Chitosan Modified Cationic Polyacrylamide Initiated by UV-H2O2 for Sludge Flocculation and New Insight on the Floc Characteristics Study
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Preparation of CTS-g-PAMD
2.3. Polymer Characterization
2.4. Sludge Flocculation Experiment
3. Results and Discussions
3.1. Single Factor Experimental Conditions
3.1.1. Effect of Ultraviolet Power
3.1.2. Influence of Illumination Time
3.1.3. Influence of pH
3.1.4. Effect of H2O2 Initiator
3.1.5. Influence of Mass Fraction of Overall Monomers
3.1.6. Influence of Mass Fraction of CTS Monomer
3.2. Characterization
3.2.1. FTIR Analysis
3.2.2. H NMR Analysis
3.2.3. XRD and XPS Analysis
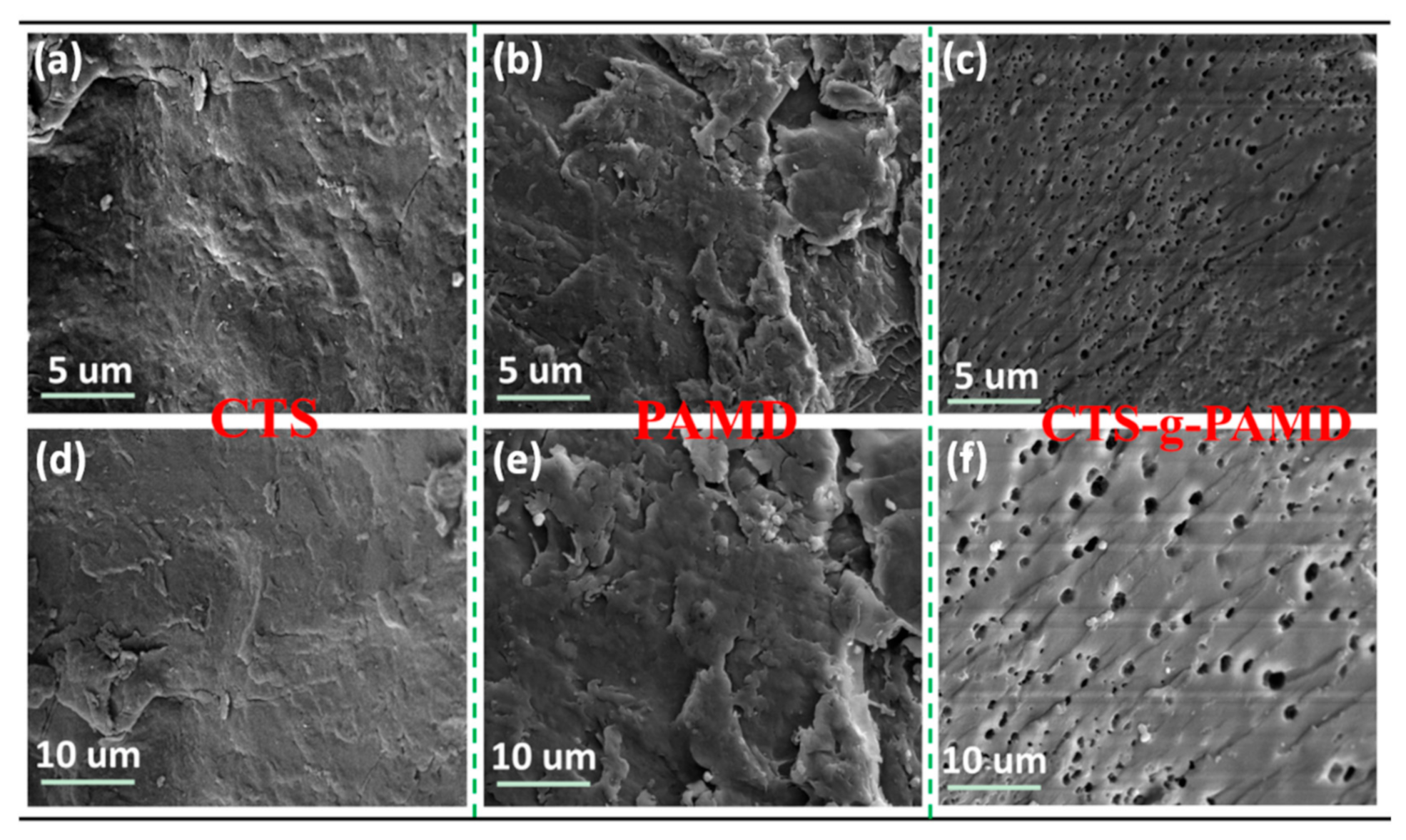
3.2.4. SEM Analysis
4. Flocculation
4.1. Effect of Flocculant Dosage
4.2. Effect of pH
4.3. Effect of CTS Grafting Efficiency
4.4. Characteristics of Sludge Flocs
4.4.1. Sludge Flocs Size and Fractal Dimension
4.4.2. Floc Formation, Breakage and Regrowth
4.4.3. Sludge Flocculation Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ohm, T.I.; Chae, J.S.; Kim, J.E.; Kim, H.K.; Moon, S.H. A study on the dewatering of industrial waste sludge by fry-drying technology. J. Hazard. Mater. 2009, 168, 445–450. [Google Scholar] [CrossRef]
- He, D.Q.; Wang, L.F.; Jiang, H.; Yu, H.Q. A fenton-like process for the enhanced activated sludge dewatering. Chem. Eng. J. 2015, 272, 128–134. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, P.; Zhang, H.; Zeng, G.; Liu, J.; Ye, J.; Fang, W.; Gou, X.Y. Possibility of sludge conditioning and dewatering with rice husk biochar modified by ferric chloride. Bioresour. Technol. 2016, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cheng, H.; Peng, S.; Li, D.; Gao, H.; Wang, D. Performance and mechanisms of wastewater sludge conditioning with slag-based hydrotalcite-like minerals (ca/mg/al-ldh). Water Res. 2020, 169, 115265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, P.; Xiao, P.; Xu, S.; Liu, Y.; Liu, F.; Wang, D. Dynamic variation in physicochemical properties of activated sludge floc from different wwtps and its influence on sludge dewaterability and settleability. Colloids Surf. A Physicochem. Eng. Asp. 2015, 467, 124–134. [Google Scholar] [CrossRef]
- Li, X.; Zheng, H.L.; Gao, B.; Zhao, C.; Sun, Y. Uv-initiated polymerization of acid- and alkali-resistant cationic flocculant p(am-maptac): Synthesis, characterization, and application in sludge dewatering. Sep. Purif. Technol. 2017, 187, 244–254. [Google Scholar] [CrossRef]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef]
- Feng, L.; Liu, S.; Zheng, H.L.; Liang, J.; Sun, Y.; Zhang, S.; Chen, X. Using ultrasonic (us)-initiated template copolymerization for preparation of an enhanced cationic polyacrylamide (cpam) and its application in sludge dewatering. Ultrason. Sonochem. 2018, 44, 53–63. [Google Scholar] [CrossRef]
- Huang, P.; Zhao, X.; Ye, L. Synthesis of hydrophobic cationic chitosan flocculant and its sludge dewatering property. J. Macromol Sci. B. 2016, 55, 299–309. [Google Scholar] [CrossRef]
