Py–FTIR–GC/MS Analysis of Volatile Products of Automobile Shredder Residue Pyrolysis
Abstract
:1. Introduction
2. Experimental Procedure and Specimens
2.1. Experimental Specimens
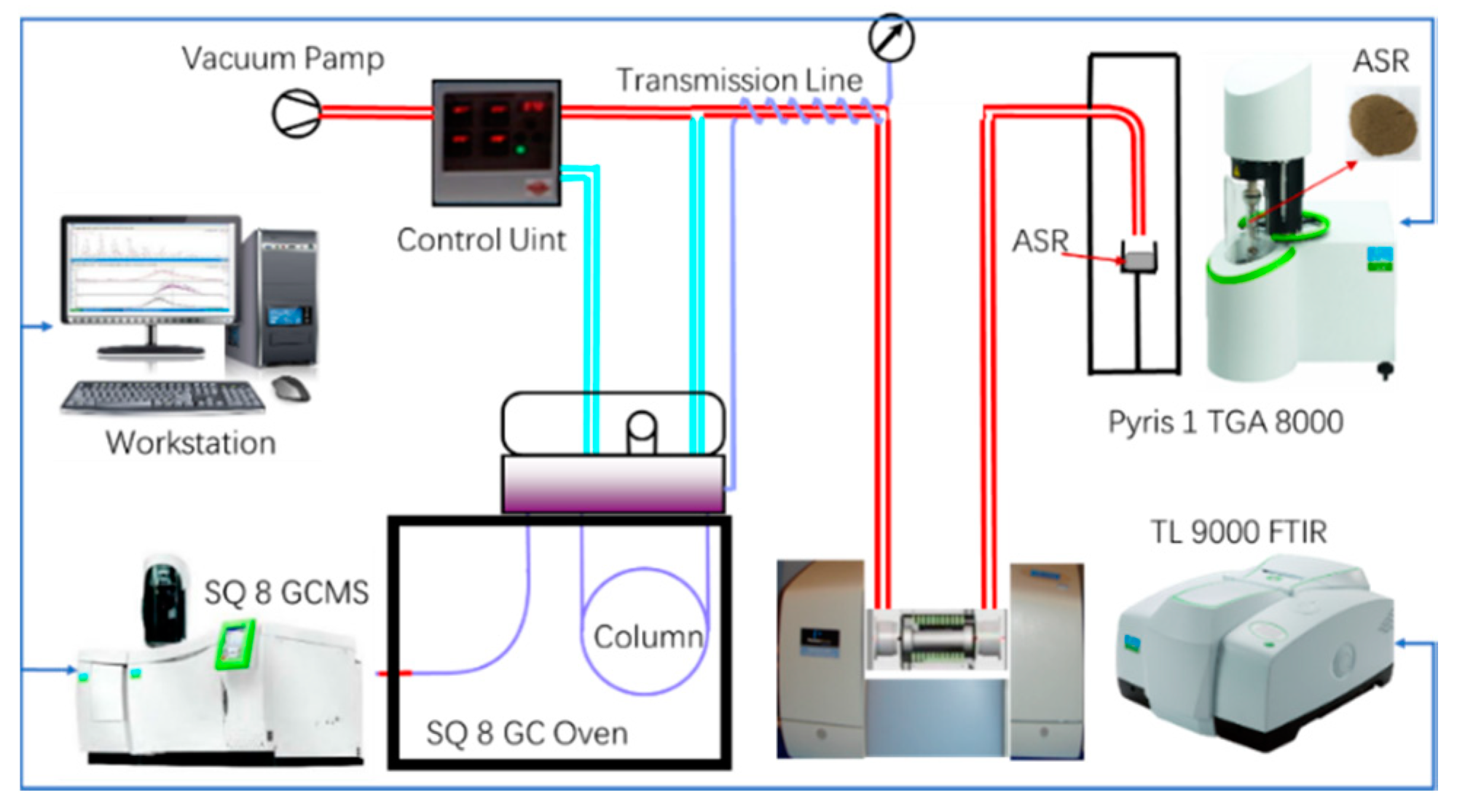
2.2. Experimental Procedure
3. Results and Discussion
3.1. FTIR Analysis of Volatile Products
3.2. GC/MS Analysis of Volatile Products of ASR Pyrolysis
- (1)
- The identified gaseous pyrolytic products were both composed of alkanes, olefins, alcohols, and benzene series, which were consistent with the analysis results of FTIR. The gaseous pyrolytic products of MixASR and ASR were not the simple superposition of pyrolytic gaseous products of its components, but main compounds of MixASR and ASR also appeared in the pyrolytic products of its main components, especially plastic and textile, e.g., styrene, 1-hexene, toluene, ethylbenzene, 2,4-dimethyl-1heptene, and 11-methyl-1-dodecanol.
- (2)
- In the volatile products of the plastics, rubber, leather, textiles, and foam pyrolysis, the total proportions of detectable macromolecular substances were 26.2634, 34.9797, 39.0640, 54.1273, and 39.2296%, respectively. The pyrolysis product of plastic contains 6.0263% olefins, 2.5443% alcohols, 15.25114% benzene series, and very few alkanes. The pyrolysis product of the rubber component contains 13.3232% alkanes, 4.7653% olefins, and 4.6502% alcohols. The leather component pyrolysis product contains 3.3224% alkanes, 7.1102% alkenes, 6.4884% alcohols, and 7.4626% ethers. The pyrolysis products of textiles components contained 1.1139% alkanes, 20.4953% olefins, 7.5055% alcohols, and 15.9611% benzene series. The pyrolysis products of foam components contained 6.8049% alkanes, 12.4046% olefins, 2.4621% ethers, 1.8138% benzene series, and 13.4151% ketones.
