Self-Assembly CNTs@PANi Coffee Rings on Poly(styrene-ethylene-butylene-styrene) Triblock Copolymer for Largely Stretchable Electronics
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of CNTs@PANi
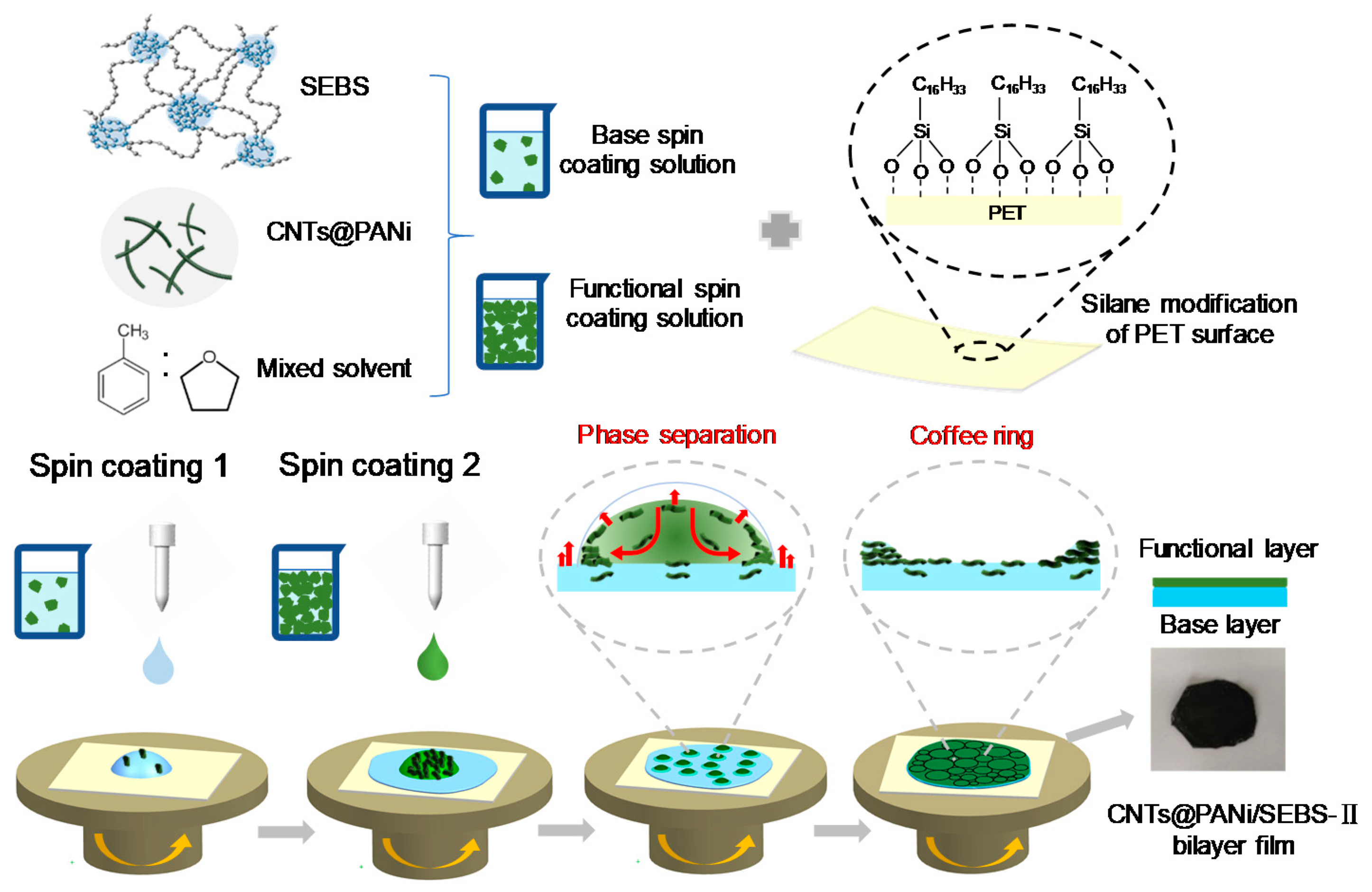
2.3. Preparation of CNTs@PANi/SEBS Film
