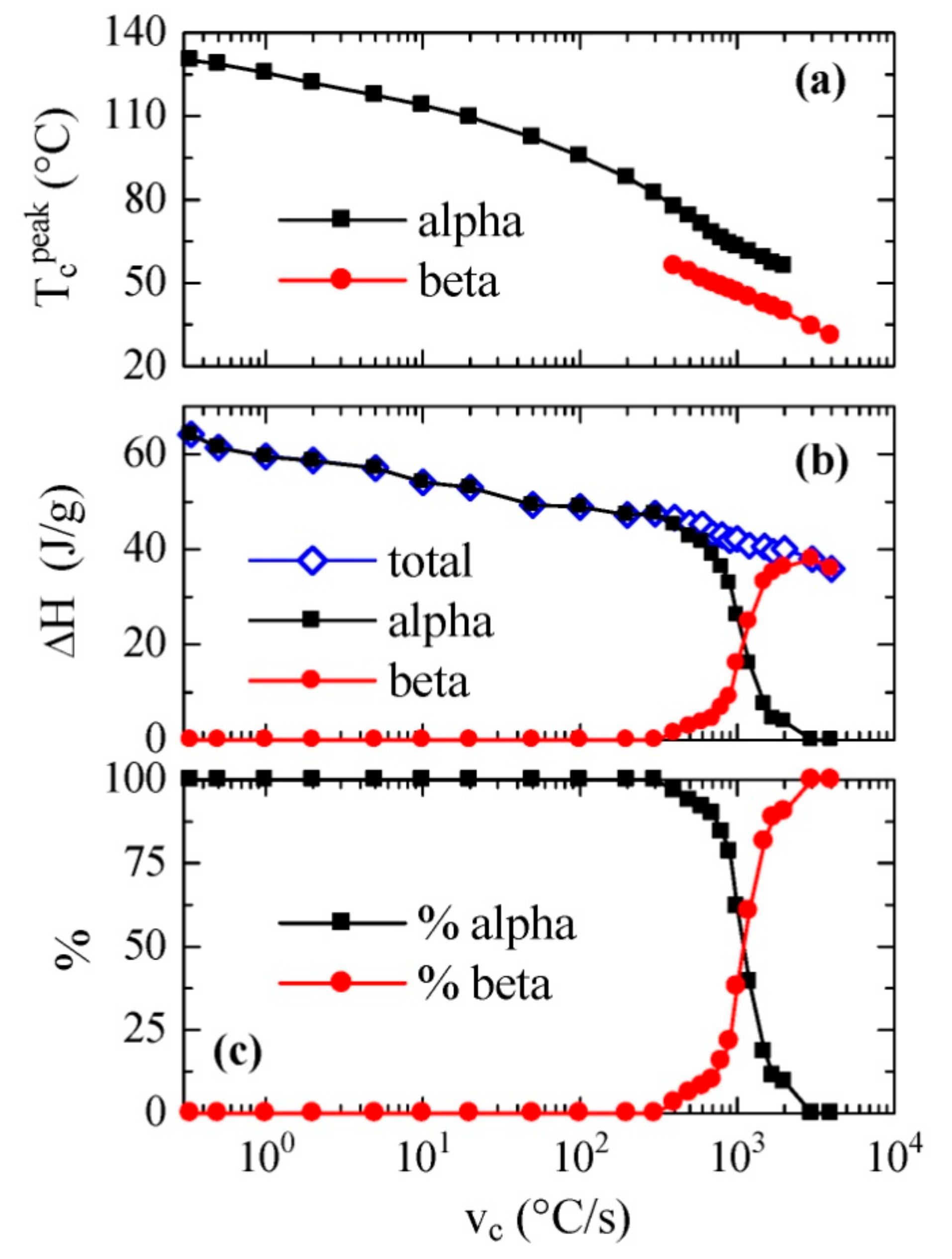
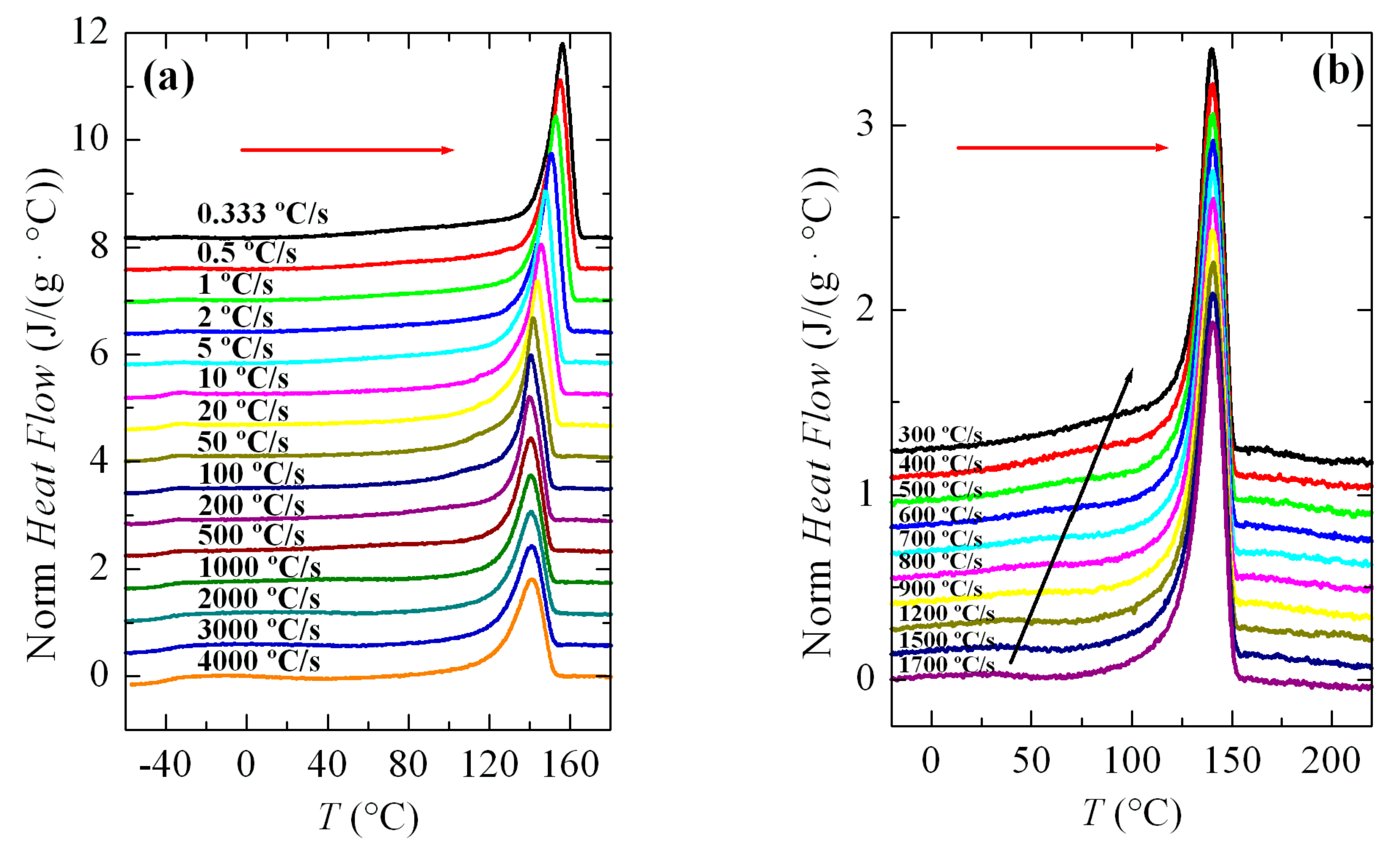
3.2. Non-Isothermal Crystallization Experiments
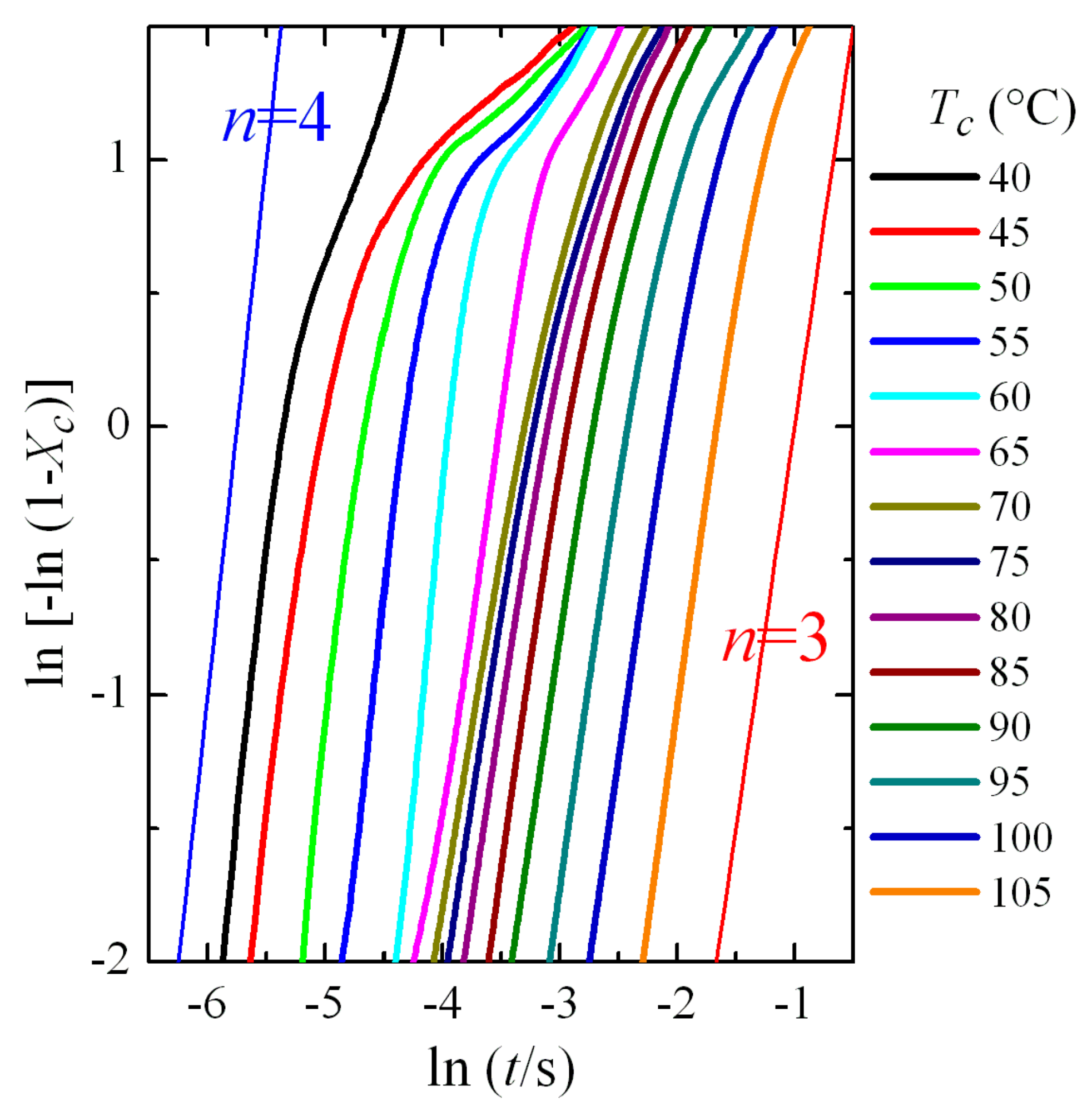
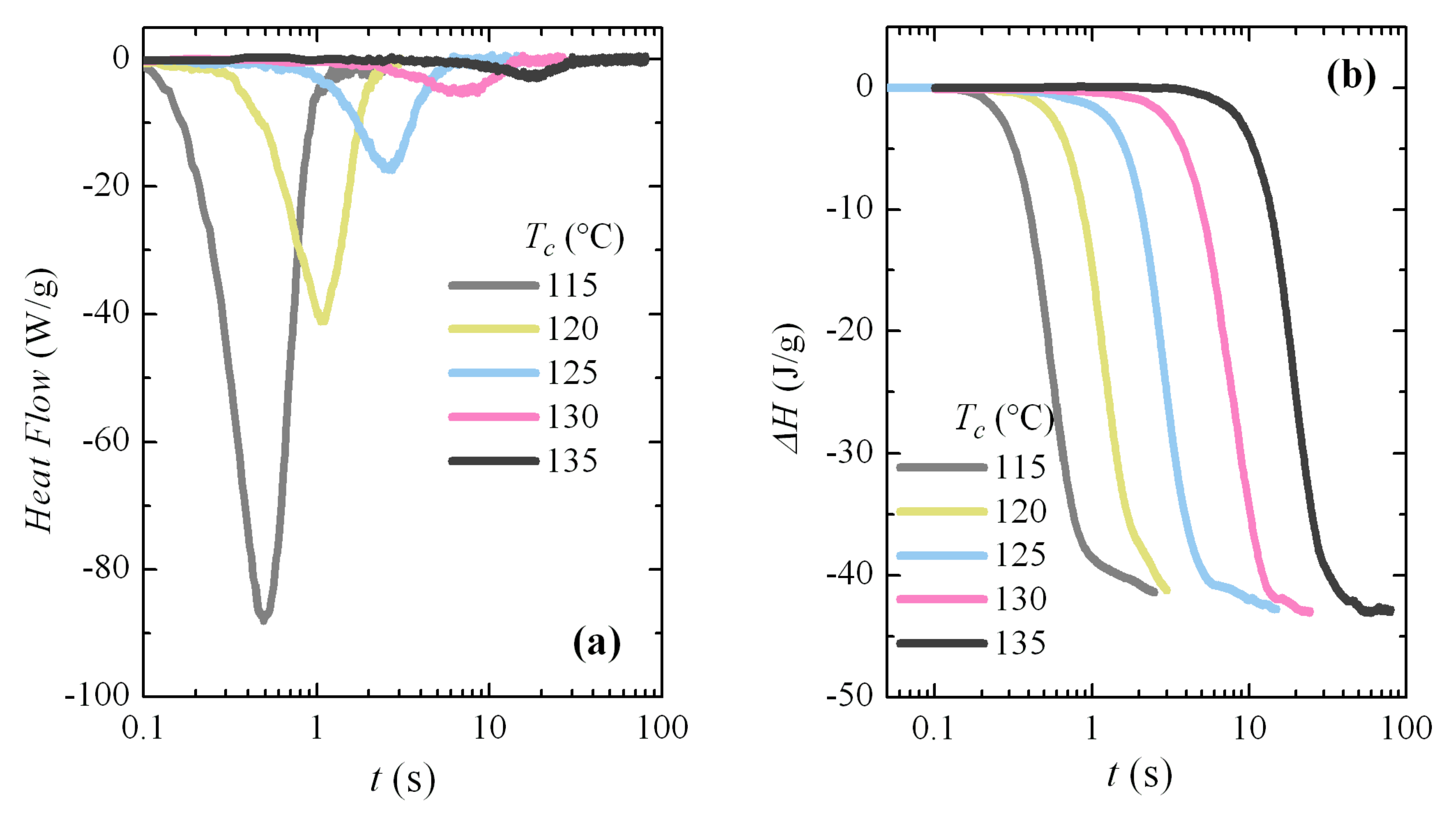
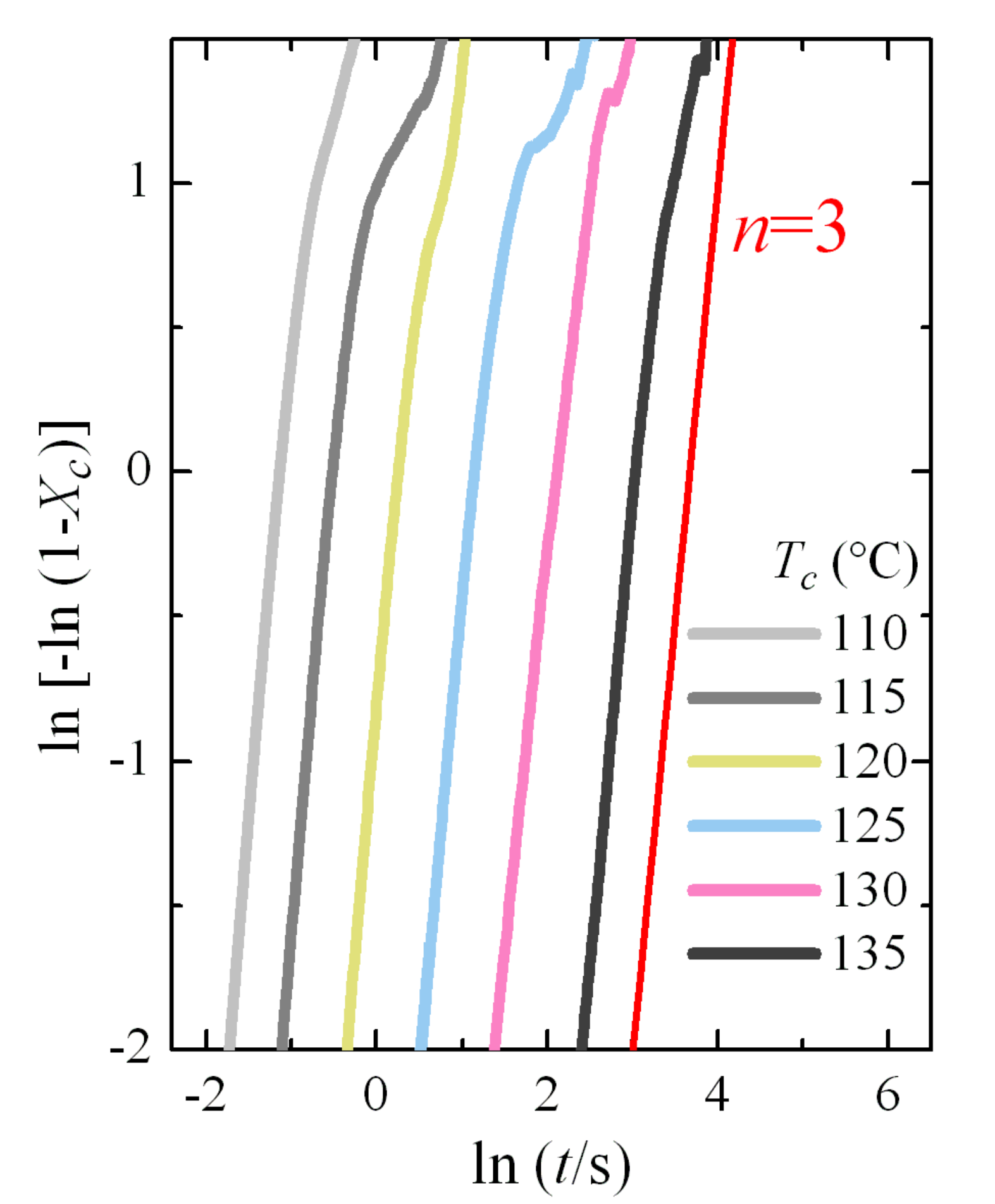
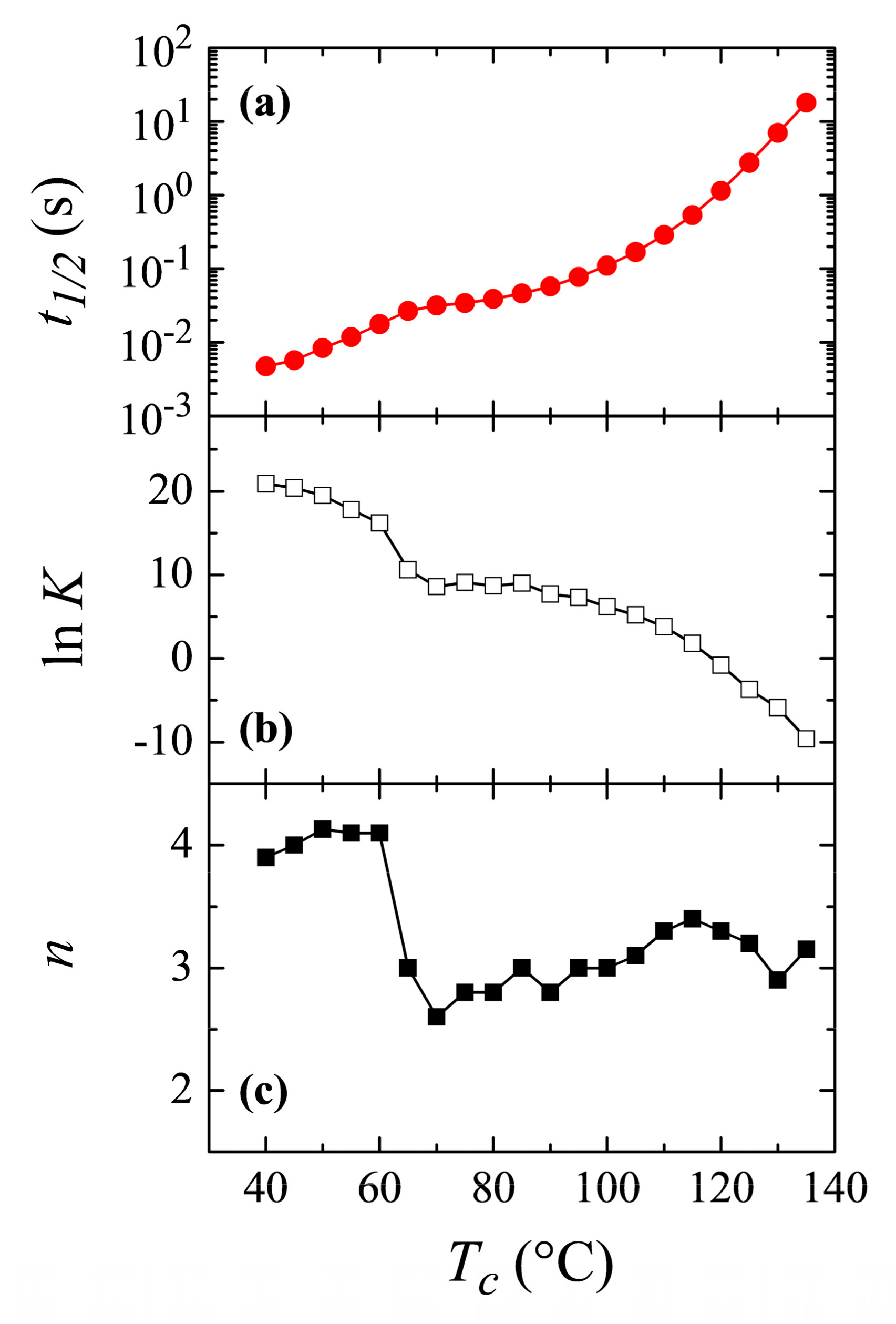
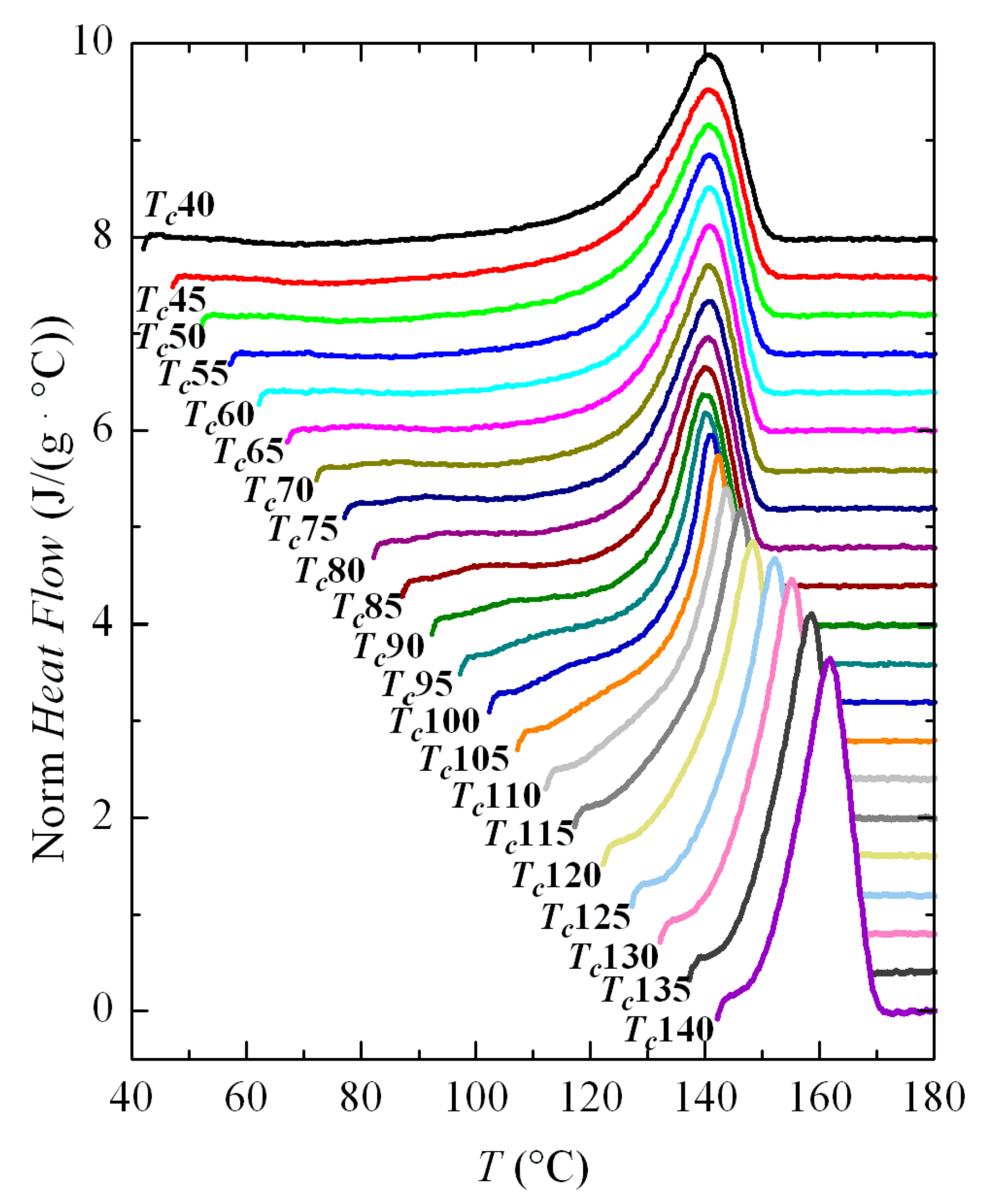
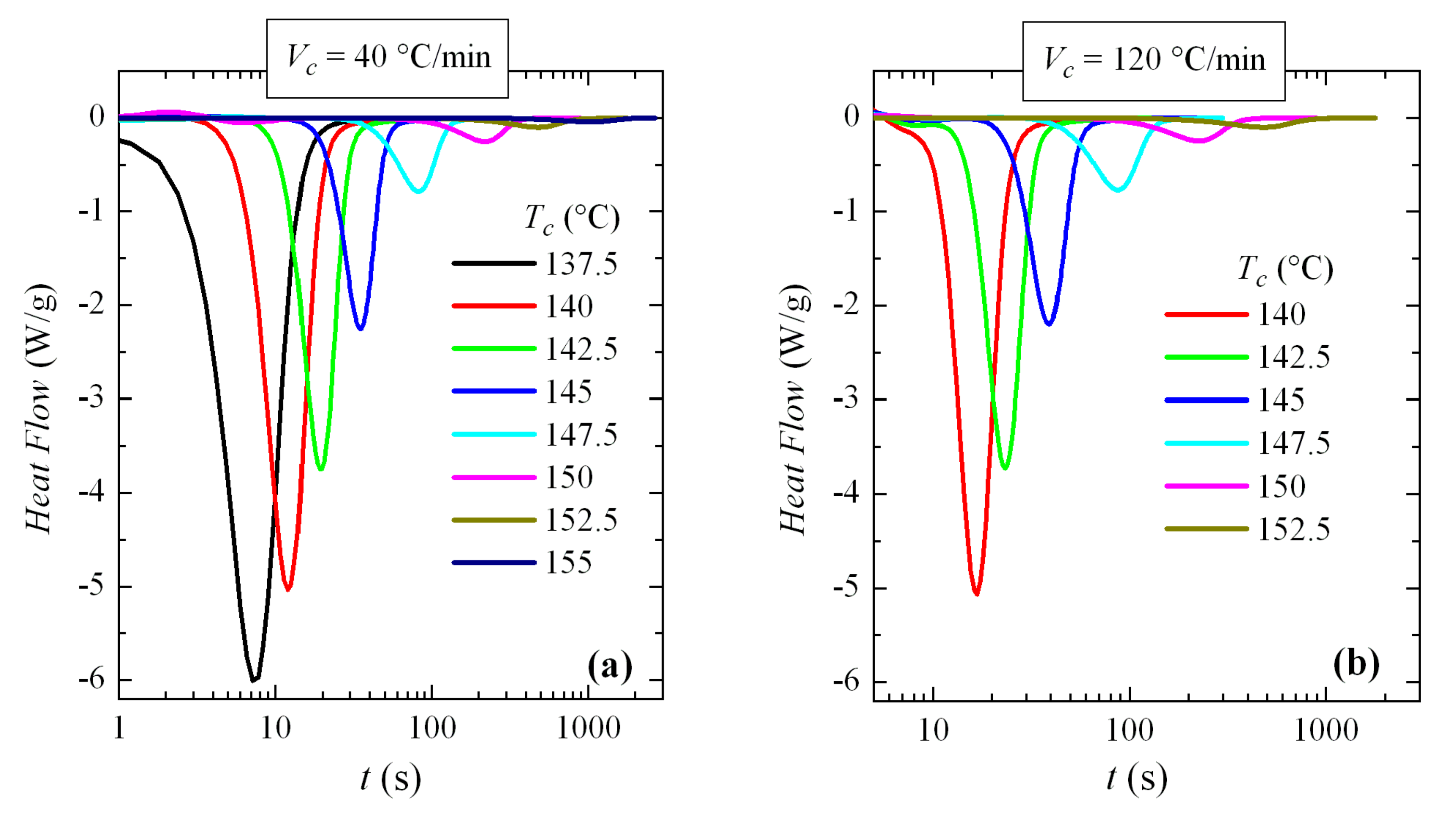
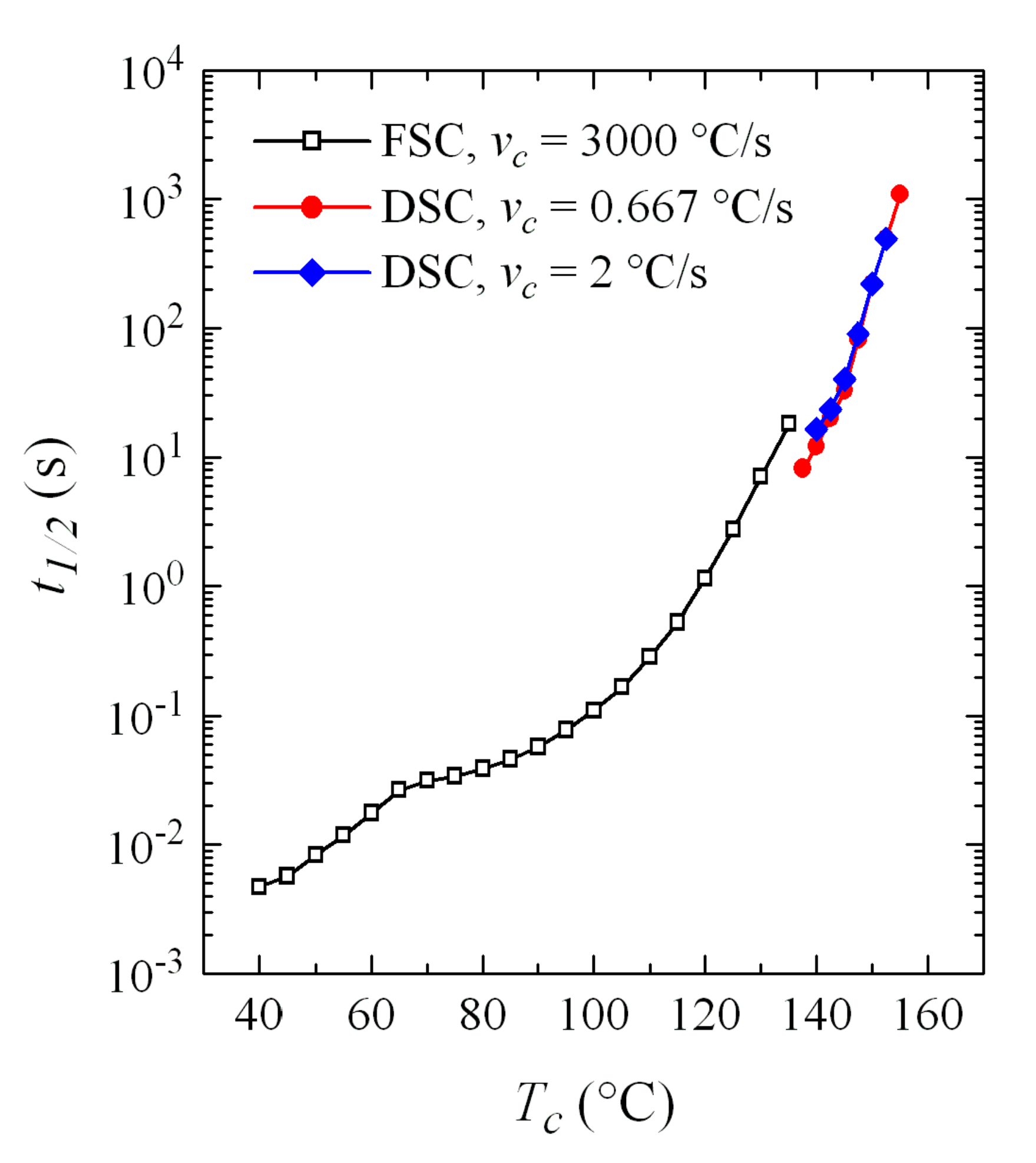
3.3. Isothermal Crystallization Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ivanov, D.A. Semicrystalline Polymers. In Polymer Science: A Comprehensive Reference; Möller, M., Matyjaszewski, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 227–258. [Google Scholar]
- Strobl, G. Crystallization and melting of bulk polymers: New observations, conclusions and a thermodynamic scheme. Prog. Polym. Sci. 2006, 31, 398–442. [Google Scholar] [CrossRef]
- Strobl, G. Colloquium: Laws controlling crystallization and melting in bulk polymers. Rev. Mod. Phys. 2009, 81, 1287–1300. [Google Scholar] [CrossRef]
