3D Nanoarchitecture of Polyaniline-MoS2 Hybrid Material for Hg(II) Adsorption Properties
Abstract
1. Introduction
2. Experimental Details
2.1. Materials and Methods
2.1.1. Chemicals and Reagents
2.1.2. Synthesis of PANI–MoS2 Hybrid Material
2.2. Material Characterization
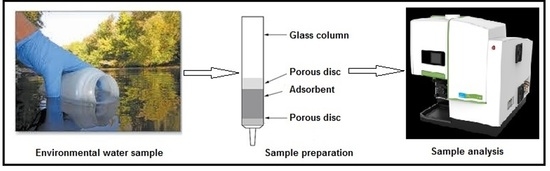
2.3. Recommended Column Procedure
3. Results and Discussion
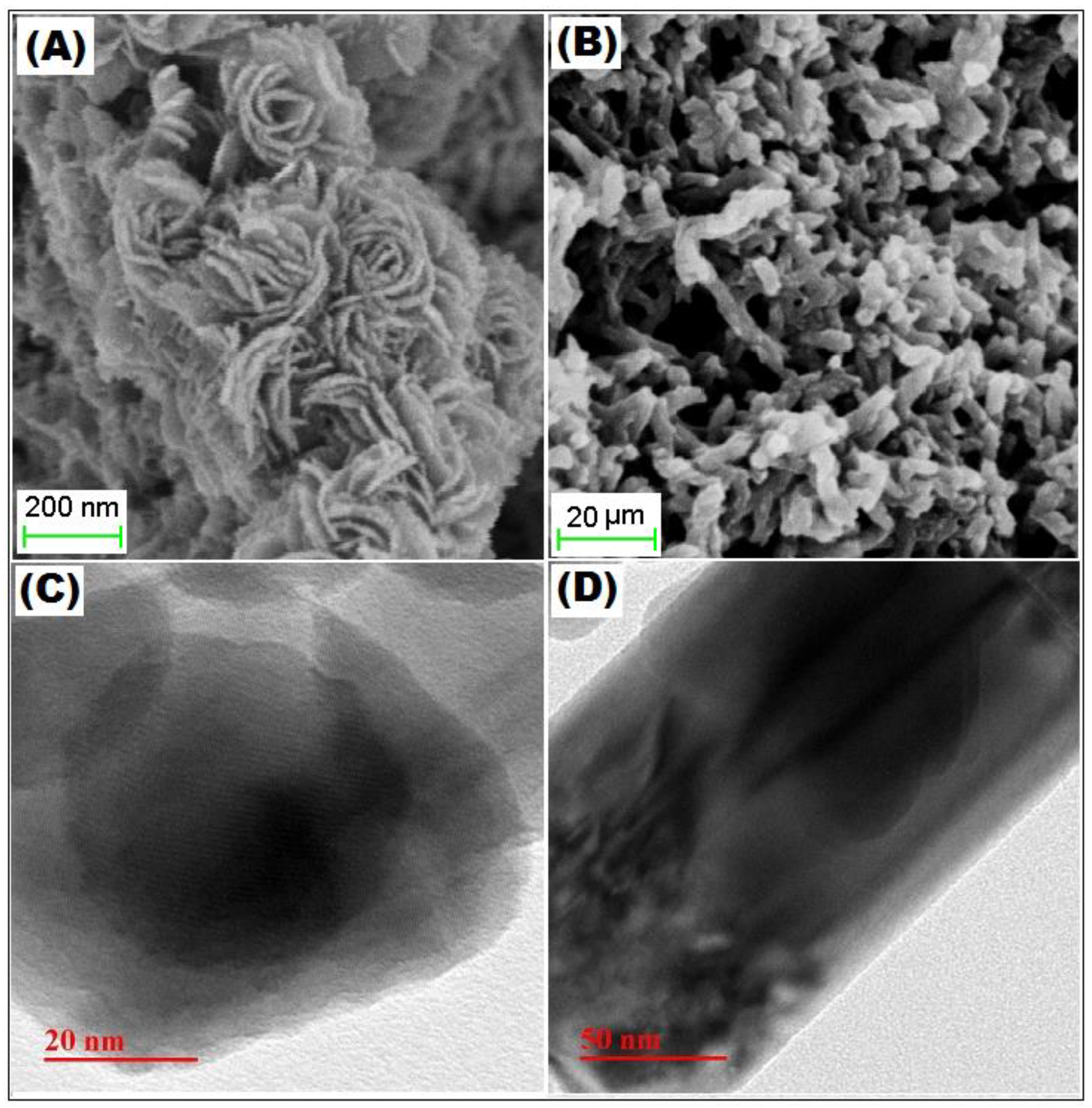
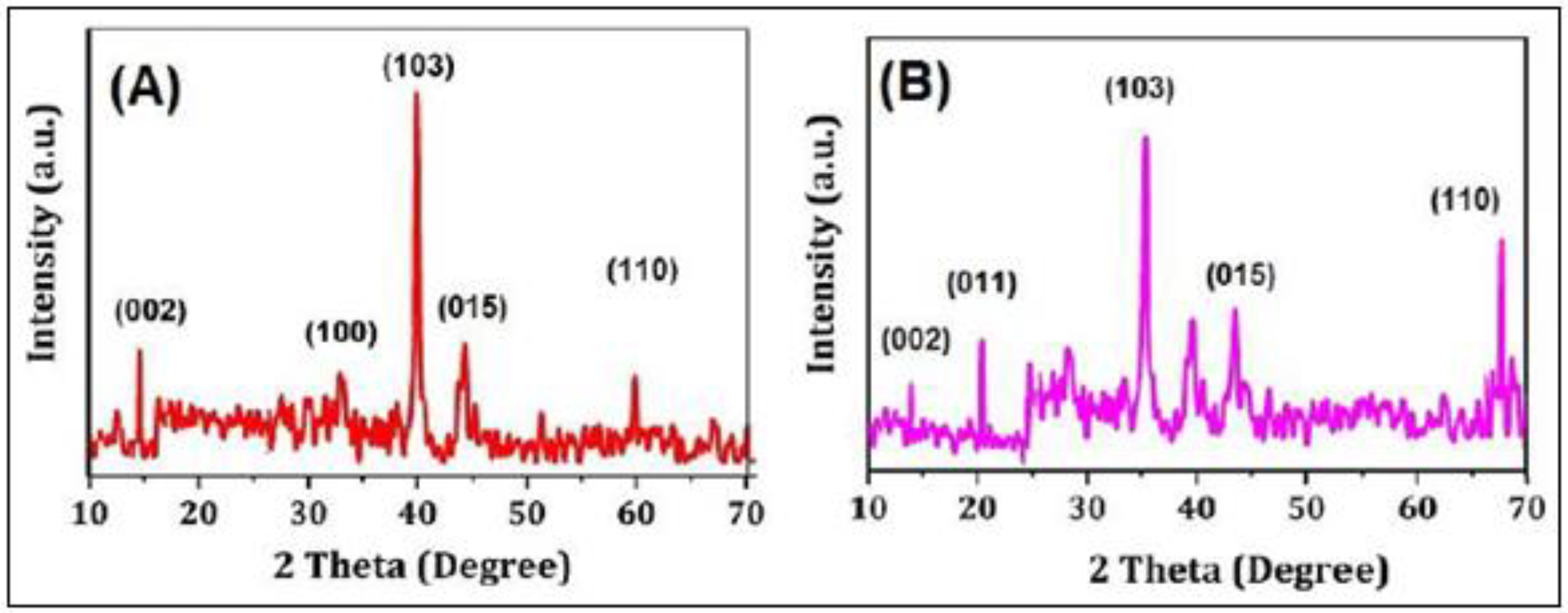
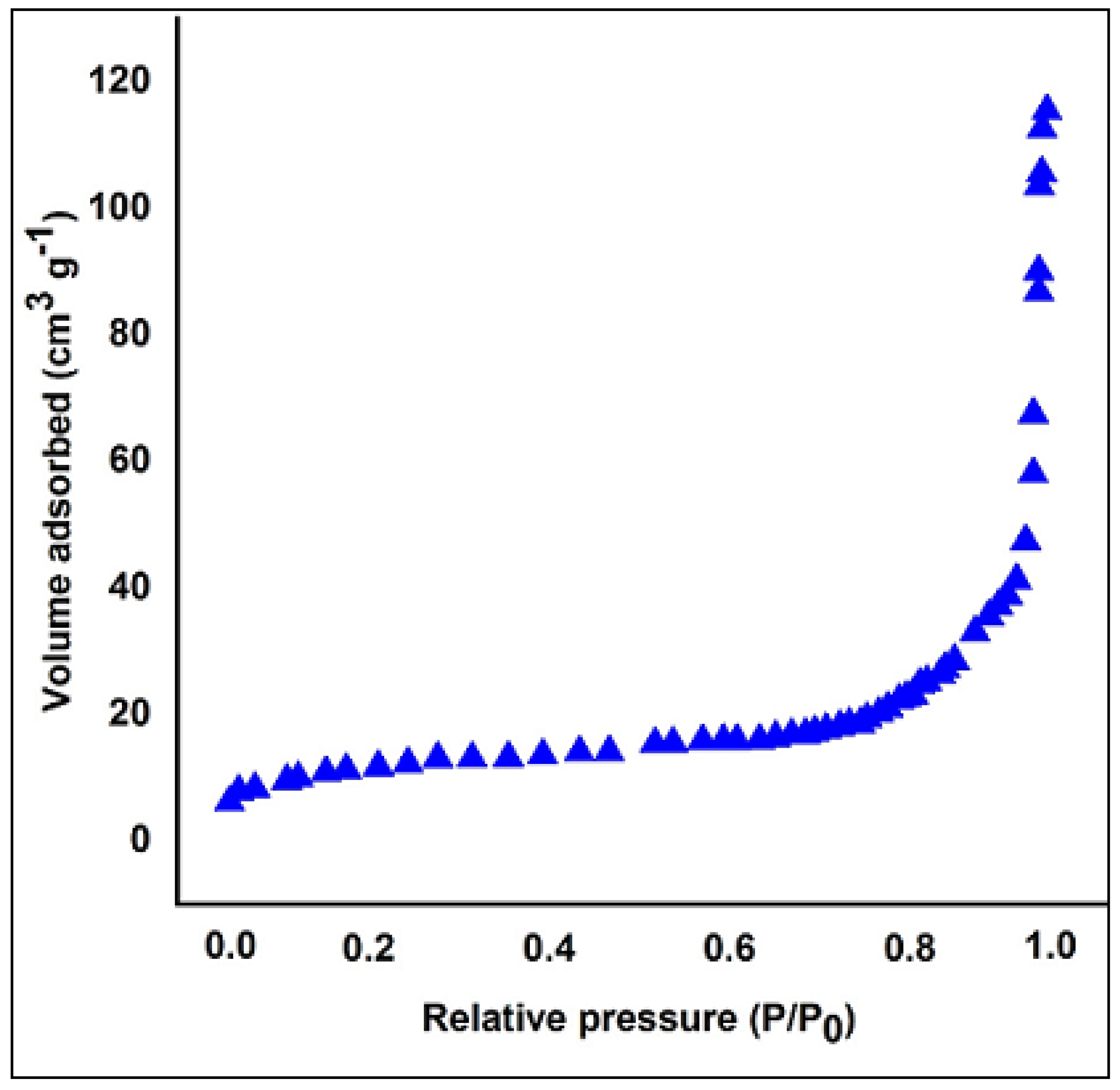
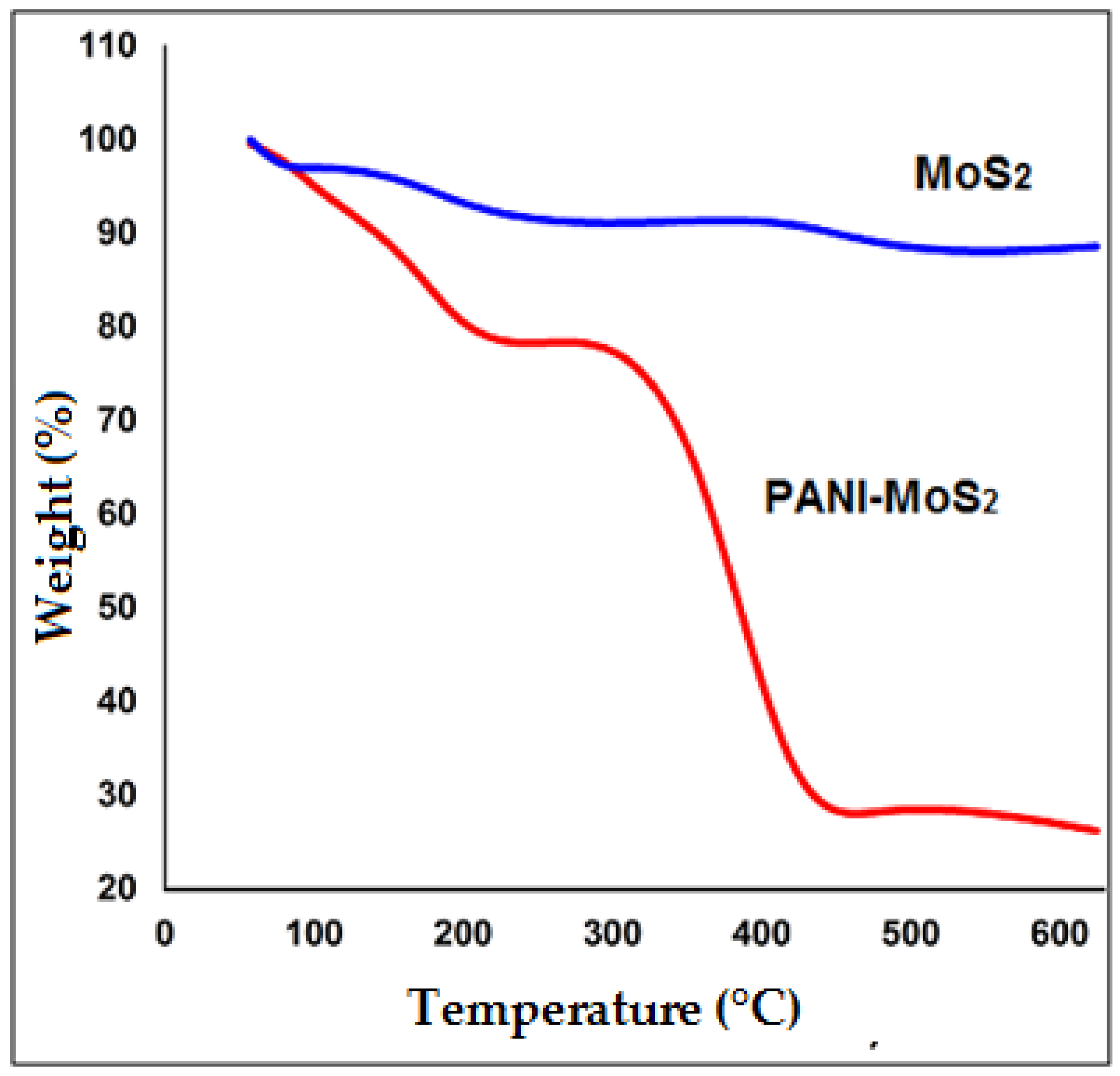
