Design and Synthesis of Bio-Inspired Polyurethane Films with High Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods and Instruments
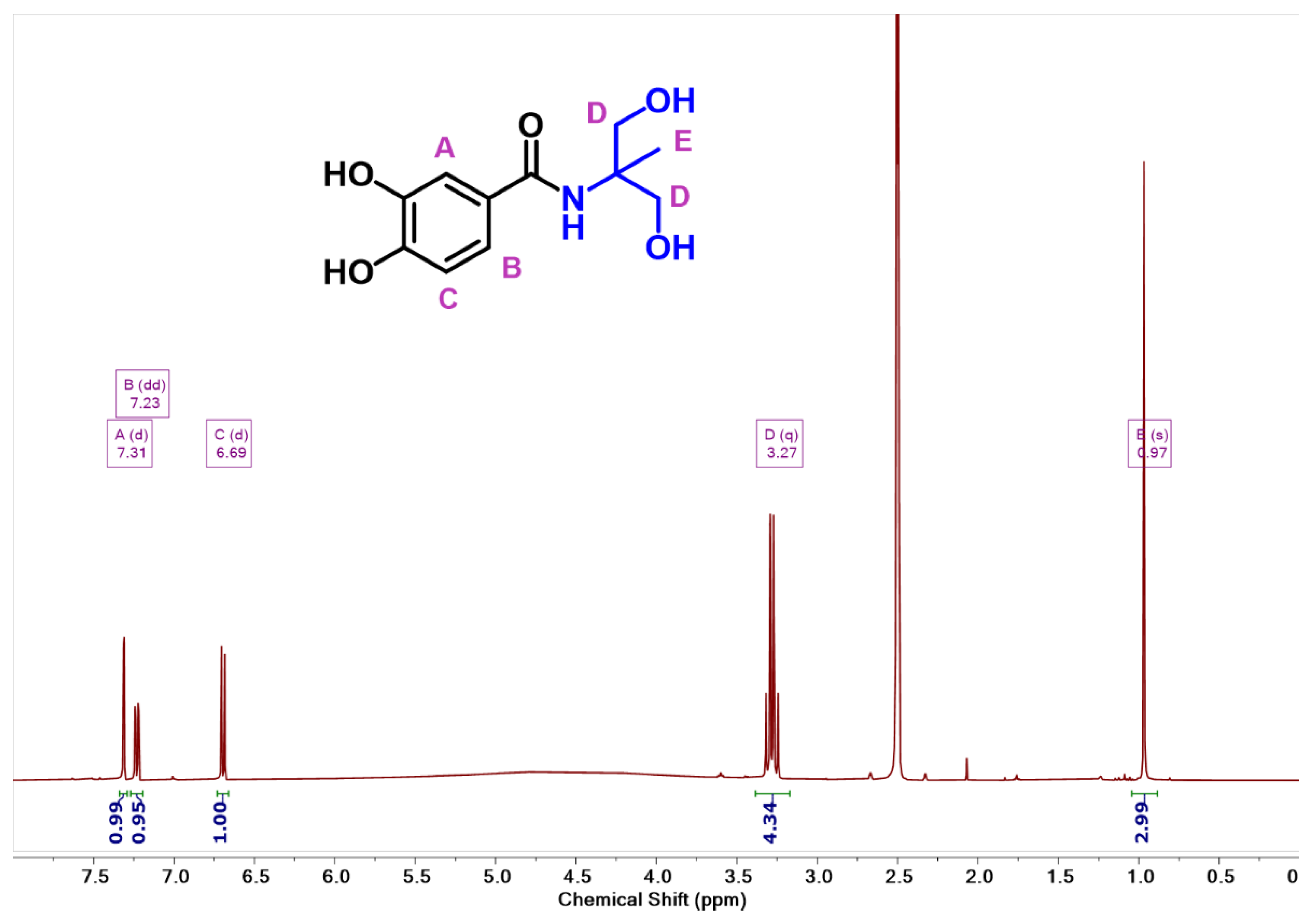
2.3. Synthesis of N-(1,3-Dihydroxy-2-Methylpropan-2-yl)-3,4-Dihydroxy-Benzamide (Cat-Fun)
2.4. Protection and Functionalization of 3,4-Dihydroxybenzoic Acid (Cat-Prot)
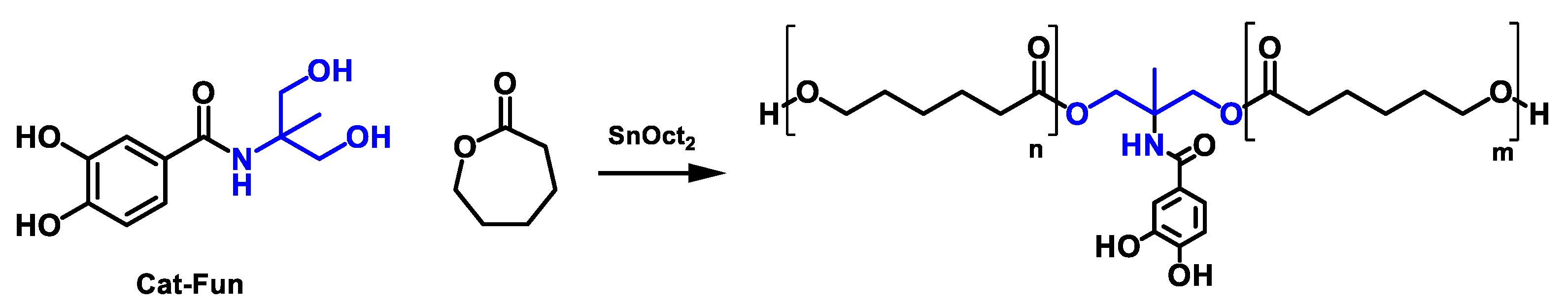
2.5. Synthesis of Functionalized Polycaprolactone-Diol Bearing Catechol Motifs (PCL-Cat)
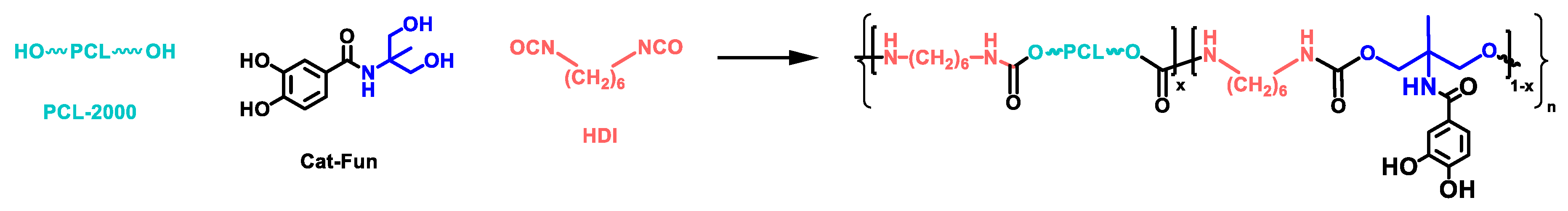
2.6. Synthesis of Polyurethanes Bearing Catechol Moieties within the Hard Segment
2.7. Synthesis of Polyurethane Functionalized with Catechol within the Soft-Segment
2.8. Preparation of Polyurethane Bearing Protected Catechol (Cat-Prot)
3. Results and Discussion
3.1. Synthesis of Functionalized Catechol
3.2. Synthesis of Polyurethanes Bearing Catecholic Moieties within the Hard Segment
3.3. Synthesis of Polyurethanes Functionalized with Catechol Units within the Soft Segment
3.4. Synthesis of Polyurethanes with Protected Catechol Units within the Hard Segment
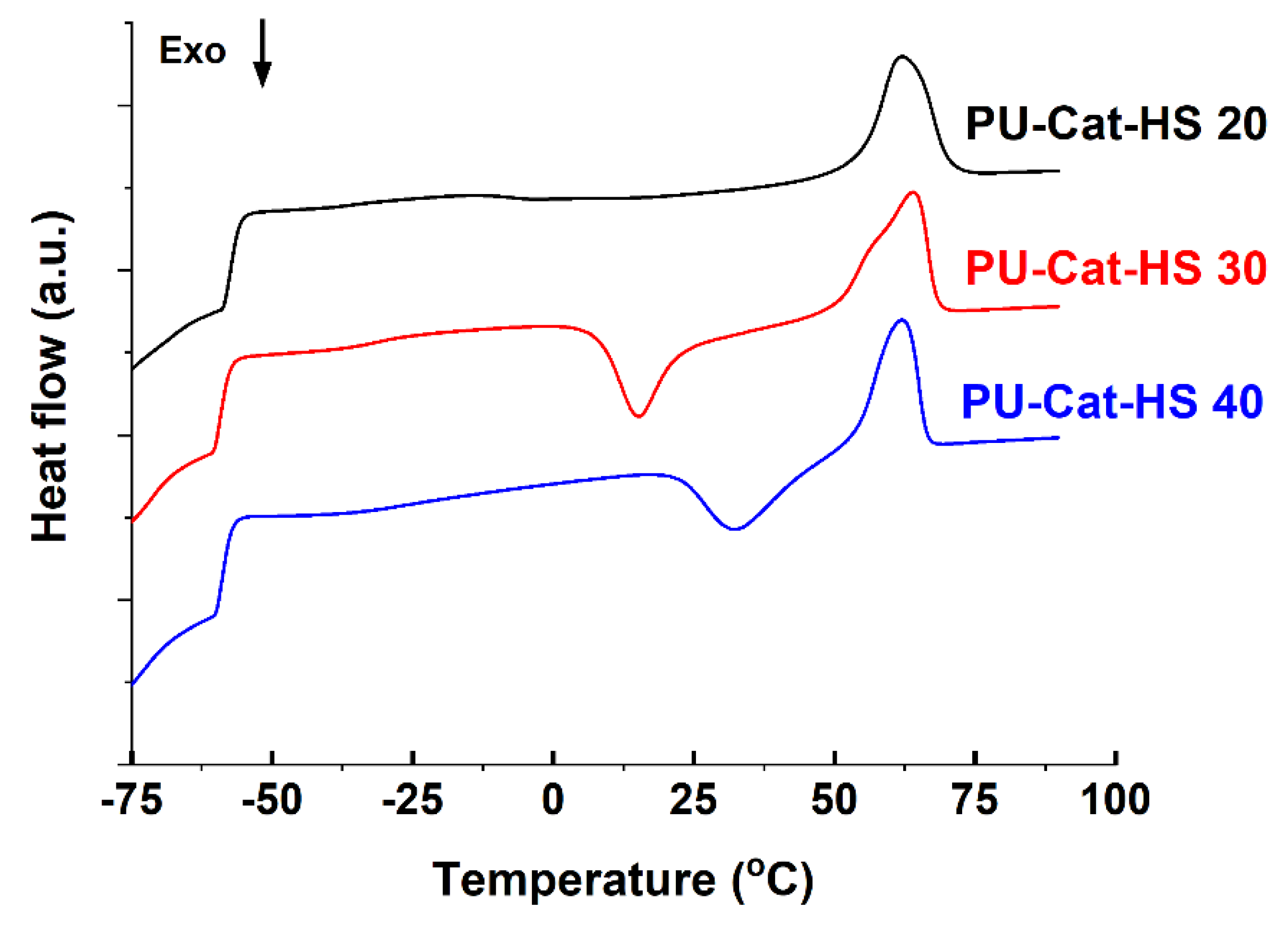
3.5. Influence of Catechol Moieties on the Polyurethane Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, J. Hydrophobic Enhancement of Dopa-Mediated Adhesion in a Mussel Foot Protein. In Adhesive Interactions of Mussel Foot Proteins; Springer: Berlin/Heidelberg, Germany, 2014; pp. 43–54. [Google Scholar]
- Wei, W.; Yu, J.; Broomell, C.; Israelachvili, J.N.; Waite, J.H. Hydrophobic enhancement of dopa-mediated adhesion in a mussel foot protein. J. Am. Chem. Soc. 2013, 135, 377–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, Z.A.; Rapp, M.V.; Wei, W.; Mullen, R.G.; Wu, C.; Zerze, G.H.; Mittal, J.; Waite, J.H.; Israelachvili, J.N.; Shea, J.