- (3)
- The total proportions of detectable macromolecular substances in the volatile products of the original ASR and MixASR pyrolysis were 41.884% and 40.2709%, respectively. The specific detected substances are shown in Table 2 and Table 3. The ASR pyrolysis product contains 3.7385% alkanes, 26.5539% alkenes, and 9.3305% benzene series. The MixASR pyrolysis products contains 0.4045% alkanes, 2.1909% olefins, and 17.739% benzene series. From the comparison of the yield of various pyrolysis products, it can also be seen that the pyrolysis products of ASR or MixASR are not the linear superposition of the pyrolysis products of its main components. This shows once again that the main components of ASR have obvious interactions in the pyrolysis process, and this effect affects the product distribution.
- (4)
- Based on the GC–MS analysis results of ASR and its main components, it can be seen that the yields of olefin and benzene series are high in the pyrolytic products, especially styrene. In pyrolytic products of plastic, textiles, foam, ASR, and MixASR, styrene accounted for 13.62, 11.64, 11.93, 17.18, and 20.68%, respectively. However, these substances are chemically unstable and can be further reacted by improving the process to generate more CO and H2.
3.3. Analysis, Summary, and Discussion
4. Conclusions
- (1)
- The main volatile products of ASR and its main components are alkanes, olefins, alcohols, and benzene series, and their proportions in the pyrolysis products are 3.7385, 26.5539, and 9.3305%, respectively. Many of these volatile products have unstable or weaker chemical bonds, such as =CH–, =CH2, –C=C–, and –C=CH2. Hence, more syngas can be obtained with further high-temperature (>800 °C) pyrolysis [39,40]. Catalytic pyrolysis and gasification are important research directions for obtaining syngas with a greater calorific value [36,37,38].
- (2)
- According to the Gram–Schmidt profiles and the 3D stack plots of MixASR, ASR, and its main components, the pyrolysis product of ASR is not a simple superposition of the pyrolysis products of its components, but the pyrolysis characteristics of the main components have the greatest influence on the product distribution, especially plastic and textiles. Some hazardous gas exists in pyrolytic products of ASR, such as benzene and toluene, which are harmful to the human body and environment. Therefore, the elimination of toxic and hazardous substances must be considered in the design of pyrolysis process.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASR | Automobile shredder residue |
| MixASR | Mixtures of plastic, textiles, leather, rubber, and foam |
| Py | Pyrolysis |
| FTIR | Fourier transform infrared spectrometry |
| GC/MS | Gas chromatography–mass spectrometry |
| PVC | Polyvinyl chloride |
| TGA | Thermogravimetry analysis |
| HHVs | Higher heating values |
Appendix A
| No. | Peaks | Formula | Compound | CAS Number | Yield/% |
|---|---|---|---|---|---|
| 1 | 2.249 | C6H10O | 6-methyl-3,6-dihydro-2H-pyran | 55230-25-6 | 3.5802 |
| 2 | 2.533 | C6H12 | 2-methyl-1-pentene | 763-29-1 | 1.0352 |
| 3 | 3.517 | C5H8O2 | methyl methacrylate | 80-62-6 | 0.1645 |
| 3 | 4.37 | C9H12O | 1-phenyl-2-propanol | 698-87-3 | 1.5010 |
| 5 | 5.558 | C8H12 | 4-vinyl-1-cyclohexene | 100-40-3 | 0.5372 |
| 6 | 5.629 | C9H18 | 2,4-dimethyl-1heptene | 19549-87-2 | 3.6554 |
| C8H16 | 5-methylhept-1-ene | 13151-04-7 | |||
| C10H20 | 3,7-dimethyloct-1-ene | 4984/1/4 | |||
| 7 | 6.601 | C8H8 | annulene | 629-20-9 | 13.6182 |
| benzocyclobutene | 694-87-1 | ||||
| styrene | 100-42-5 | ||||
| 8 | 8.277 | C9H10 | 2-phenyl-1-propene | 98-83-9 | 0.1321 |