- Grebowicz, J.; Lau, J.F.; Wunderlich, B. The thermal-properties of polypropylene. J. Polym. Sci. Polym. Symp. 1984, 71, 19. [Google Scholar] [CrossRef]
- Corradini, P.; de Rosa, C.; Guerra, G.; Petraccone, V. Comments on the possibility that the mesomorphic form of isotactic polypropylene is composed of small crystals of the beta-crystalline form. Polym. Commun. 1989, 30, 281–285. [Google Scholar]
- Brückner, S.; Meille, S.V.; Petraccone, V.; Pirozzi, B. Polymorphism in isotactic polypropylene. Prog. Polym. Sci. 1991, 16, 361–404. [Google Scholar] [CrossRef]
- Lotz, B.; Kopp, S.; Dorset, D.C.R. An original crystal-structure of polymers with ternary helices. C. R. Acad. Sci. Ser. II 1994, 319, 187–192. [Google Scholar]
- Rotter, G.; Ishida, H. FTIR separation of PA6 chain conformations: Clarification of the mesomorphous and crystalline phases. J. Polym. Sci. Part B Polym. Phys. 1992, 30, 489–495. [Google Scholar] [CrossRef]
- Fu, Y.G.; Annis, B.; Boller, A.; Jin, Y.M.; Wunderlich, B. Analysis of structure and properties of poly(ethyleneterephthalate) fibers. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 2289–2306. [Google Scholar]