- Ma, C.; Pei, H.; Hu, W.; Cheng, J.; Xu, H.; Jin, Y. Significantly enhanced dewatering performance of drinking water sludge from a coagulation process using a novel chitosan–aluminum chloride composite coagulant in the treatment of cyanobacteria-laden source water. RSC Adv. 2016, 6, 61047–61056. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, T.; Yan, L.; Mi, Z.; Gu, Q.; Zhang, Y. Synthesis, characterization and evaluation of dewatering properties of chitosan-grafting dmdaac flocculants. Int. J. Biol. Macromol. 2016, 92, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, Q.; Li, J.; Wang, D.; Zheng, R.; Gu, Q.; Zhang, Y.M. Preparation and dewatering property of two sludge conditioners chitosan/am/aa and chitosan/am/aa/dmdaac. J. Polym. Environ. 2019, 27, 275–285. [Google Scholar] [CrossRef]
- Lu, X.; Xu, Y.; Sun, W.; Sun, Y.; Zheng, H.L. Uv-initiated synthesis of a novel chitosan-based flocculant with high flocculation efficiency for algal removal. Sci. Total Environ. 2017, 609, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, H.L.; Wang, Y.; Sun, Y.; Xu, B.; Zhao, C. Fabricating an enhanced sterilization chitosan-based flocculants: Synthesis, characterization, evaluation of sterilization and flocculation. Chem. Eng. J. 2017, 319, 119–130. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, A.; Pan, S.Y.; Sun, W.; Zhu, C.; Shah, K.J.; Zheng, H.L. Novel chitosan-based flocculants for chromium and nickle removal in wastewater via integrated chelation and flocculation. J. Environ. Manage. 2019, 248, 109241. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.L.; Feng, L.; Wang, Y.L.; Zhang, S.X.; Chen, N. Ultrasonic-template technology inducing and regulating cationic microblocks in cpam: Characterization, mechanism and sludge flocculation performance. Rsc Adv. 2017, 7, 23444–23456. [Google Scholar]
- Cheng, Z.; Dong, Z.; Su, M.; Zhang, Y.; Wang, Z.; He, P. Synthesis of cationic polyacrylamide via inverse emulsion polymerization method for the application in water treatment. J. Macromol. Sci. A 2019, 56, 1–10. [Google Scholar] [CrossRef]
- Hernandez-Ortiz, J.C.; Jaramillo-Soto, G.; Palacios-Alquisira, J.; Vivaldo-Lima, E. Modeling of polymerization kinetics and molecular weight development in the microwave-activated nitroxide-mediated radical polymerization of styrene. Macromol. React. Eng. 2010, 3, 101–107. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, C.; Xu, Y.; Zheng, H.L.; Ren, M. Comparison of initiation methods in the structure of cpam and sludge flocs properties. J. Appl Polym. Sci. 2016, 133, 44071. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, H.; Zheng, H.L.; Liang, J. UV-H2O2 preparation of cationic polyacrylamide (cpam) and its application in sludge dewatering. Sci. Adv. Mater. 2019, 11, 842–853. [Google Scholar] [CrossRef]
- Greenwood, J.; Rainey, T.; Doherty, W. Light scattering study on the size and structure of calcium phosphate/hydroxyapatite flocs formed in sugar solutions. J. Colloid Interface Sci. 2007, 306, 66–71. [Google Scholar] [CrossRef]