| 9 | 8.717 | C10H22 | 3-ethyl-3-methyl heptane | 17302-01-1 | 0.1538 |
| 10 | 8.793 | C11H24 | 2,6-dimethylnonane | 17302-28-2 | 0.1761 |
| 11 | 9.148 | C10H16 | (3R)-(+)-Isosylvestren | 1461-27-4 | 0.6663 |
| 12 | 9.91 | C13H28O | 11-methyl-1-dodecanol | 85763-57-1 | 0.3317 |
| 13 | 9.978 | C13H28O | 11-methyl-1-dodecanol | 85763-57-1 | 0.2899 |
| 14 | 13.352 | C13H28O | 11-methyl-1-dodecanol | 85763-57-1 | 0.1570 |
| 15 | 13.474 | C13H28O | 11-methyl-1-dodecanol | 85763-57-1 | 0.0933 |
| 16 | 13.596 | C13H28O | 11-methyl-1-dodecanol | 85763-57-1 | 0.1712 |
| No. | Peaks | Formula | Compound | CAS Number | Yield/% |
|---|---|---|---|---|---|
| 1 | 2.249 | C5H12 | 2-methylbutane | 78-78-4 | 7.2044 |
| 2 | 2.439 | C6H14O | 2-methyl-1-pentanol | 105-30-6 | 0.5324 |
| 3 | 2.544 | C6H12 | 1-hexene | 592-41-6 | 3.9098 |
| C6H12 | methylcyclopentane | 96-37-7 | |||
| C6H13Cl | 2-chlorohexane | 638-28-8 | |||
| 4 | 2.958 | C6H10 | bipropenyl | 592-46-1 | 0.5624 |
| 5 | 3.054 | C6H6 | benzene | 71-43-2 | 0.6028 |
| 6 | 3.304 | C7H14 | 1-heptene | 592-76-7 | 1.8165 |
| 7 | 3.406 | C7H16 | heptane | 142-82-5 | 0.4222 |
| 8 | 3.599 | C7H12 | 2,4-dimethyl-1,3-pentadiene | 1000-86-8 | 0.1327 |
| 9 | 3.999 | C7H10 | 1-methyl-1,4-cyclohexadiene | 4313-57-9 | 0.5747 |
| 10 | 4.197 | C10H16O | 3-hydroxy-2-methyl-6-methylene-1,7-octadiene | 22459-10-5 | 0.6420 |
| 11 | 4.376 | C9H12O | 1-phenyl-2-propanol | 698-87-3 | 1.8206 |
| 12 | 4.662 | C8H16 | 2-methyl-1-heptene | 15870-10-7 | 0.4028 |
| 13 | 4.747 | C8H16 | oct-1-ene | 111-66-0 | 0.6065 |
| C8H18O | octan-1-ol | 111-87-5 | |||
| 14 | 4.9 | C8H18 | octane | 111-65-9 | 0.4613 |
| 15 | 5.065 | C8H14O | oct-2-yn-1-ol | 20739-58-6 | 0.1743 |
| 16 | 5.635 | C9H18 | 2,4-dimethyl-1-heptene | 19549-87-2 | 6.3153 |
| 17 | 6.037 | C8H10 | ethylbenzene | 100-41-4 | 0.2325 |
| 18 | 6.21 | C12H16O | 2-phenyl-hex-5-en-3-ol | 77383-06-3 | 0.5773 |
| 19 | 6.601 | C12H14O2 | 2,2-dimethyl-5-phenyloxolan-3-one | 63678-00-2 | 3.2998 |
| 20 | 6.752 | C9H20 | nonane | 111-84-2 | 0.3576 |
| 21 | 7.903 | C21H26O2 | benzoic acid-(2,4-di-tert-butyl-phenyl ester) | 39000-49-2 | 0.1512 |
| 22 | 8.439 | C10H20 | 1-decene | 872-05-9 | 0.5524 |
| 23 | 8.595 | C10H22 | decane | 124-18-5 | 0.2374 |
| 24 | 8.722 | C10H22 | 3,3,5-trimethylheptane | 7154-80-5 | 0.1590 |
| 25 | 8.819 | C16H34 | hexadecane | 544-76-3 | 0.4068 |
| 26 | 9.068 | C8H18O | 2-ethylhexan-1-ol | 104-76-7 | 0.4132 |
| 27 | 9.261 | C10H12O | 2-phenylbut-3-en-1-ol | 6052-63-7 | 0.1071 |
| 28 | 9.91 | C13H28O | 11-methyl-1-dodecanol | 85763-57-1 | 0.4746 |
| 29 | 10.163 | C11H22 | 1-undecene | 821-95-4 | 0.3116 |
| 30 | 10.302 | C11H24 | undecane | 1120-21-4 | 0.1645 |
| 31 | 10.69 | C15H32O | 3,7,11-trimethyldodecan-1-ol | 6750-34-1 | 0.1266 |
| 32 | 10.798 | C20H40O | phytol | 150-86-7 | 0.0440 |
| 33 | 11.064 | C16H26O3 | 2-dodecen-1-ylsuccinic anhydride | 19780-11-1 | 0.0765 |
| C10H20O | cyclohexanemethanol, 2,4,6-trimethyl- | 13702-56-2 | |||
| 34 | 11.172 | C13H18O | ether,isopropyl 2-benzyl-2-propenyl | 900152-79-5 | 0.1764 |
| 35 | 11.645 | C12H24 | 5-undecene,2-methyl-,(Z)- | 74630-63-0 | 0.0857 |
| 36 | 11.753 | C12H24 | 1-dodecene | 112-41-4 | 0.1798 |
| 37 | 11.878 | C17H37N | 1-aminoheptadecane | 4200-95-7 | 0.0804 |
| 38 | 13.355 | C18H38O | 2-hexyldodecan-1-ol | 110225-00-8 | 0.5847 |
| C13H28O | 11-methyl-1-dodecanol | 85763-57-1 |
| No. | Peaks | Formula | Compound | CAS Number | Yield/% |
|---|---|---|---|---|---|
| 1 | 2.246 | C8H16O | 1-prop-2-enoxypentane | 23186-70-1 | 7.4626 |
| 2 | 2.439 | C5H10O | 3-penten-2-ol | 1569-50-2 | 0.7402 |