3.1. Characterization
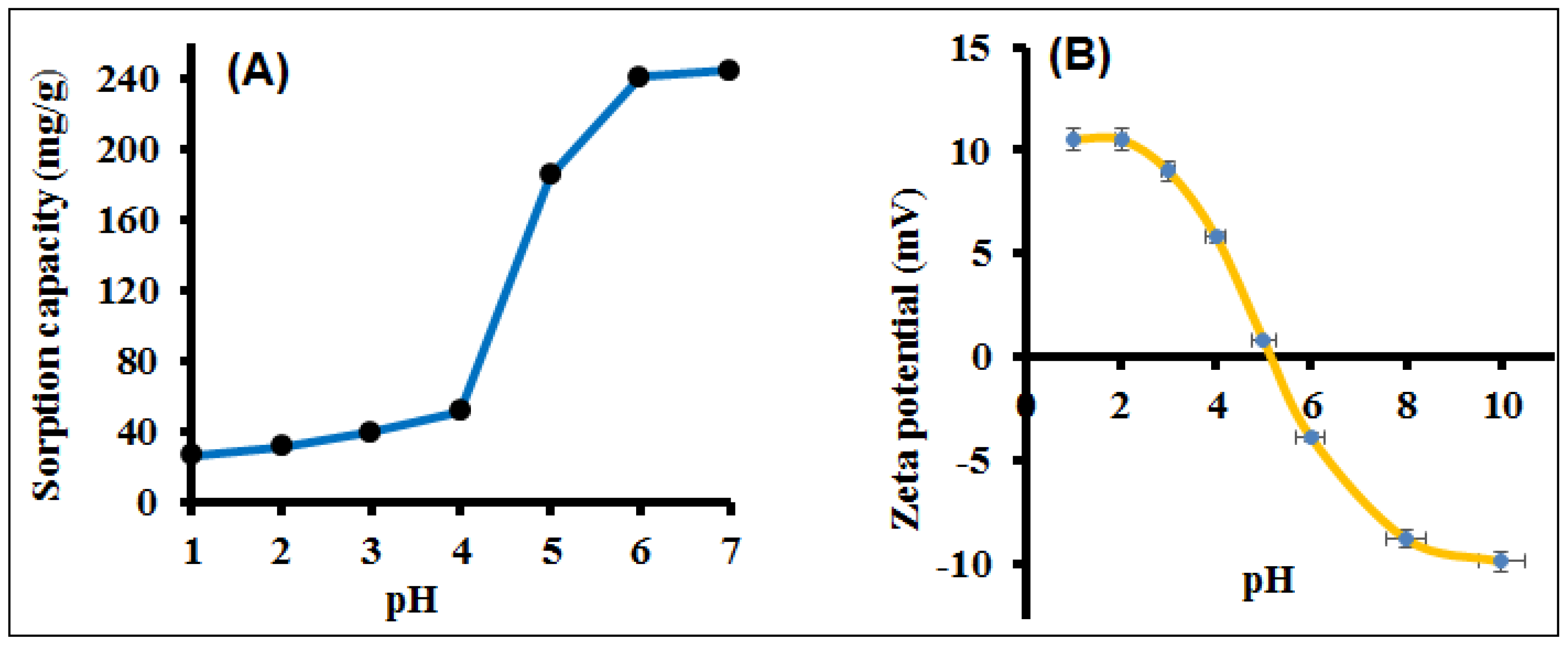
3.2. Optimized Sample pH and Adsorption Mechanism
3.3. Preconcentration and Breakthrough Studies
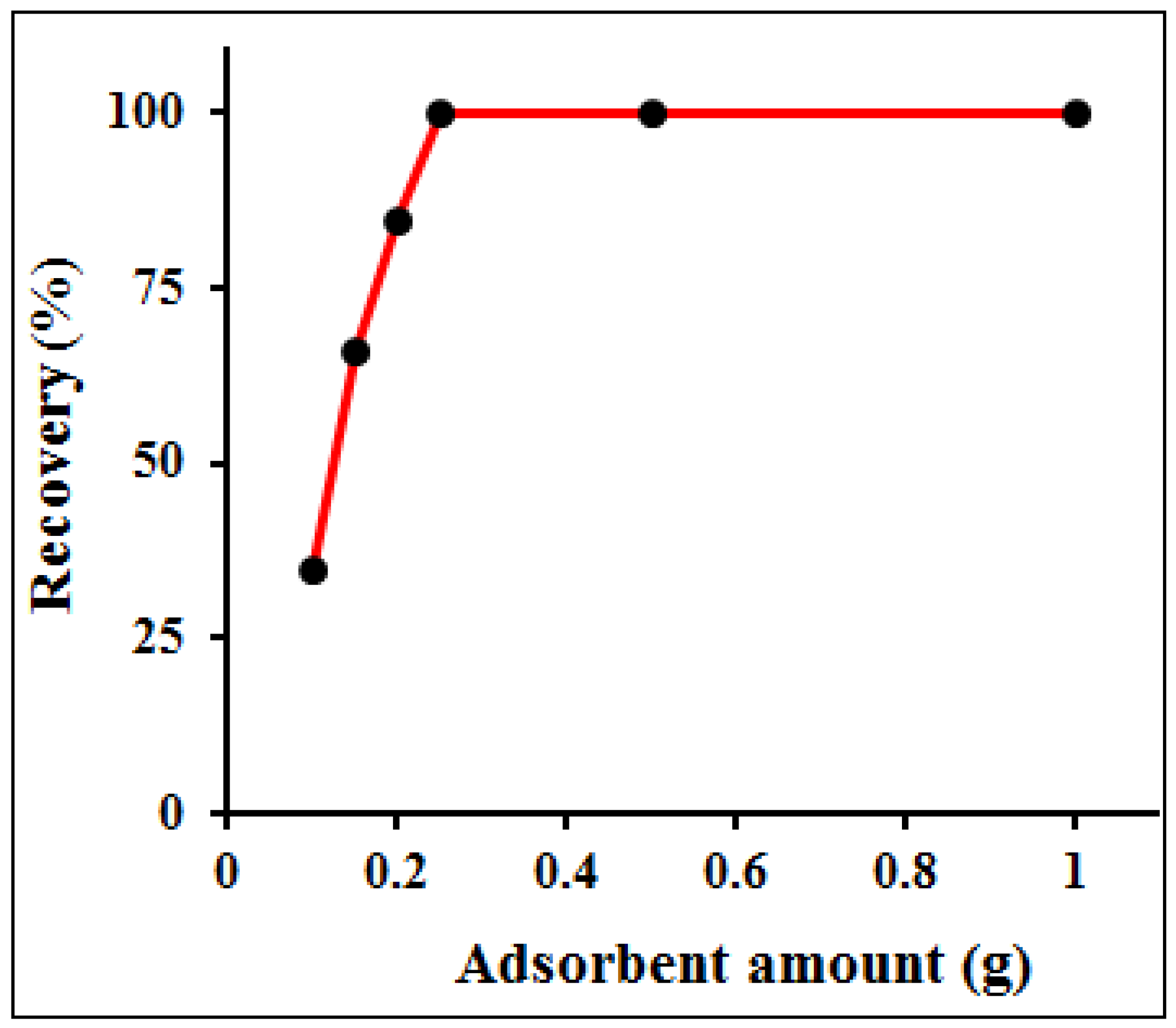
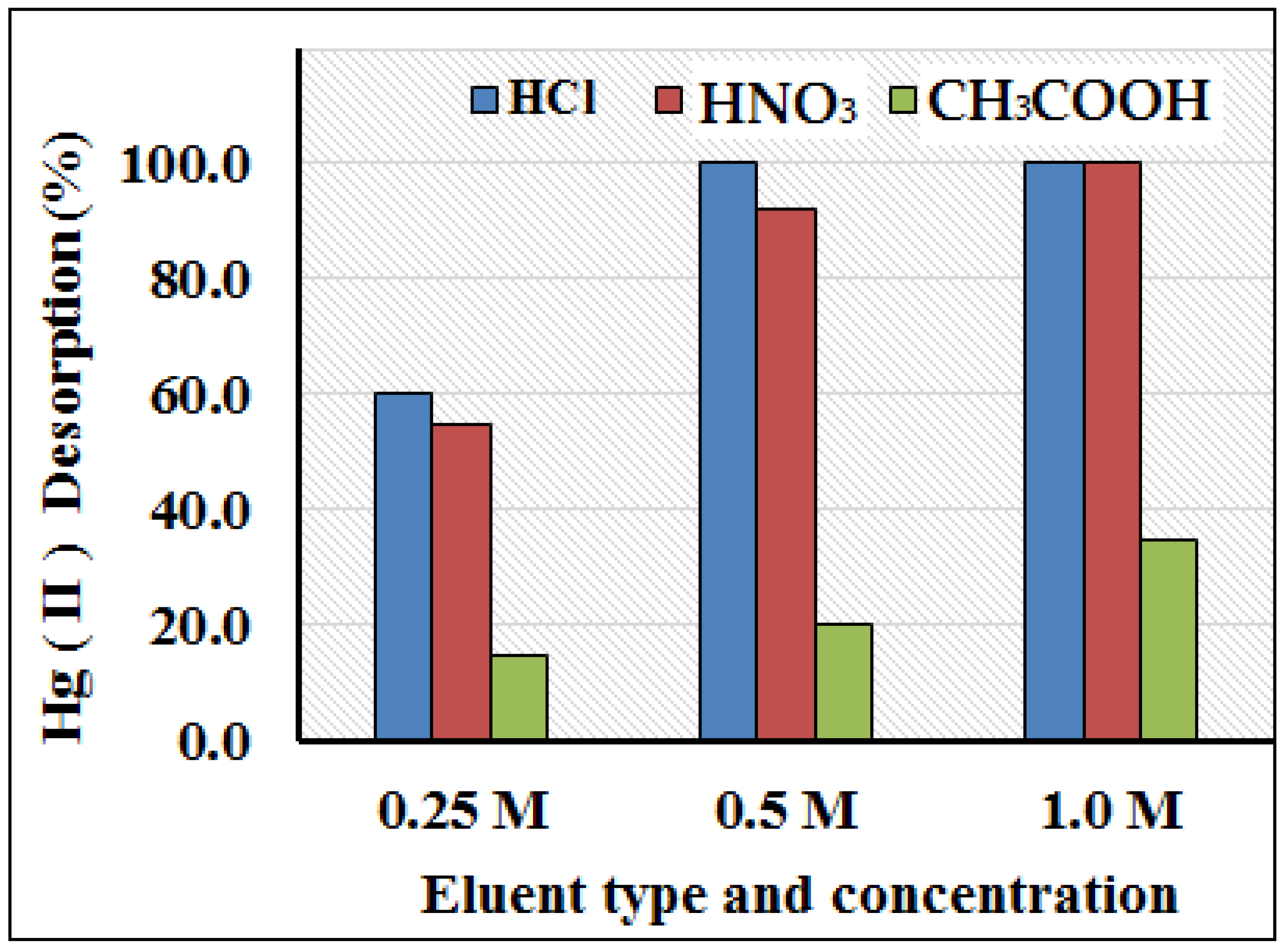