-E. Surface force measurements and simulations of mussel-derived peptide adhesives on wet organic surfaces. Proc. Natl. Acad. Sci. USA 2016, 113, 4332–4337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J. Effects of interfacial redox in mussel adhesive protein films on mica. In Adhesive Interactions of Mussel Foot Proteins; Springer: Berlin/Heidelberg, Germany, 2014; pp. 21–30. [Google Scholar]
- Yu, M.; Hwang, J.; Deming, T.J. Role of L-3, 4-dihydroxyphenylalanine in mussel adhesive proteins. J. Am. Chem. Soc. 1999, 121, 5825–5826. [Google Scholar] [CrossRef]
- Patil, N.; Jerome, C.; Detrembleur, C. Recent advances in the synthesis of catechol-derived (bio) polymers for applications in energy storage and environment. Prog. Polym. Sci. 2018, 82, 34–91. [Google Scholar] [CrossRef]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Krumenacker, L.; Costantini, M.; Pontal, P.; Sentenac, J. Hydroquinone, resorcinol, and catechol. Encycl. Chem. Technol. 2000. [Google Scholar] [CrossRef]
- Oyeneye, O.O.; Xu, W.Z.; Charpentier, P.A. Designing catechol-end functionalized poly (DMAm-co-NIPAM) by RAFT with tunable LCSTs. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 4062–4070. [Google Scholar] [CrossRef]
- Boulding, N.A.; Millican, J.M.; Hutchings, L.R. Understanding copolymerisation kinetics for the design of functional copolymers via free radical polymerisation. Polym. Chem. 2019, 10, 5665–5675. [Google Scholar] [CrossRef]
- Na, Y.; Chen, C. Catechol-Functionalized Polyolefins. Angew. Chem. Int. Ed. 2020, 59, 7953–7959. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 11. [Google Scholar]
- Seoane Rivero, R.; Solaguren, P.B.; Gondra Zubieta, K.; Peponi, L.; Marcos-Fernández, A. Synthesis, kinetics of photo-dimerization/photo-cleavage and physical properties of coumarin-containing branched polyurethanes based on polycaprolactones. Express Polym. Lett. 2016, 10, 84. [Google Scholar] [CrossRef]