| 3 | 2.53 | C6H12 | 2-methyl-1-pentene | 763-29-1 | 2.7042 |
| 1-hexene | 592-41-6 | ||||
| 4 | 2.952 | C6H10 | bipropenyl | 592-46-1 | 0.4749 |
| 5 | 3.052 | C6H6 | benzene | 71-43-2 | 0.5936 |
| 6 | 3.307 | C7H16O | (s)-3,4-dimethylpentanol | 900143-83-9 | 0.3582 |
| 7 | 3.4 | C7H16 | heptane | 142-82-5 | 0.5957 |
| 8 | 4.361 | C9H12O | 1-phenyl-2-propanol | 698-87-3 | 2.6530 |
| 9 | 4.722/4.88/5.039 | C8H16 | trans-1-butyl-2-methylcyclopropane | 38851-70-6 | 2.5389 |
| cis-1-butyl-2-methylcyclopropane | 38851-69-3 | ||||
| trans-2-octene | 13389-42-9 | ||||
| trans-4-octene | 14850-23-8 | ||||
| C8H17Cl | 4-chlorooctane | 999-07-5 | |||
| 3-chlorooctane | 1117-79-9 | ||||
| 10 | 5.618 | C9H18 | 2,4-dimethyl-1-heptene | 19549-87-2 | 9.8874 |
| 11 | 6.017 | C8H10 | ethylbenzene | 100-41-4 | 1.1988 |
| 12 | 6.59 | C8H8 | styrene | 100-42-5 | 4.8862 |
| 13 | 7.894 | C9H12 | 3-ethyltoluene | 620-14-4 | 0.2492 |
| 14 | 8.215 | C9H12 | 1-ethyl-4-methylbenzene | 622-96-8 | 0.1529 |
| 1-ethyl-2-methylbenzene | 611-14-3 | ||||
| 3-ethyltoluene | 620-14-4 | ||||
| 15 | 8.269 | C9H10 | 2-phenyl-1-propene | 98-83-9 | 0.2948 |
| 16 | 8.427 | C14H28 | (e)-tetradec-3-ene | 41446-68-8 | 0.1934 |
| 17 | 8.586 | C10H22 | decane | 124-18-5 | 0.1247 |
| 18 | 8.708 | C10H22 | 3-ethyl-3-methylheptane | 17302-01-1 | 0.1877 |
| 19 | 8.807 | C20H17NO2 | 6-methyl-dodecane | 6044-71-9 | 0.4697 |
| 20 | 9.054 | C8H18O | 2-ethylhexan-1-ol | 104-76-7 | 0.8314 |
| 21 | 9.244 | C9H10 | indane | 496-11-7 | 0.2027 |
| 22 | 9.902/9.973/13.349 | C13H28O | 11-methyl-1-dodecanol | 85763-57-1 | 2.2638 |
| No. | Peaks | Formula | Compound | CAS Number | Yield/% |
|---|---|---|---|---|---|
| 1 | 2.238 | C4H11NO | O-(2-methylpropyl)hydroxylamine | 5618-62-2 | 6.9797 |
| 2 | 2.45 | C6H14O | 2,3-dimethylbutyl alcohol | 19550-30-2 | 0.4687 |
| 3 | 2.533 | C6H12 | 2-methyl-1-pentene | 763-29-1 | 3.7191 |
| 1-hexene | 592-41-6 | ||||
| 4 | 3.043 | C6H6 | benzene | 71-43-2 | 3.4949 |
| 5 | 4.191 | C8H16 | 2,3-dimethylhex-1-ene | 16746-86-4 | 0.3236 |
| 2,5-dimethyl-2-hexene | 3404-78-2 | ||||
| (E)-2,3-dimethylhex-3-ene | 7145-23-5 | ||||
| 6 | 4.364 | C9H12O | 1-phenyl-2-propanol | 698-87-3 | 2.9873 |
| 7 | 5.133 | C9H18 | cis-1,1,3,4-tetramethylcyclopentane | 53907-60-1 | 0.2179 |
| 8 | 5.272 | C9H20 | 2,4-dimethyl-heptane | 2213-23-2 | 0.0875 |
| 9 | 5.459 | C10H18 | isocitronellene | 85006-04-8 | 0.3957 |
| 10 | 5.629 | C8H16 | 5-methylhept-1-ene | 13151-04-7 | 16.0668 |
| 11 | 5.972 | C9H18 | 1,3,5-trimethyl-cyclohexane | 1839-63-0 | 0.3468 |
| 12 | 6.026 | C8H10 | ethylbenzene | 100-41-4 | 0.3153 |
| 13 | 6.599 | C8H8 | styrene | 100-42-5 | 11.6357 |
| 14 | 6.973 | C10H18 | 2,5-dimethyl-1,trans-6-octadien | 68702-25-0 | 0.1709 |
| 15 | 7.208 | C9H12 | cumene | 98-82-8 | 0.0322 |
| 16 | 7.616 | C9H10 | prop-2-enylbenzene | 300-57-2 | 0.0838 |
| 17 | 7.767 | C13H18O2 | phenylacetic acid isoamyl ester | 102-19-2 | 0.0935 |
| 18 | 7.92 | C7H6O | benzaldehyde | 100-52-7 | 0.1585 |
| 19 | 8.277 | C9H10 | 2-phenyl-1-propene | 98-83-9 | 0.9701 |
| 20 | 8.43 | C16H32 | hexadec-3-ene | 34303-81-6 | 0.3858 |
| 21 | 8.717/8.787 | C14H30 | dodecane,4,6-dimethyl | 61141-72-8 | 0.6796 |
| C11H24 | 2,6-dimethylnonane | 17302-28-2 | |||
| 22 | 9.907/9.981 | C13H28O | 11-methyl-1-dodecanol | 85763-57-1 | 2.3873 |
| 23 | 10.236 | C8H8O2 | methyl benzoate | 93-58-3 | 0.0960 |
| 24 | 10.684/10.792 | C15H32O | 3,7,11-trimethyldodecan-1-ol | 6750-34-1 | 0.1598 |
| 25 | 10.894 | C9H8O2 | vinyl benzoate | 769-78-8 | 0.1427 |
| 26 | 11.064 | C10H20O | dihydoisocyclogeraniol | 13702-56-2 | 0.1177 |
| 27 | 11.45 | C9H10O2 | ethyl benzoate | 93-89-0 | 0.1083 |