3.4. Amount of Sorbent and Choice of Eluent and Concentration
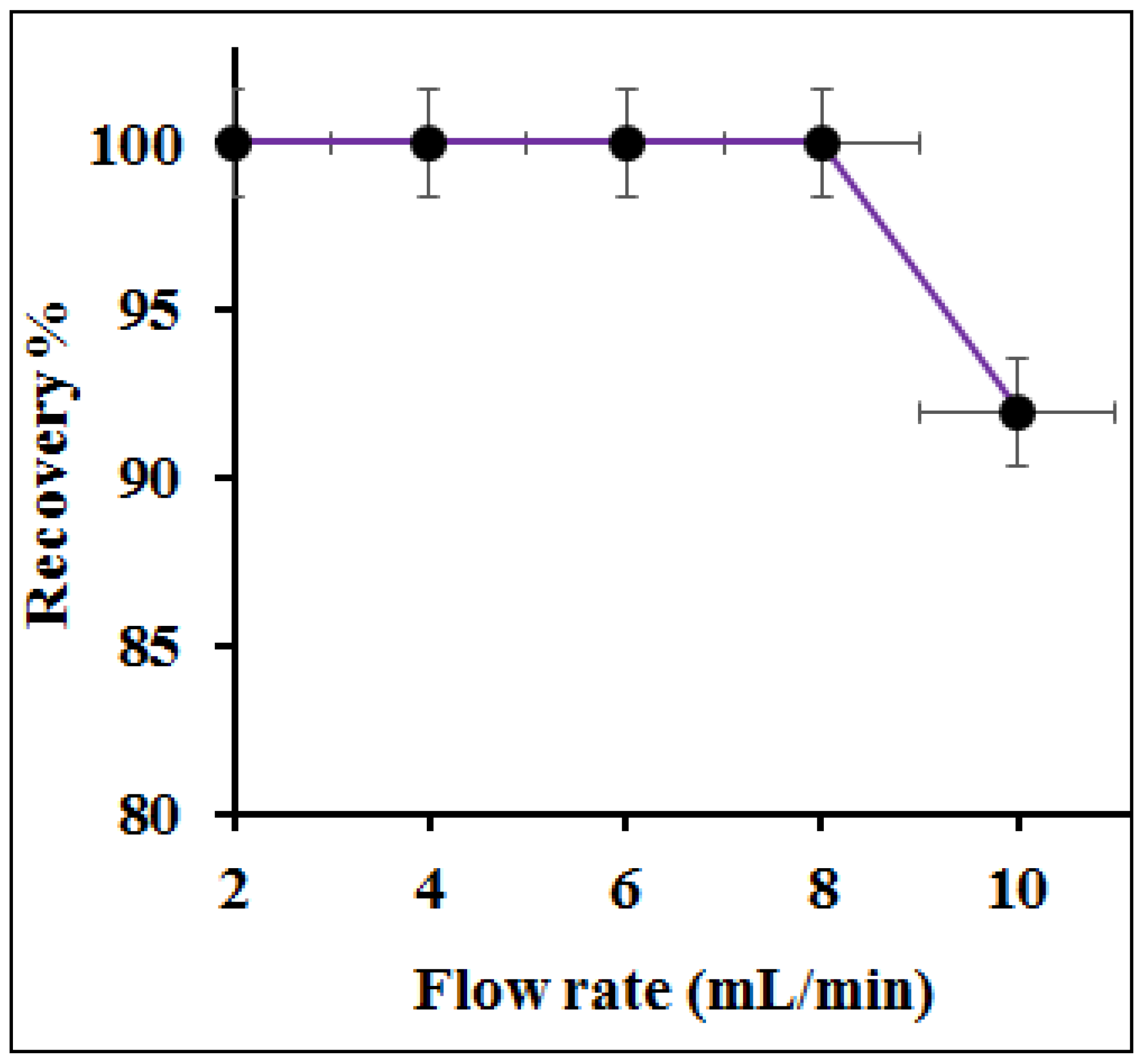
3.5. Influence of Column Flow Rate on Preconcentration Efficiency
3.6. Interference Studies
3.7. Analytical Figures of Merit and Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Available online: https://www.atsdr.cdc.gov/spl/index.html#2019spl (accessed on 8 October 2020).
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef]
- Lohren, H.; Blagojevic, L.; Fitkau, R.; Ebert, F.; Schildknecht, S.; Leist, M.; Schwerdtle, T. Toxicity of organic and inorganic mercury species in differentiated human neurons and human astrocytes. J. Trace Elem. Med. Biol. 2015, 32, 200–208. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Inorganic (accessed on 8 October 2020).