- Kolb, R.; Wutz, C.; Stribeck, N.; von Krosigk, G.; Riekel, C. Investigation of secondary crystallization of polymers by means of microbeam X-ray scattering. Polymer 2001, 42, 5257–5266. [Google Scholar] [CrossRef]
- Tashiro, K.; Sasaki, S. Structural changes in the ordering process of polymers as studied by an organized combination of the various measurement techniques. Prog. Polym. Sci. 2003, 28, 451–519. [Google Scholar] [CrossRef]
- Schick, C. Differential scanning calorimetry (DSC) of semicrystalline polymers. Anal. Bioanal. Chem. 2009, 395, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Abate, L.; Bottino, F.A. Mono substituted octaphenyl POSSs: The effects of substituents on thermal properties and solubility. Thermochim. Acta 2017, 655, 117–123. [Google Scholar] [CrossRef]
- Lai, S.L.; Ramanath, G.; Allen, L.H.; Infante, P.; Ma, Z. High-speed (10(4)-degrees-C/s) scanning microcalorimetry with monolayer sensitivity (J/M(2)). Appl. Phys. Lett. 1995, 67, 1229–1231. [Google Scholar] [CrossRef]
- Lai, S.L.; Guo, J.Y.; Petrova, V.; Ramanath, G.; Allen, L.H. Size dependent melting properties of small tin particles: Nanocalorimetric measurements. Phys. Rev. Lett. 1996, 77, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Efremov, M.Y.; Olson, E.A.; Zhang, M.; Lai, S.L.; Schienekatte, F.; Zhang, Z.S.; Allen, L.H. Thin-film differential scanning nanocalorimetiy: Heat capacify analysis. Thermochim. Acta 2004, 412, 13–23. [Google Scholar] [CrossRef]
- Hasegawa, R.; Takahashi, Y.; Tadokoro, H.; Chatani, Y. Crystal-structures of three crystalline forms of poly(vinylidene fluoride). Polym. J. 1972, 3, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Lovinger, A.J. Annealing of poly(vinylidene fluoride) and formation of a 5th phase. Macromolecules 1982, 15, 40–44. [Google Scholar] [CrossRef]
- Gregorio, R.; Cestari, M. Effect of crystallization temperature on the crystalline phase content and morphology of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1994, 32, 857–870. [Google Scholar] [CrossRef]
- Oka, Y.; Koizumi, N. Formation of Unoriented Form I Poly (vinylidene fluoride) by High-Rate Quenching and its Electrical Properties. Bull. Inst. Chem. Res. Kyoto Univ. 1985, 63, 192–206. [Google Scholar]