| 28 | 13.355/13.474/13.590 | C13H28O | 11-methyl-1-dodecanol | 85763-57-1 | 1.5021 |
| No. | Peaks | Formula | Compound | CAS Number | Yield/% |
|---|---|---|---|---|---|
| 1 | 2.226 | C6H12O2 | 1-isopropoxy-propan-2-one | 42781-12-4 | 6.6008 |
| 2 | 2.399 | C3H6N2O2 | cycloserine | 68-41-7 | 0.6897 |
| 3 | 2.884 | C6H14O | 1-propan-2-yloxypropane | 627-08-7 | 1.3509 |
| 4 | 3.188 | C6H12O | 2,2,3,3-tetramethyloxirane | 5076-20-0 | 1.9050 |
| 5 | 3.502 | C17H34O2 | 2-(tetradecoxymethyl)oxirane | 38954-75-5 | 0.4383 |
| 6 | 3.689 | C6H12O2 | 2,2,4-Trimethyl-1,3-dioxolane | 1193-11-9 | 1.2745 |
| 7 | 3.908 | C6H10O2 | 4-methylpentane-2,3-dione | 7493-58-5 | 0.3843 |
| 8 | 4.067 | C11H22O2 | 2-ethylhexyl glycidyl ether | 2461-15-6 | 0.7434 |
| 9 | 4.361 | C7H8 | toluene | 108-88-3 | 1.3903 |
| 10 | 4.79 | C8H18O2 | 1-(1-propoxyethoxy)propane | 105-82-8 | 2.6692 |
| 11 | 5.135 | C6H12O2 | 1-isopropoxy-propan-2-one | 42781-12-4 | 5.3007 |
| 12 | 5.532 | C6H12O2 | 2,2,4-trimethyl-1,3-dioxolane | 1193-11-9 | 0.1558 |
| 13 | 5.62 | C10H20O | decanal | 112-31-2 | 0.2558 |
| 14 | 5.728 | C6H13NO | hexanamide | 628-02-4 | 0.3094 |
| 15 | 6.023 | C8H10 | ethylbenzene | 100-41-4 | 0.4235 |
| 16 | 6.599 | C8H8 | annulene | 629-20-9 | 11.9324 |
| styrene | 100-42-5 | ||||
| 17 | 7.078 | C7H16O | 4-methylhexan-3-ol | 615-29-2 | 0.0498 |
| 18 | 7.738 | C7H16O | 2-propan-2-yloxybutane | 18641-81-1 | 0.3678 |
| 19 | 7.869 | C8H18O | 4-methyl-3-heptanol | 14979-39-6 | 0.2974 |
| 20 | 8.274 | C9H10 | 2-phenyl-1-propene | 98-83-9 | 0.4722 |
| 21 | 8.337 | C6H12O2 | 5-methoxypentan-2-one | 17429-04-8 | 1.5136 |
| 22 | 9.071 | C8H18O | 6-methyl-1-heptanol | 1653-40-3 | 0.3426 |
| 23 | 9.13 | C8H17Cl | 3-chlorooctane | 1117-79-9 | 0.3620 |
References
- Nourreddine, M. Recycling of auto shredder residue. J. Hazard. Mater. 2007, 139, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Fiore, S.; Ruffino, B.; Zanetti, M.C. Automobile Shredder Residues in Italy: Characterization and valorization opportunities. Waste Manag. 2012, 32, 1548–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cossu, R.; Fiore, S.; Lai, T.; Luciano, A.; Mancini, G.; Ruffino, B.; Viotti, P.; Zanetti, M.C. Review of Italian experience on automotive shredder residue characterization and management. Waste Manag. 2014, 34, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, B.; Fiore, S.; Zanetti, M.C. Strategies for the enhancement of automobile shredder residues (ASRs) recycling: Results and cost assessment. Waste Manag. 2014, 34, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Wenzel, H.; Hansen, J.B. Characterization of Shredder Residues generated and deposited in Denmark. Waste Manag. 2014, 34, 1279–1288. [Google Scholar] [CrossRef]
- Cossu, R.; Lai, T. Automotive shredder residue (ASR) management: An overview. Waste Manag. 2015, 45, 143–151. [Google Scholar] [CrossRef]
- Morselli, L.; Santini, A.; Passarini, F.; Vassura, I. Automotive shredder residue (ASR) characterization for a valuable management. Waste Manag. 2010, 30, 2228–2234. [Google Scholar] [CrossRef]
- Passarini, F.; Ciacci, L.; Santini, A.; Vassura, I.; Morselli, L. Auto shredder residue LCA: Implications of ASR composition evolution. J. Clean. Prod. 2012, 23, 28–36. [Google Scholar] [CrossRef]
- Mancini, G.; Viotti, P.; Luciano, A.; Fino, D. On the ASR and ASR thermal residues characterization of full scale treatment plant. Waste Manag. 2014, 34, 448–457. [Google Scholar] [CrossRef]