- Duan, J.; Wu, W.; Wei, Z.; Zhu, D.; Tu, H.; Zhang, A. Synthesis of functional catechols as monomers of mussel-inspired biomimetic polymers. Green Chem. 2018, 20, 912–920. [Google Scholar] [CrossRef]
- Yin, J.; Yang, L.; Mou, L.; Dong, K.; Jiang, J.; Xue, S.; Xu, Y.; Wang, X.; Lu, Y.; Ye, H. A green tea—Triggered genetic control system for treating diabetes in mice and monkeys. Sci. Transl. Med. 2019, 11, eaav8826. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Thi, T.V.; Nguyen, T.-T.; Le, T.D.; Vo, D.M.H.; Nguyen, D.H.; Nguyen, C.K.; Nguyen, D.C.; Nguyen, T.T.; Bach, L.G.; et al. Investigation of Chitosan Nanoparticles Loaded with Protocatechuic Acid (PCA) for the Resistance of Pyricularia oryzae Fungus against Rice Blast. Polymers 2019, 11, 177. [Google Scholar]
- Tsai, T.-H.; Yu, C.-H.; Chang, Y.-P.; Lin, Y.-T.; Huang, C.-J.; Kuo, Y.-H.; Tsai, P.-J. Protective effect of caffeic acid derivatives on tert-butyl hydroperoxide-induced oxidative hepato-toxicity and mitochondrial dysfunction in HepG2 cells. Molecules 2017, 22, 702. [Google Scholar] [CrossRef]
- Montalbetti, C.A.G.N.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Bellare, M.; Kadambar, V.K.; Bollella, P.; Katz, E.; Melman, A. Electrochemically stimulated molecule release associated with interfacial pH changes. Chem. Commun. 2019, 55, 7856–7859. [Google Scholar] [CrossRef]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Stenström, P.; Andrén, O.C.J.; Malkoch, M. Fluoride-promoted esterification (FPE) chemistry: A robust route to Bis-MPA dendrons and their postfunctionalization. Molecules 2016, 21, 366. [Google Scholar] [CrossRef]