- Arranz-Andrés, J.; Pérez, E.; Cerrada, M.L. Effect of copper nanoparticles incorporation on the polar beta-phase development in polyvinylidene fluoride. Mater. Chem. Phys. 2015, 162, 794–800. [Google Scholar] [CrossRef] [Green Version]
- Gradys, A.; Sajkiewicz, P.; Adamovsky, S.; Minakov, A.; Schick, C. Crystallization of poly(vinylidene fluoride) during ultra-fast cooling. Thermochim. Acta 2007, 461, 153–157. [Google Scholar] [CrossRef]
- Gradys, A.; Sajkiewicz, P.; Zhuravlev, E.; Schick, C. Kinetics of isothermal and non-isothermal crystallization of poly(vinylidene fluoride) by fast scanning calorimetry. Polymer 2016, 82, 40–48. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Q.D.; Hu, W. Primary and secondary crystallization of fast-cooled poly(vinylidene fluoride) studied by Flash DSC, wide-angle X-ray diffraction and Fourier transform infrared spectroscopy. Polym. Int. 2016, 65, 387–392. [Google Scholar] [CrossRef]
- Neef, A.; Samuel, C.; Stoclet, G.; Rguiti, M.; Courtois, C.; Dubois, P.; Soulestin, J.; Raquez, J.M. Processing of PVDF-based electroactive/ferroelectric films: Importance of PMMA and cooling rate from the melt state on the crystallization of PVDF beta-crystals. Soft Matter. 2018, 14, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, X.; Zhou, D.; Shen, Q.D.; Hu, W. Low temperature crystallization of P(VDF-TrFE-CFE) studied by Flash DSC. Polymer 2016, 84, 319–327. [Google Scholar] [CrossRef]
- Chen, Y.X.; Lu, H.W.; Shen, Z.W.; Li, Z.L.; Shen, Q.D. Cooling Rate Controlled Microstructure Evolution through Flash DSC and Enhanced Energy Density in P(VDF–CTFE) for Capacitor Application. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 1245–1253. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, L.; Yang, C.; Zhang, L.; Zheng, P.; Liu, A.; Shen, Q.D. In-depth understanding of interfacial crystallization via Flash DSC and enhanced energy storage density in ferroelectric P(VDF-CTFE)/Au NRs nanocomposites for capacitor application. Soft Matter. 2018, 14, 7714–7723. [Google Scholar] [CrossRef]