- Vermeulen, I.; Van Caneghem, J.; Block, C.; Baeyens, J.; Vandecasteele, C. Automotive shredder residue (ASR): Reviewing its production from end-of-life vehicles (ELVs) and its recycling, energy or chemicals’ valorisation. J. Hazard. Mater. 2011, 190, 8–27. [Google Scholar] [CrossRef]
- Roy, C.; Chaala, A. Vacuum pyrolysis of automobile shredder residues. Resour. Conserv. Recycl. 2001, 32, 1–27. [Google Scholar] [CrossRef]
- Zolezzi, M.; Nicolella, C.; Ferrara, S.; Iacobucci, C.; Rovatti, M. Conventional and fast pyrolysis of automobile shredder residues (ASR). Waste Manag. 2004, 24, 691–699. [Google Scholar] [CrossRef]
- Santini, A.; Passarini, F.; Vassura, I.; Serrano, D.; Dufour, J.; Morselli, L. Auto shredder residue recycling: Mechanical separation and pyrolysis. Waste Manag. 2012, 32, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Harder, M.K.; Forton, O.T. A critical review of developments in the pyrolysis of automotive shredder residue. J. Anal. Appl. Pyrolysis 2007, 79, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Ni, F.J.; Chen, M. Research on ASR in China and its energy recycling with pyrolysis method. J. Mater. Cycles Waste Manag. 2015, 17, 107–117. [Google Scholar] [CrossRef]
- Haydary, J.; Susa, D.; Gelinger, V.; Čacho, F. Pyrolysis of automobile shredder residue in a laboratory scale screw type reactor. J. Environ. Chem. Eng. 2016, 4, 965–972. [Google Scholar] [CrossRef]
- Mayyas, M.; Pahlevani, F.; Handoko, W.; Sahajwalla, V. Preliminary investigation on the thermal conversion of automotive shredder residue into value-added products: Graphitic carbon and nano-ceramics. Waste Management 2016, 50, 173–183. [Google Scholar] [CrossRef]
- Anzano, M.; Collina, E.; Piccinelli, E.; Lasagni, M. Lab-scale pyrolysis of the Automotive Shredder Residue light fraction and characterization of tar and solid products. Waste Manag. 2017, 64, 263–271. [Google Scholar] [CrossRef]
- Notarnicola, M.; Cornacchia, G.; De Gisi, S.; Di Canio, F.; Freda, C.; Garzone, P.; Martino, M.; Valerio, V.; Villone, A. Pyrolysis of automotive shredder residue in a bench scale rotary kiln. Waste Manag. 2017, 65, 92–103. [Google Scholar] [CrossRef]
- Evangelopoulos, P.; Sophonrat, N.; Jilvero, H.; Yang, W. Investigation on the low-temperature pyrolysis of automotive shredder residue (ASR) for energy recovery and metal recycling. Waste Manag. 2018, 76, 507–515. [Google Scholar] [CrossRef]
- Yang, B.; Chen, M. Influence of Interactions among Polymeric Components of Automobile Shredder Residue on the Pyrolysis Temperature and Characterization of Pyrolytic Products. Polymers 2020, 12, 1682. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Hu, Z.; Xiao, B.; Li, J.; Guo, X.; Luo, S.; Yang, F.; Feng, Y.; Yang, G.; Liu, S. Hydrogen-rich gas from catalytic steam gasification of municipal solid waste (MSW): Influence of catalyst and temperature on yield and product composition. Int. J. Hydrog. Energy 2009, 34, 195–203. [Google Scholar] [CrossRef]
- He, M.; Xiao, B.; Liu, S.; Hu, Z.; Guo, X.; Luo, S.; Yang, F. Syngas production from pyrolysis of municipal solid waste (MSW) with dolomite as downstream catalysts. J. Anal. Appl. Pyrolysis 2010, 87, 181–187. [Google Scholar] [CrossRef]
- Blanco, P.H.; Wu, C.; Onwudili, J.A.; Williams, P.T. Characterization and evaluation of Ni/SiO2 catalysts for hydrogen production and tar reduction from catalytic steam pyrolysis-reforming of refuse derived fuel. Appl. Catal. B Environ. 2013, 134–135, 238–250. [Google Scholar] [CrossRef]
- He, M.; Xiao, B.; Liu, S.; Guo, X.; Luo, S.; Xu, Z.; Feng, Y.; Hu, Z. Hydrogen-rich gas from catalytic steam gasification of municipal solid waste (MSW): Influence of steam to MSW ratios and weight hourly space velocity on gas production and composition. Int. J. Hydrog. Energy 2009, 34, 2174–2183. [Google Scholar] [CrossRef]