- García-Gallego, S.; Hult, D.; Olsson, J.V.; Malkoch, M. Fluoride-Promoted Esterification with Imidazolide-Activated Compounds: A Modular and Sustainable Approach to Dendrimers. Angew. Chem. Int. Ed. 2015, 54, 2416–2419. [Google Scholar] [CrossRef]
- Engels, H.-W.; Pirkl, H.-G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile materials and sustainable problem solvers for today’s challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Bruechert, S.; Andrade, S.L.A.; Hellwig, P. Secondary Structure Determination by Means of ATR-FTIR Spectroscopy. In Membrane Protein Structure and Function Characterization; Springer: Berlin/Heidelberg, Germany, 2017; pp. 195–203. [Google Scholar]
- Borah, J.M.; Sarma, J.; Mahiuddin, S. Adsorption comparison at the α-alumina/water interface: 3,4-Dihydroxybenzoic acid vs. catechol. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 387, 50–56. [Google Scholar] [CrossRef]
- Hu, X.; Bürgi, T. In Situ ATR-FTIR Investigation of Photodegradation of 3,4-Dihydroxybenzoic Acid on TiO2. Catal. Lett. 2016, 146, 2215–2220. [Google Scholar] [CrossRef]
- Ren, Z.; Ma, D.; Wang, Y.; Zhao, G. Molecular structure and hydrogen bonds in solid dimethylol propionic acid (DMPA). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2713–2722. [Google Scholar] [CrossRef]
- Kord Forooshani, P.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.; Yao, X.; Peng, Y.; Tu, X.; Du, P.; Zheng, Z.; Wang, X. Facile preparation of mussel-inspired polyurethane hydrogel and its rapid curing behavior. ACS Appl. Mater. Interfaces 2014, 6, 12495–12504. [Google Scholar] [CrossRef]