- Mathot, V.; Pyda, M.; Pijpers, T.; Vanden Poel, G.; van de Kerkhof, E.; van Herwaarden, S.; van Herwaarden, F.; Leenaers, A. The Flash DSC 1, a power compensation twin-type, chip-based fast scanning calorimeter (FSC): First findings on polymers. Thermochim. Acta 2011, 522, 36–45. [Google Scholar] [CrossRef]
- Gregorio, R. Determination of the α, β, and γ Crystalline Phases of Poly(vinylidene fluoride) Films Prepared at Different Conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- Wu, T.; Pan, Y.; Li, L. Fabrication of superhydrophobic hybrids from multiwalled carbon nanotubes and poly(vinylidene fluoride). Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 47–52. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I. General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II. Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. III. Granulation, phase change and microstructure. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
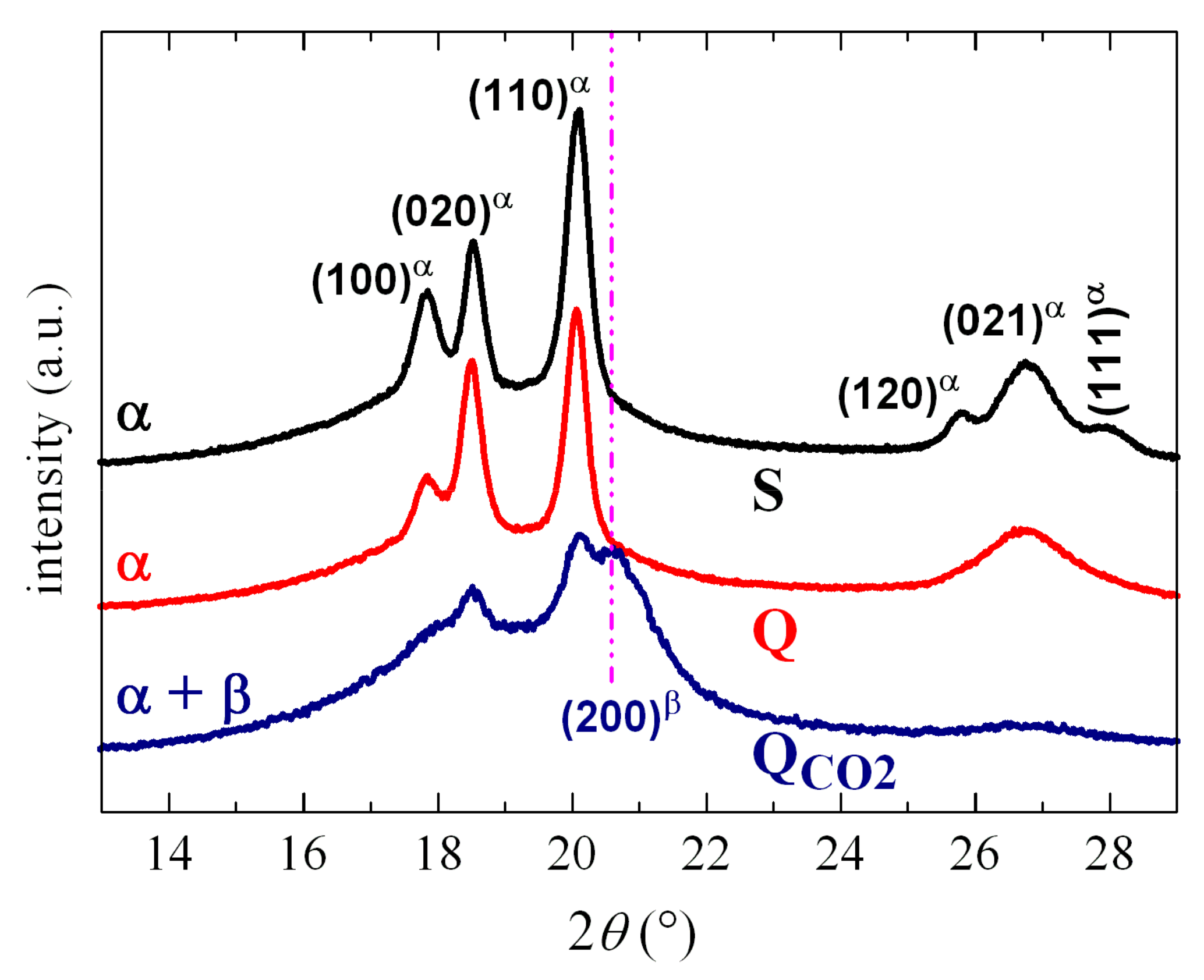
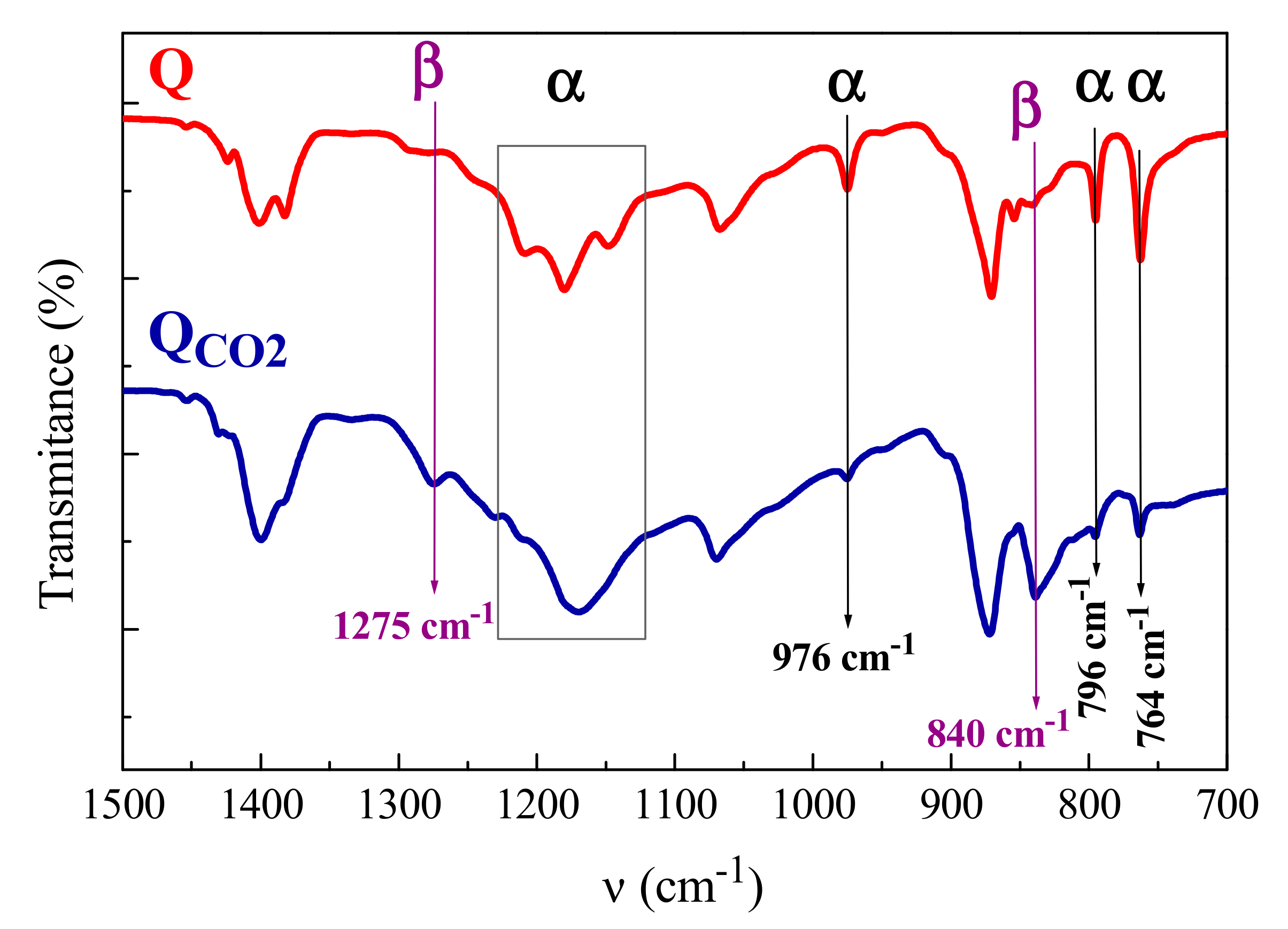


| α Form | β Form | γ Form | |
|---|---|---|---|