- Acomb, J.C.; Wu, C.; Williams, P.T. Control of steam input to the pyrolysis-gasification of waste plastics for improved production of hydrogen or carbon nanotubes. Appl. Catal. B Environ. 2014, 147, 571–584. [Google Scholar] [CrossRef]
- Donaj, P.; Blasiak, W.; Yang, W.; Forsgren, C. Conversion of microwave pyrolysed ASR’s char using high temperature agents. J. Hazard. Mater. 2011, 185, 472–481. [Google Scholar] [CrossRef]
- Lin, K.-S.; Chowdhury, S.; Wang, Z.-P. Catalytic gasification of automotive shredder residues with hydrogen generation. J. Power Sources 2010, 195, 6016–6023. [Google Scholar] [CrossRef]
- Kai, X.; Li, R.; Yang, T.; Shen, S.; Ji, Q.; Zhang, T. Study on the co-pyrolysis of rice straw and high density polyethylene blends using TG-FTIR-MS. Energy Convers. Manag. 2017, 146, 20–33. [Google Scholar] [CrossRef]
- Wu, J.; Chen, T.; Luo, X.; Han, D.; Wang, Z.; Wu, J. TG/FTIR analysis on co-pyrolysis behavior of PE, PVC and PS. Waste Manag. 2014, 34, 676–682. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Wu, J.; Wu, J. TG-MS analysis and kinetic study for thermal decomposition of six representative components of municipal solid waste under steam atmosphere. Waste Manag. 2015, 43, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Wu, C.; Williams, P.T. Pyrolysis of waste materials using TGA-MS and TGA-FTIR as complementary characterisation techniques. J. Anal. Appl. Pyrolysis 2012, 94, 99–107. [Google Scholar] [CrossRef]
- Guo, Q.J.; Zhang, X.; Li, C.; Liu, X.M.; Li, J.H. TG-MS study of the thermo-oxidative behavior of plastic automobile shredder residues. J. Hazard. Mater. 2012, 209, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, M.; Pahlevani, F.; Bucknall, M.; Maroufi, S.; You, Y.; Liu, Z.; Sahajwalla, V. Thermocatalytic Conversion of Automotive Shredder Waste and Formation of Nanocarbons as a Process Byproduct. Acs Sustain. Chem. Eng. 2017, 5, 5440–5448. [Google Scholar] [CrossRef]
- Gao, Z.; Nakada, M.; Amasaki, I. A consideration of errors and accuracy in the isoconversional methods. Thermochim. Acta 2001, 369, 137–142. [Google Scholar] [CrossRef]
- Guan, Y.; Luo, S.; Liu, S.; Xiao, B.; Cai, L. Steam catalytic gasification of municipal solid waste for producing tar-free fuel gas. Int. J. Hydrog. Energy 2009, 34, 9341–9346. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Artetxe, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Hydrogen-rich gas production by continuous pyrolysis and in-line catalytic reforming of pine wood waste and HDPE mixtures. Energy Convers. Manag. 2017, 136, 192–201. [Google Scholar] [CrossRef]
- Duman, G.; Yanik, J. Two-step steam pyrolysis of biomass for hydrogen production. Int. J. Hydrog. Energy 2017, 42, 17000–17008. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Almeida, D.; Marques, M.d.F. Thermal and Catalytic Pyrolysis of Plastic Waste. Polímeros 2016, 26, 44–51. [Google Scholar] [CrossRef]







| Sample | Material Composition (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| Metals | Plastics | Textiles | Leather | Rubber | Foam | Others | |
| ASR | 3.0 | 39.7 | 28.1 | 3.3 | 2.2 | 2.1 | 21.6 |
| MixASR | 0 | 53 | 37 | 3 | 4 | 3 | 0 |
| No. | Peaks | Formula | Compound | CAS Number | Yield/% |
|---|---|---|---|---|---|
| 1 | 2.226 | C8H16O | 1-prop-2-enoxypentane | 23186-70-1 | 2.8681 |
| 2 | 2.445 | C3H9NO | 1-aminopropan-2-ol | 78-96-6 | 0.4912 |
| 3 | 2.544 | C6H12 | 1-hexene | 592-41-6 | 0.8056 |
| C6H12 | 4-methyl-1-Pentene | 691-37-2 | |||
| C6H13Cl | 2-chlorohexane | 638-28-8 | |||