- Panchireddy, S.; Grignard, B.; Thomassin, J.-M.; Jerome, C.; Detrembleur, C. Catechol containing polyhydroxyurethanes as high-performance coatings and adhesives. ACS Sustain. Chem. Eng. 2018, 6, 14936–14944. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, Q.; Liu, Q.; Du, L. Mussel-inspired fabrication of pH-sensitive biomimetic hydrogels based on greenhouse gas carbon dioxide. New J. Chem. 2019, 43, 4757–4764. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wu, G. Effects of soft segment characteristics on the properties of biodegradable amphiphilic waterborne polyurethane prepared by a green process. J. Mater. Sci. 2020, 55, 3139–3156. [Google Scholar] [CrossRef]
- Yeganeh, H.; Lakouraj, M.M.; Jamshidi, S. Synthesis and properties of biodegradable elastomeric epoxy modified polyurethanes based on poly (ε-caprolactone) and poly (ethylene glycol). Eur. Polym. J. 2005, 41, 2370–2379. [Google Scholar] [CrossRef]
- Cooper, S.L.; Guan, J. Advances in Polyurethane Biomaterials; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Heath, D.E.; Guelcher, S.A.; Cooper, S.L. Polyurethanes. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 103–108. [Google Scholar]
- Lee, B.P.; Huang, K.; Nunalee, F.N.; Shull, K.R.; Messersmith, P.B. Synthesis of 3, 4-dihydroxyphenylalanine (DOPA) containing monomers and their co-polymerization with PEG-diacrylate to form hydrogels. J. Biomater. Sci. Polym. Ed. 2004, 15, 449–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Li, S.; Li, M.; Xu, L.; Ding, H.; Xia, J.; Zhang, M.; Huang, K. A thermal self-healing polyurethane thermoset based on phenolic urethane. Polym. J. 2017, 49, 775–781. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, T.; Guo, B.; Xu, J. Solvent-free thermo-reversible and self-healable crosslinked polyurethane with dynamic covalent networks based on phenol-carbamate bonds. Polymer (Guildf.) 2019, 181, 121788. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, T.; Zhang, Y.; Guo, B.; Xu, J. Reprocessable Crosslinked Polyurethane with Dynamic and Tunable Phenol-Carbamate Network. ACS Sustain. Chem. Eng. 2020, 8, 1207–1218. [Google Scholar] [CrossRef]