| Wavenumber (cm−1) | 408; 532; 614; 764; 796; 855; 976 | 445; 510; 840; 1275 | 431; 512; 776; 812; 1220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, E.; Angulo, I.; Blázquez-Blázquez, E.; Cerrada, M.L. Characteristics of the Non-Isothermal and Isothermal Crystallization for the β Polymorph in PVDF by Fast Scanning Calorimetry. Polymers 2020, 12, 2708. https://doi.org/10.3390/polym12112708
Pérez E, Angulo I, Blázquez-Blázquez E, Cerrada ML. Characteristics of the Non-Isothermal and Isothermal Crystallization for the β Polymorph in PVDF by Fast Scanning Calorimetry. Polymers. 2020; 12(11):2708. https://doi.org/10.3390/polym12112708
Chicago/Turabian StylePérez, Ernesto, Irene Angulo, Enrique Blázquez-Blázquez, and María L. Cerrada. 2020. "Characteristics of the Non-Isothermal and Isothermal Crystallization for the β Polymorph in PVDF by Fast Scanning Calorimetry" Polymers 12, no. 11: 2708. https://doi.org/10.3390/polym12112708
APA StylePérez, E., Angulo, I., Blázquez-Blázquez, E., & Cerrada, M. L. (2020). Characteristics of the Non-Isothermal and Isothermal Crystallization for the β Polymorph in PVDF by Fast Scanning Calorimetry. Polymers, 12(11), 2708. https://doi.org/10.3390/polym12112708