- Gómez-Ariza, J.L.; Lorenzo, F.; García-Barrera, T. Comparative study of atomic fluorescence spectroscopy and inductively coupled plasma mass spectrometry for mercury and arsenic multispeciation. Anal. Bioanal. Chem. 2005, 382, 485–492. [Google Scholar] [CrossRef]
- Nakadi, F.V.; Garde, R.; da Veiga, M.A.M.S.; Cruces, J.; Resano, M. A simple and direct atomic absorption spectrometry method for the direct determination of Hg in dried blood spots and dried urine spots prepared using various microsampling devices. J. Anal. At. Spectrom. 2020, 35, 136–144. [Google Scholar] [CrossRef]
- Vicentino, P.D.O.; Brum, D.M.; Cassella, R.J. Development of a method for total Hg determination in oil samples by cold vapor atomic absorption spectrometry after its extraction induced by emulsion breaking. Talanta 2015, 132, 733–738. [Google Scholar] [CrossRef]
- Haseen, U.; Ahmad, H. Preconcentration and Determination of Trace Hg(II) Using a Cellulose Nanofiber Mat Functionalized with MoS2 Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 3198–3204. [Google Scholar] [CrossRef]
- Rastegarifard, F.; Ghanemi, K.; Fallah-Mehrjardi, M. A deep eutectic solvent-based extraction method for fast determination of Hg in marine fish samples by cold vapor atomic absorption spectrometry. Anal. Methods 2017, 9, 5741–5748. [Google Scholar] [CrossRef]
- Ojeda, C.B.; Rojas, F.S. Separation and preconcentration by cloud point extraction procedures for determination of ions: Recent trends and applications. Microchim. Acta 2012, 177, 1–21. [Google Scholar] [CrossRef]
- De Diego, A.; Pécheyran, C.; Tseng, C.M.; Donard, O.F.X. Chapter 12—Cryofocusing for on-line metal and metalloid speciation in the environment. In Analytical Spectroscopy Library; Sanz-Medel, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 9, pp. 375–406. [Google Scholar]
- Zheng, H.; Hong, J.; Luo, X.; Li, S.; Wang, M.; Yang, B.; Wang, M. Combination of sequential cloud point extraction and hydride generation atomic fluorescence spectrometry for preconcentration and determination of inorganic and methyl mercury in water samples. Microchem. J. 2019, 145, 806–812. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Vieira, M.A.; Curtius, A.J. Determination of hydride forming elements (As, Sb, Se, Sn) and Hg in environmental reference materials as acid slurries by on-line hydride generation inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2004, 59, 243–253. [Google Scholar] [CrossRef]
- Liška, I. Fifty years of solid-phase extraction in water analysis – historical development and overview. J. Chromatogr. A 2000, 885, 3–16. [Google Scholar] [CrossRef]
- Buszewski, B.; Szultka, M. Past, Present, and Future of Solid Phase Extraction: A Review. Crit. Rev. Anal. Chem. 2012, 42, 198–213. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Deemer, E.; Fernandez-Delgado, O.; Wang, H.; Curry, M.L.; El-Gendy, A.A.; Noveron, J.C. Fe nanoparticles encapsulated in MOF-derived carbon for the reduction of 4-nitrophenol and methyl orange in water. Catal. Commun. 2019, 130, 105753. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Fernandez-Delgado, O.; Deemer, E.; Wang, H.; El-Gendy, A.A.; Curry, M.L.; Noveron, J.C. Carbonization of Co-BDC MOF results in magnetic C@Co nanoparticles that catalyze the reduction of methyl orange and 4-nitrophenol in water. J. Mol. Liq. 2019, 290, 111059. [Google Scholar] [CrossRef]
- Vasconcelos, I.; Fernandes, C. Magnetic solid phase extraction for determination of drugs in biological matrices. Trac Trends Anal. Chem. 2017, 89, 41–52. [Google Scholar] [CrossRef]
- Herrero Latorre, C.; Álvarez Méndez, J.; Barciela García, J.; García Martín, S.; Peña Crecente, R.M. Carbon nanotubes as solid-phase extraction sorbents prior to atomic spectrometric determination of metal species: A review. Anal. Chim. Acta 2012, 749, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.A.; Jabbari, V.; Imam, M.A.; Castro, E.; Kim, H.; Curry, M.L.; Valles-Rosales, D.J.; Noveron, J.C. Nanoscale nickel metal organic framework decorated over graphene oxide and carbon nanotubes for water remediation. Sci. Total Environ. 2020, 698, 134214. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Zuo, W.; Chen, F.; Wang, B. 3D MoS2 Composition Aerogels as Chemosensors and Adsorbents for Colorimetric Detection and High-Capacity Adsorption of Hg2+. Acs Sustain. Chem. Eng. 2016, 4, 3398–3408. [Google Scholar] [CrossRef]
- Li, J.-R.; Wang, X.; Yuan, B.; Fu, M.-L.; Cui, H.-J. Robust removal of heavy metals from water by intercalation chalcogenide [CH3NH3]2xMnxSn3−xS6·0.5H2O. Appl. Surf. Sci. 2014, 320, 112–119. [Google Scholar] [CrossRef]
- Li, J.-R.; Wang, X.; Yuan, B.; Fu, M.-L. Layered chalcogenide for Cu2+ removal by ion-exchange from wastewater. J. Mol. Liq. 2014, 200, 205–212. [Google Scholar] [CrossRef]