- Rodríguez-deLeón, E.; Bah, M.; Jiménez-Halla, J.O.C.; Bonilla-Cruz, J.; Estévez, M.; Báez, J.E. Synthesis and characterization of segmented poly (ester-urethane) s (PEUs) containing carotenoids. Polym. Chem. 2019, 10, 6580–6587. [Google Scholar] [CrossRef]
- García, K.E.; Navarro, R.; Ramírez-Hernández, A.; Marcos-Fernández, Á. New routes to difunctional macroglycols using ethylene carbonate: Reaction with bis-(2-hydroxyethyl) terephthalate and degradation of poly (ethylene terephthalate). Polym. Degrad. Stab. 2017, 144, 195–206. [Google Scholar] [CrossRef]
- Báez, J.E.; Ramírez, D.; Valentín, J.L.; Marcos-Fernández, A. Biodegradable poly (ester-urethane-amide) s based on poly (ε-caprolactone) and diamide—Diol chain extenders with crystalline hard segments. Synthesis and characterization. Macromolecules 2012, 45, 6966–6980. [Google Scholar] [CrossRef]
- Steponaviciute, M.; Klimkevicius, V.; Makuska, R. Synthesis and Stability against Oxidation of Random Brush Copolymers Carrying PEO Side Chains and Catechol Moieties. Mater. Today Commun. 2020, 25, 101262. [Google Scholar] [CrossRef]
- Berberich, O.; Blöhbaum, J.; Hölscher-Doht, S.; Meffert, R.H.; Teßmar, J.; Blunk, T.; Groll, J. Catechol-modified poly (oxazoline) s with tunable degradability facilitate cell invasion and lateral cartilage integration. J. Ind. Eng. Chem. 2019, 80, 757–769. [Google Scholar] [CrossRef]
- Nam, K.-H.; Jin, J.-U.; Lee, J.H.; Kim, J.; Chung, Y.S.; Yeo, H.; You, N.-H.; Ku, B.-C. Highly efficient thermal oxidation and cross-linking reaction of catechol functionalized polyacrylonitrile copolymer composites for halogen-free flame retardant. Compos. Part B Eng. 2020, 184, 107687. [Google Scholar] [CrossRef]