| 4 | 2.771 | C4H8O | tetrahydrofuran | 109-99-9 | 0.2440 |
| C5H10O2 | 4-ethyl-1,3-dioxolane | 29921-38-8 | |||
| 5 | 3.037 | C6H11ClO | α-chlorohexanal | 762-39-0 | 2.6292 |
| 6 | 3.508 | C7H12O3 | 2-methylacrylic acid 3-hydroxypropyl ester | 2761-09-3 | 0.8626 |
| C7H12O3 | 2-hydroxypropyl methacrylate | 923-26-2 | |||
| 7 | 4.364 | C7H8 | toluene | 108-88-3 | 4.1164 |
| 8 | 4.798 | C8H18O | 2,3-dimethylhexan-3-ol | 4166-46-5 | 0.2205 |
| C6H14O | 4-Methyl-2-pentanol | 108-11-2 | |||
| 9 | 5.147 | C6H12O2 | 1-isopropoxy-propan-2-one | 42781-12-4 | 0.3793 |
| 10 | 5.623 | C9H18 | 2,4-dimethyl-1heptene | 19549-87-2 | 1.6540 |
| 11 | 6.026 | C8H10 | ethylbenzene | 100-41-4 | 1.2742 |
| o-Xylene | 95-47-6 | ||||
| 12 | 6.593 | C8H8 | annulene | 629-20-9 | 20.6849 |
| benzocyclobutene | 694-87-1 | ||||
| styrene | 100-42-5 | ||||
| 13 | 7.205 | C9H12 | cumene | 98-82-8 | 0.0749 |
| 14 | 7.614 | C9H10 | prop-2-enylbenzene | 300-57-2 | 0.1990 |
| 15 | 8.127 | C6H6O | phenol | 108-95-2 | 0.3113 |
| 16 | 8.181 | C8H14O2 | butyl methacrylate | 97-88-1 | 0.2616 |
| 17 | 8.274 | C9H10 | 2-phenyl-1-propene | 98-83-9 | 3.0575 |
| 18 | 8.717 | C14H30 | 7-methyltridecane | 26730-14-3 | 0.0597 |
| C10H22 | 3-ethyl-3-methyl heptane | 17302-01-1 | |||
| 19 | 9.06 | C8H18O | 2-ethylhexan-1-ol | 104-76-7 | 0.8311 |
| 2-propylpentan-1-ol | 58175-57-8 | ||||
| 20 | 9.142 | C8H16 | 3,4,4-trimethyl-2-pentene | 598-96-9 | 0.2199 |
| 2,4,4-trimethyl-2-pentene | 107-40-4 | ||||
| 21 | 9.36 | C10H12 | 4-phenyl-1-butene | 768-56-9 | 0.1319 |
| 22 | 9.601 | C10H12 | alpha,p-dimethylstyrene | 1195-32-0 | 0.2256 |
| 23 | 9.754 | C8H8O | acetophenone | 98-86-2 | 0.0718 |
| 24 | 9.907 | C13H28O | 11-methyl-1-dodecanol | 85763-57-1 | 0.2098 |
| No. | Peaks | Formula | Compound | CAS Number | Yield/% |
|---|---|---|---|---|---|
| 1 | 2.229 | C6H12O | 1-prop-2-enoxypropane | 1471-03-0 | 8.1671 |
| 2 | 2.431 | C7H10O6 | methanetricarboxylic acid, 1,1,1-trimethyl ester | 1186-73-8 | 0.4723 |
| 3 | 2.521 | C6H12 | 2-methyl-1-pentene | 763-29-1 | 1.9019 |
| 1-hexene | 592-41-6 | ||||
| 4 | 4.347 | C9H12O | 1-phenyl-2-propanol | 698-87-3 | 2.0945 |
| 5 | 4.784 | C6H14O | 4-methyl-2-pentanol | 108-11-2 | 0.8483 |
| 6 | 5.13 | C6H12O2 | 1-isopropoxy-propan-2-one | 42781-12-4 | 0.7124 |
| 7 | 5.609 | C9H18 | 2,4-dimethyl-1heptene | 19549-87-2 | 6.9013 |
| 8 | 6.0009 | C8H10 | ethylbenzene | 100-41-4 | 0.2366 |
| o-xylene | 95-47-6 | ||||
| 9 | 6.579 | C8H8 | styrene | 100-42-5 | 17.1758 |
| 10 | 8.266 | C9H10 | 2-phenyl-1-propene | 98-83-9 | 0.3269 |
| 11 | 8.702 | C10H22 | 3-ethyl-3-methyl heptane | 17302-01-1 | 0.4045 |
| C11H24 | 2,6-dimethylnonane | 17302-28-2 | |||
| C14H30 | dodecane,4,6-dimethyl | 61141-72-8 | |||
| 12 | 9.136 | C10H16 | (3R)-(+)-isosylvestren | 1461-27-4 | 0.2890 |
| 13 | 9.902 | C13H28O | 11-methyl-1-dodecanol | 85763-57-1 | 0.7403 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Chen, M. Py–FTIR–GC/MS Analysis of Volatile Products of Automobile Shredder Residue Pyrolysis. Polymers 2020, 12, 2734. https://doi.org/10.3390/polym12112734
Yang B, Chen M. Py–FTIR–GC/MS Analysis of Volatile Products of Automobile Shredder Residue Pyrolysis. Polymers. 2020; 12(11):2734. https://doi.org/10.3390/polym12112734
Chicago/Turabian StyleYang, Bin, and Ming Chen. 2020. "Py–FTIR–GC/MS Analysis of Volatile Products of Automobile Shredder Residue Pyrolysis" Polymers 12, no. 11: 2734. https://doi.org/10.3390/polym12112734
APA StyleYang, B., & Chen, M. (2020). Py–FTIR–GC/MS Analysis of Volatile Products of Automobile Shredder Residue Pyrolysis. Polymers, 12(11), 2734. https://doi.org/10.3390/polym12112734