- Bag, S.; Arachchige, I.U.; Kanatzidis, M.G. Aerogels from metal chalcogenides and their emerging unique properties. J. Mater. Chem. 2008, 18, 3628–3632. [Google Scholar] [CrossRef]
- Gao, M.-R.; Xu, Y.-F.; Jiang, J.; Yu, S.-H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef]
- Cheng, W.; Rechberger, F.; Niederberger, M. Three-Dimensional Assembly of Yttrium Oxide Nanosheets into Luminescent Aerogel Monoliths with Outstanding Adsorption Properties. ACS Nano 2016, 10, 2467–2475. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.-L.; Xu, W.-H.; Huang, X.-J.; Liu, J.-H.; Xu, A.-W. Stable Organic–Inorganic Hybrid of Polyaniline/α-Zirconium Phosphate for Efficient Removal of Organic Pollutants in Water Environment. ACS Appl. Mater. Interfaces 2012, 4, 2686–2692. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, C.; Chen, H.; Zhang, R.; Wang, X. Synthesis of a novel organic–inorganic hybrid of polyaniline/titanium phosphate for Re(vii) removal. Dalton Trans. 2015, 44, 8917–8925. [Google Scholar] [CrossRef]
- Pearson, R.G. The HSAB principle—more quantitative aspects. Inorg. Chim. Acta 1995, 240, 93–98. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581. [Google Scholar] [CrossRef]
- Lara, R.; Cerutti, S.; Salonia, J.A.; Olsina, R.A.; Martinez, L.D. Trace element determination of Argentine wines using ETAAS and USN-ICP-OES. Food Chem. Toxicol. 2005, 43, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Li, X.; Wang, C.; Yu, A.; Zhang, S.; Ouyang, G.; Cui, Y. Adsorption behavior and mechanism of Hg (II) on a porous core-shell copper hydroxy sulfate@MOF composite. Appl. Surf. Sci. 2021, 538, 148054. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Hu, Y.; Zhao, H.; Zhou, J.; Liu, Y.; Lou, Z.; Xu, X. Functional nanomaterials: Study on aqueous Hg(II) adsorption by magnetic Fe3O4@SiO2-SH nanoparticles. J. Taiwan Inst. Chem. Eng. 2016, 60, 394–402. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, X.; Wang, T.; Fu, J.; Ni, J. Selective and irreversible adsorption of mercury(ii) from aqueous solution by a flower-like titanate nanomaterial. J. Mater. Chem. A 2015, 3, 17676–17684. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zhao, Y.; Xia, K.; Guo, Y.; Qu, Z.; Bai, R. A mild and facile synthesis of amino functionalized CoFe2O4@ SiO2 for Hg (II) removal. Nanomaterials 2018, 8, 673. [Google Scholar] [CrossRef]



| Preconcentration Studies | Breakthrough Studies | ||||||
|---|---|---|---|---|---|---|---|
| Sample Volume (mL) | Hg(II) Amount (µg L−1) | E(%) a | PL b (µg L−1) | PF c | Column Adsorption Capacity (mg g−1) | Breakthrough Volume (mL) | Breakthrough Capacity (mg g−1) |
| 1500 | 0.66 | 100 | 0.66 | 300 | 4000 | 160.5 | |
| 2000 | 0.50 | 100 | 0.50 | 400 | 240.0 | ||
| 2700 | 0.37 | 100 | 0.37 | 540 | |||
| 3200 | 0.31 | 100 | 0.31 | 640 | |||
| 3500 | 0.29 | 90 | - | - | |||
| 4000 | 0.25 | 85 | - | - | |||
| Interfering Ions | Salt Added | Amount Added (×103 µg L−1) | Recovery % (RSD) |
|---|---|---|---|
| Hg(II) | |||
| Na+ | NaCl | 6000 | 98.0 (4.15) |
| K+ | KCl | 5600 | 98.9 (4.65) |
| Ca2+ | CaCl2 | 900 | 97.0 (3.00) |
| Mg2+ | MgCl2 | 1500 | 99.7 (4.00) |
| Cl− | NaCl | 9000 | 100 (4.23) |
| Br− | NaBr | 8000 | 99.8 (3.54) |
| CO32− | Na2CO3 | 4500 | 98.7 (4.18) |
| SO42− | Na2SO4 | 4200 | 98.6 (4.25) |
| NO3− | NaNO3 | 3500 | 100.4 (4.05) |
| CH3COO− | CH3COONa | 4000 | 96.5 (4.94) |
| C6H5O73− | Na3C6H5O7 | 3300 | 99.5 (4.16) |
| Samples | Analyte | Certified Values (µg g−1) | Values Found by Proposed Method (µg g−1) a ± Standard Deviation | Value of t-Test b |
|---|---|---|---|---|
| NIST SRM 1641d | Hg(II) | 1.56 ± 0.02 | 1.55 ± 0.06 | 1.37 |
| Adsorbent | Metal Ion | Adsorption Capacity (mg g−1) | References |
|---|---|---|---|
| PANI–MoS2 | Hg(II) | 240 | This work |
| MOF | Hg(II) | 627.6 | [38] |
| Fe3O4@SiO2SH | Hg(II) | 132.0 | [39] |
| MSCFM | Hg(II) | 160.4 | [12] |
| Titanate nanoflowers | Hg(II) | 454.5 | [40] |
| Magnetic composite | Hg(II) | 149.3 | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, H.; BinSharfan, I.I.; Khan, R.A.; Alsalme, A. 3D Nanoarchitecture of Polyaniline-MoS2 Hybrid Material for Hg(II) Adsorption Properties. Polymers 2020, 12, 2731. https://doi.org/10.3390/polym12112731
Ahmad H, BinSharfan II, Khan RA, Alsalme A. 3D Nanoarchitecture of Polyaniline-MoS2 Hybrid Material for Hg(II) Adsorption Properties. Polymers. 2020; 12(11):2731. https://doi.org/10.3390/polym12112731
Chicago/Turabian StyleAhmad, Hilal, Ibtisam I. BinSharfan, Rais Ahmad Khan, and Ali Alsalme. 2020. "3D Nanoarchitecture of Polyaniline-MoS2 Hybrid Material for Hg(II) Adsorption Properties" Polymers 12, no. 11: 2731. https://doi.org/10.3390/polym12112731
APA StyleAhmad, H., BinSharfan, I. I., Khan, R. A., & Alsalme, A. (2020). 3D Nanoarchitecture of Polyaniline-MoS2 Hybrid Material for Hg(II) Adsorption Properties. Polymers, 12(11), 2731. https://doi.org/10.3390/polym12112731