- Milite, C.; Feoli, A.; Sasaki, K.; La Pietra, V.; Balzano, A.L.; Marinelli, L.; Mai, A.; Novellino, E.; Castellano, S.; Tosco, A.; et al. A novel cell-permeable, selective, and noncompetitive inhibitor of KAT3 histone acetyltransferases from a combined molecular pruning/classical isosterism approach. J. Med. Chem. 2015, 58, 2779–2798. [Google Scholar] [CrossRef] [PubMed]
- Weibel, N.; Błaszczyk, A.; Von Hänisch, C.; Mayor, M.; Pobelov, I.; Wandlowski, T.; Chen, F.; Tao, N. Redox-Active Catechol-Functionalized Molecular Rods: Suitable Protection Groups and Single-Molecule Transport Investigations. Eur. J. Org. Chem. 2008, 2008, 136–149. [Google Scholar] [CrossRef]
- Yang, J.; Stuart, M.A.C.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, H.; Sader, F.; Vadhavkar, N.; Njus, D. Oxidation of 4-methylcatechol: Implications for the oxidation of catecholamines. Biochemistry 2007, 46, 6978–6983. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Kawabata, J. Synergistic effects of thiols and amines on antiradical efficiency of protocatechuic acid. J. Agric. Food Chem. 2004, 52, 8163–8168. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Okamoto, Y.; Kawabata, J. Effects of alcoholic solvents on antiradical abilities of protocatechuic acid and its alkyl esters. Biosci. Biotechnol. Biochem. 2004, 68, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Filippidi, E.; Cristiani, T.R.; Eisenbach, C.D.; Waite, J.H.; Israelachvili, J.N.; Ahn, B.K.; Valentine, M.T. Toughening elastomers using mussel-inspired iron-catechol complexes. Science 2017, 358, 502–505. [Google Scholar] [CrossRef] [Green Version]





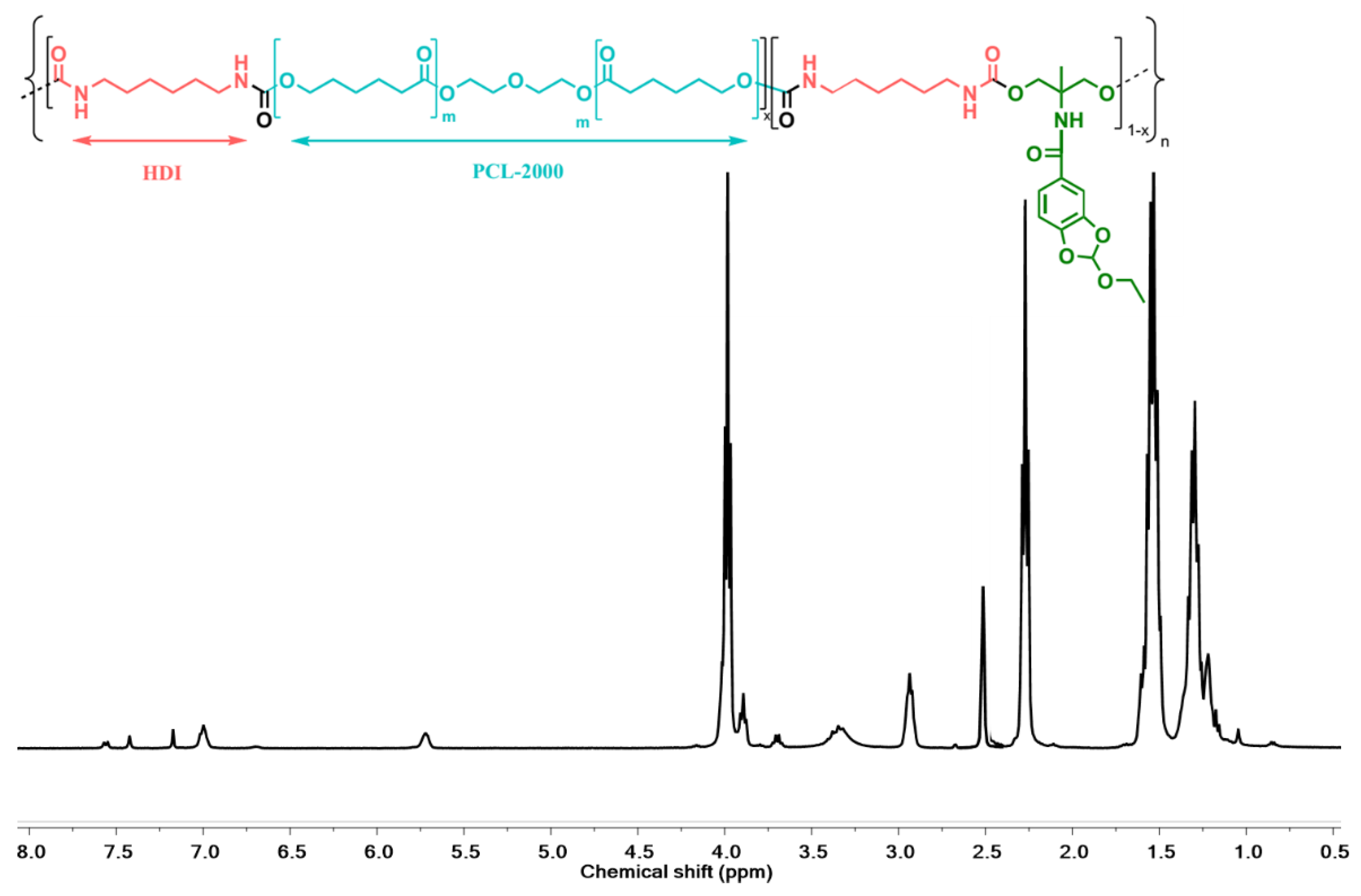
| Sample | PCL2000 a | HDI a | Cat-Fun a | %HS | Mn (kDa) | Mw (kDa) | Ð |