- Jarvis, P.; Jefferson, B.; Parsons, S.A. Breakage, regrowth, and fractal nature of natural organic matter flocs. Environ. Sci. Technol. 2005, 39, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, J.K.; Yu, W.B.; Luo, S.; Peng, L.; Shen, X.X.; Shi, Y.F.; Zhang, S.N.; Song, J.; Ye, N.; et al. Mechanism of red mud combined with Fenton’s reagent in sewage sludge conditioning. Water Res. 2014, 59, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Vilhunen, S.; Puton, J.; Virkutyte, J.; Sillanp, M. Efficiency of hydroxyl radical formation and phenol decomposition using uv light emitting diodes and H2O2. Environ. Technol. 2011, 32, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tang, P.; Zhao, C.; Zhang, Z.; Sun, G. An environmentally friendly bleaching process for cotton fabrics: Mechanism and application of UV/H2O2 system. Cellulose 2020, 27, 1071–1083. [Google Scholar] [CrossRef]
- Zheng, H.L.; Sun, Y.; Zhu, C.; Guo, J.; Zhao, C.; Liao, Y.; Guang, Q.Q. Uv-initiated polymerization of hydrophobically associating cationic flocculants: Synthesis, characterization, and dewatering properties. Chem. Eng. J. 2013, 234, 318–326. [Google Scholar] [CrossRef]
- Liao, Y.; Zheng, H.L.; Qian, L.; Sun, Y.; Dai, L.; Xue, W. Uv-initiated polymerization of hydrophobically associating cationic polyacrylamide modified by a surface-active monomer: A comparative study of synthesis, characterization, and sludge dewatering performance. Ind. Eng. Chem. Res. 2014, 53, 11193–11203. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.L.; Wang, Y.; Zheng, X.; Zhao, C. Synthesis of a cationic polyacrylamide by a photocatalytic surface-initiated method and evaluation of its flocculation and dewatering performance: Nano-TiO2 as a photo initiator. RSC Adv. 2018, 8, 28329–28340. [Google Scholar] [CrossRef]
- Kokufuta, E.; Shimizu, N.; Nakamura, I. Preparation of polyelectrolyte-coated ph-sensitive poly(styrene) microcapsules and their application to initiation-cessation control of an enzyme reaction. Biotechnol. Bioeng. 2010, 32, 289–294. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Du, F.S.; Li, Z.C. Facile synthesis of H2O2-cleavable poly(ester-amide)s by passerini multicomponent polymerization. ACS Macro Lett. 2016, 6, 11–15. [Google Scholar] [CrossRef]
- Ma, J.Y.; Fu, K.; Shi, J.; Sun, Y.J.; Zhang, X.X.; Ding, L. Ultraviolet-assisted synthesis of polyacrylamide-grafted chitosan nanoparticles and flocculation performance. Carbohydr. Polym. 2016, 151, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, J.; Ding, H.; Zhu, G.; Fu, K.; Fu, X. Synthesis of cationic polyacrylamide by low-pressure UV initiation for turbidity water flocculation. Chem. Eng. J. 2017, 312, 20–29. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Lu, W.; Liu, Z.; Xu, C.; Chen, Y.; Zheng, H. Preparation of a graft modified flocculant based on chitosan by ultrasonic initiation and its synergistic effect with kaolin for the improvement of acid blue 83 (AB 83) removal. Int. J. Biol. Macromol. 2020, 150, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Ren, M.; Sun, W.; Xiao, X.; Xu, Y.; Zheng, H.L.; Wu, H.F.; Liu, Z.Y.; Zhu, H. Plasma-induced synthesis of chitosan-g-polyacrylamide and its flocculation performance for algae removal. Environ. Technol. 2019, 40, 954–968. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, M.; Zhu, C.; Xu, Y.; Zheng, H.L.; Xiao, X.; Wu, H.F.; Xia, T.; You, Z.Y. Uv-initiated graft copolymerization of cationic chitosan-based flocculants for treatment of zinc phosphate-contaminated wastewater. Ind. Eng. Chem. Res. 2016, 55, 10025–10035. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, H.; Tang, X.; Zheng, X.; Liu, S.; Qiang Sun, Q.; Moxi Wang, M. The investigation of the specific behavior for the cationic block structure and its excellent flocculation performance in high turbidity water treatment. RSC Adv. 2018, 8, 15119–15133. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, H.L.; Deng, X.; Xu, B.; Sun, Y.; Liu, Y.; Liang, J.J. Formation of cationic hydrophobic micro-blocks in p(am-dmc) by template assembly: Characterization and application in sludge dewatering. RSC Adv. 2017, 7, 6114–6122. [Google Scholar] [CrossRef]
- Wang, M.; Feng, L.; Fan, X.W.; Li, D.M.; Qu, W.Q.; Jiang, S.X.; Li, S.X. Fabrication of bifunctional chitosan-based flocculants: Characterization, assessment of flocculation, and sterilization performance. Materials 2018, 11, 2009. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.F.; Kan, J.; Tang, Y.Q.; Jin, C.H. Preparation, characterization and antioxidant activity of phenolic acids grafted carboxymethyl chitosan. Int. J. Biol. Macromol. 2013, 62, 85–93. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, H.; Sun, Y.J.; Chiang, P.C.; Sun, W.Q.; Xu, Y.H. Characterization and sludge dewatering performance evaluation of the photo-initiated cationic flocculant pdd. J. Taiwan Inst. Chem. E 2018, 93, 253–262. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zheng, H.L.; Sun, Y.J.; Ren, J.; Zheng, X.Y.; Sun, Q.; Jiang, S.J.; Ding, W. Synthesis of novel chitosan-based flocculants with amphiphilic structure and its application in sludge dewatering: Role of hydrophobic groups. J. Clean. Prod. 2020, 249, 119350. [Google Scholar] [CrossRef]
- Yang, Z.L.; Gao, B.Y.; Li, C.X.; Yue, Q.Y.; Liu, B. Synthesis and characterization of hydrophobically associating cationic polyacrylamide. Chem. Eng. J. 2010, 161, 27–33. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zheng, H.L.; An, Y.Y.; Ren, J.; Zheng, X.Y.; Zhao, C.; Zhang, S.X. Ultrasound-assisted synthesis of the β-cyclodextrin based cationic polymeric flocculants and evaluation of flocculation performance: Role of β-cyclodextrin. Sep. Purif. Technol. 2019, 228, 115735. [Google Scholar] [CrossRef]
- Feng, L.; Liu, J.; Xu, C.; Lu, W.; Li, D.; Zhao, C.; Liu, B.Z.; Li, X.; Sarfaraz, K.; Zheng, H.L.; et al. Better understanding the polymerization kinetics of ultrasonic-template method and new insight on sludge floc characteristics research. Sci. Total Environ. 2019, 689, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Dong, S.; Zhang, G.; Han, T.; Zhang, Y.; Li, B. Enhancing the auto-flocculation of photosynthetic bacteria to realize biomass recovery in brewery wastewater treatment. Environ. Technol. 2019, 40, 2147–2156. [Google Scholar] [CrossRef]
- Heiderscheidt, E.; Leivisk, T. Evaluating the influence of ph adjustment on chemical purification efficiency and the suitability of industrial by-products as alkaline agents. J. Environ. Manage. 2018, 213, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Long, J.; Zhao, W.; Wang, L.; Chen, G. Ph-responsive chitosan-mediated graphene dispersions. Langmuir 2014, 26, 16771–16774. [Google Scholar] [CrossRef] [PubMed]