|---|---|---|---|---|---|---|---|
| PU-Cat HS 20 | 80.0 | 12.1 | 7.9 | 20 | 42.6 | 60.5 | 1.52 |
| PU-Cat HS 30 | 70.0 | 15.7 | 14.3 | 30 | 26.4 | 38.2 | 1.44 |
| PU-Cat HS 40 | 60.0 | 19.4 | 20.6 | 40 | 19.0 | 24.4 | 1.28 |
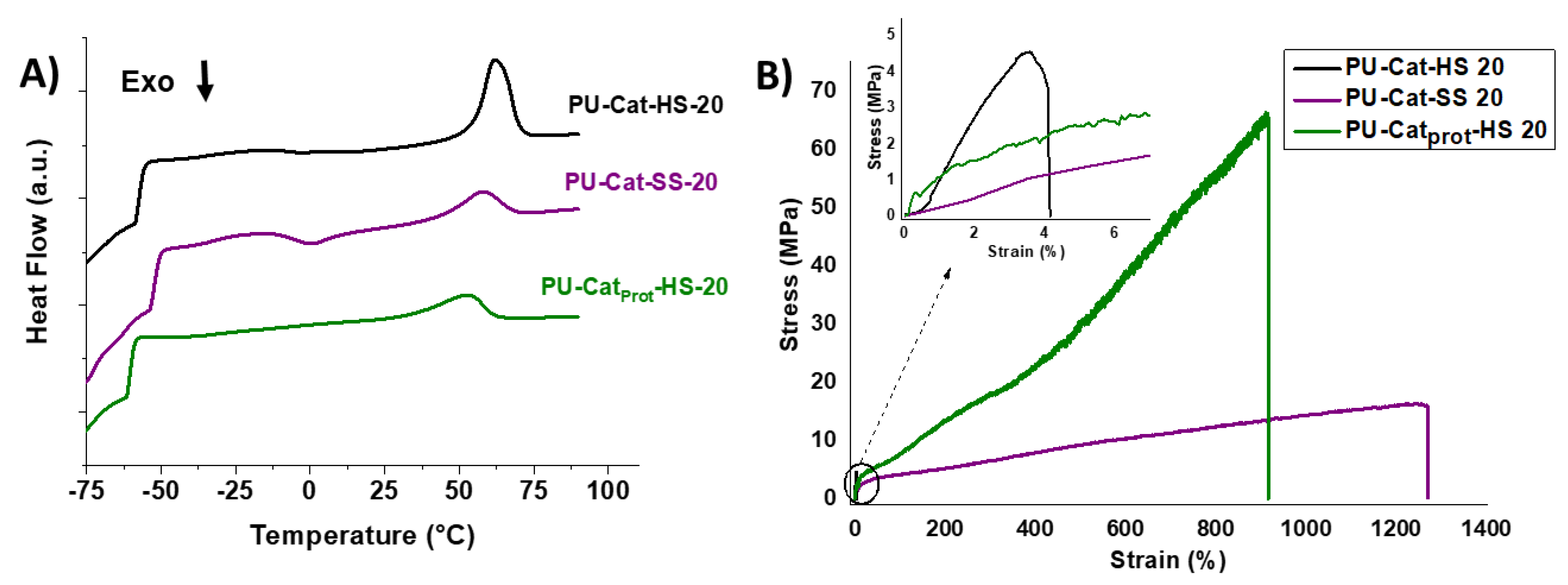
| PU | Mn (kDa) | Mw (kDa) | Ð | Tg (°C) | Tc (°C) | Tm (°C) | Elastic Modulus (MPa) | Stress (MPa) | Strain (%) |
|---|---|---|---|---|---|---|---|---|---|
| PU-Cat-HS 20 | 42.6 | 60.5 | 1.52 | −57.2 | - | 62.3 | 152.9 ± 2.9 | 4.5 ± 0.14 | 4.12 ± 0.52 |
| PU-Cat-SS 20 | 66.4 | 150.2 | 2.26 | −52.4 | 0.1 | 57.9 | 9.58 ± 2.6 | 16.3 ± 1.1 | 1250 ± 160 |
| PU-Catprot-HS 20 | 180.9 | 280.0 | 1.54 | −59.7 | - | 53.7 | 9.07 ± 1.5 | 66 ± 8 | 910 ± 120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briz-López, E.M.; Navarro, R.; Martínez-Hernández, H.; Téllez-Jurado, L.; Marcos-Fernández, Á. Design and Synthesis of Bio-Inspired Polyurethane Films with High Performance. Polymers 2020, 12, 2727. https://doi.org/10.3390/polym12112727
Briz-López EM, Navarro R, Martínez-Hernández H, Téllez-Jurado L, Marcos-Fernández Á. Design and Synthesis of Bio-Inspired Polyurethane Films with High Performance. Polymers. 2020; 12(11):2727. https://doi.org/10.3390/polym12112727
Chicago/Turabian StyleBriz-López, Eva Marina, Rodrigo Navarro, Héctor Martínez-Hernández, Lucía Téllez-Jurado, and Ángel Marcos-Fernández. 2020. "Design and Synthesis of Bio-Inspired Polyurethane Films with High Performance" Polymers 12, no. 11: 2727. https://doi.org/10.3390/polym12112727
APA StyleBriz-López, E. M., Navarro, R., Martínez-Hernández, H., Téllez-Jurado, L., & Marcos-Fernández, Á. (2020). Design and Synthesis of Bio-Inspired Polyurethane Films with High Performance. Polymers, 12(11), 2727. https://doi.org/10.3390/polym12112727