- Polexe, R.; Delair, T. Elaboration of stable and antibody functionalized positively charged colloids by polyelectrolyte complexation between chitosan and hyaluronic acid. Molecules 2013, 18, 8563–8578. [Google Scholar] [CrossRef]
- Cheng, W.P.; Kao, Y.P.; Yu, R.F. A novel method for on-line evaluation of floc size in coagulation process. Water Res. 2008, 42, 2691–2697. [Google Scholar] [CrossRef]
- Koh, P.T.L.; Andrews, J.R.G.; Uhlherr, P.H.T. Floc-size distribution of scheelite treated by shear-flocculation. Int. J. Miner. Process. 1986, 17, 45–65. [Google Scholar] [CrossRef]
- Xu, W.; Gao, B.; Du, B.; Xu, Z.; Zhang, Y.; Wei, D. Influence of shear force on floc properties and residual aluminum in humic acid treatment by nano-al13. J. Hazard Mater. 2014, 271, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, R.; Jiang, Y.; Chow, C.W.K. Characterization of floc structure and strength: Role of changing shear rates under various coagulation mechanisms. Colloids Surf. A: Physicochem. Eng. Asp. 2011, 379, 36–42. [Google Scholar] [CrossRef]
- Barbot, E.; Moustier, S.; Bottero, J.Y.; Moulin, P. Coagulation and ultrafiltration: Understanding of the key parameters of the hybrid process. J. Membrane Sci. 2008, 325, 520–527. [Google Scholar] [CrossRef]
- Zheng, H.L.; Feng, L.; Gao, B.Y.; Zhou, Y.H.; Zhang, S.X.; Xu, B.C. Effect of cationic block structure on the characteristics of sludge flocs formed by charge neutralization and patching. Materials 2017, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Lan, S.; Li, X.; Xie, Y.; Liang, Y.; Yan, P.; Chen, Z.Y.; Xin, Y.X. Characterization and coagulation-flocculation performance of a composite flocculant in high-turbidity drinking water treatment. Chemosphere 2018, 206, 701–708. [Google Scholar] [CrossRef] [PubMed]











| pH | Density (g·mL−1) | Zeta Potential (mV) | SRF (×1012 m·kg−1) | Moisture (%) | VSS/TSS | Appearance |
|---|---|---|---|---|---|---|
| 7.3 | 1.018 | −25.48 | 44.7 | 97.9 | 0.714 | Yellow and fresh |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Xu, X.; Nie, R.; Feng, L.; Li, X.; Liu, B. Chitosan Modified Cationic Polyacrylamide Initiated by UV-H2O2 for Sludge Flocculation and New Insight on the Floc Characteristics Study. Polymers 2020, 12, 2738. https://doi.org/10.3390/polym12112738
Chen J, Xu X, Nie R, Feng L, Li X, Liu B. Chitosan Modified Cationic Polyacrylamide Initiated by UV-H2O2 for Sludge Flocculation and New Insight on the Floc Characteristics Study. Polymers. 2020; 12(11):2738. https://doi.org/10.3390/polym12112738
Chicago/Turabian StyleChen, Jie, Xiaojun Xu, Rui Nie, Li Feng, Xuhao Li, and Bingzhi Liu. 2020. "Chitosan Modified Cationic Polyacrylamide Initiated by UV-H2O2 for Sludge Flocculation and New Insight on the Floc Characteristics Study" Polymers 12, no. 11: 2738. https://doi.org/10.3390/polym12112738
APA StyleChen, J., Xu, X., Nie, R., Feng, L., Li, X., & Liu, B. (2020). Chitosan Modified Cationic Polyacrylamide Initiated by UV-H2O2 for Sludge Flocculation and New Insight on the Floc Characteristics Study. Polymers, 12(11), 2738. https://doi.org/10.3390/polym12112738