2.3.1. PET Substrate Modification
2.3.2. Preparation of Spin Coating Solution
2.3.3. Preparation of CNTs@PANi/SEBS Composite Film
2.4. Characterization
3. Results and Discussion
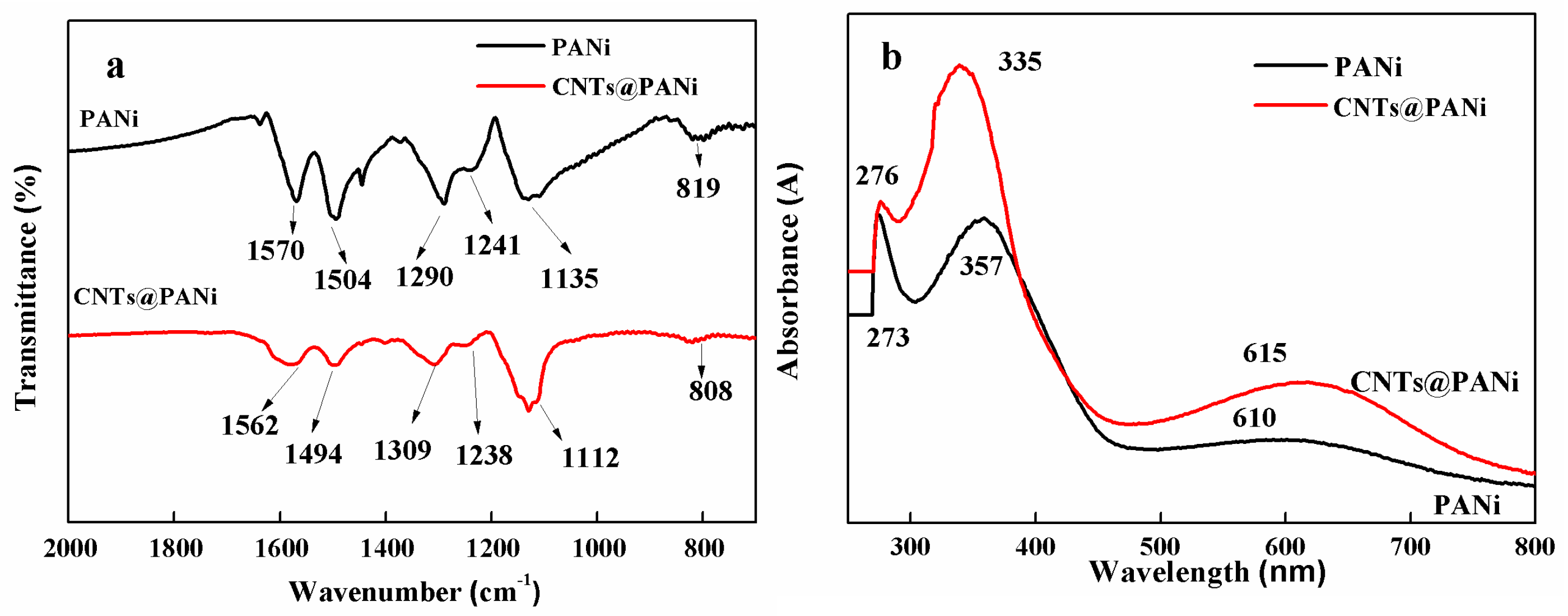
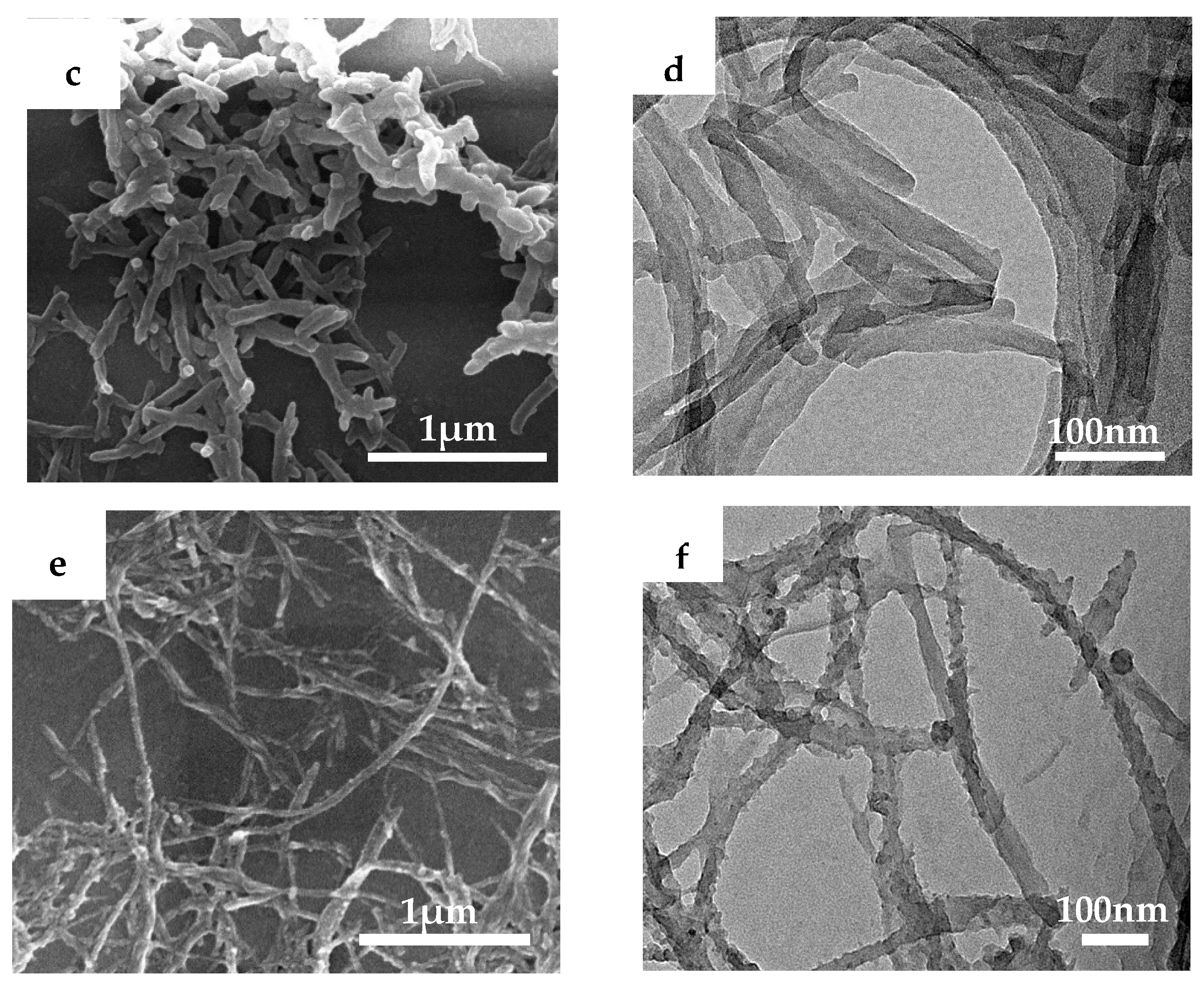
3.1. Structure and Morphology of CNTs@PANi
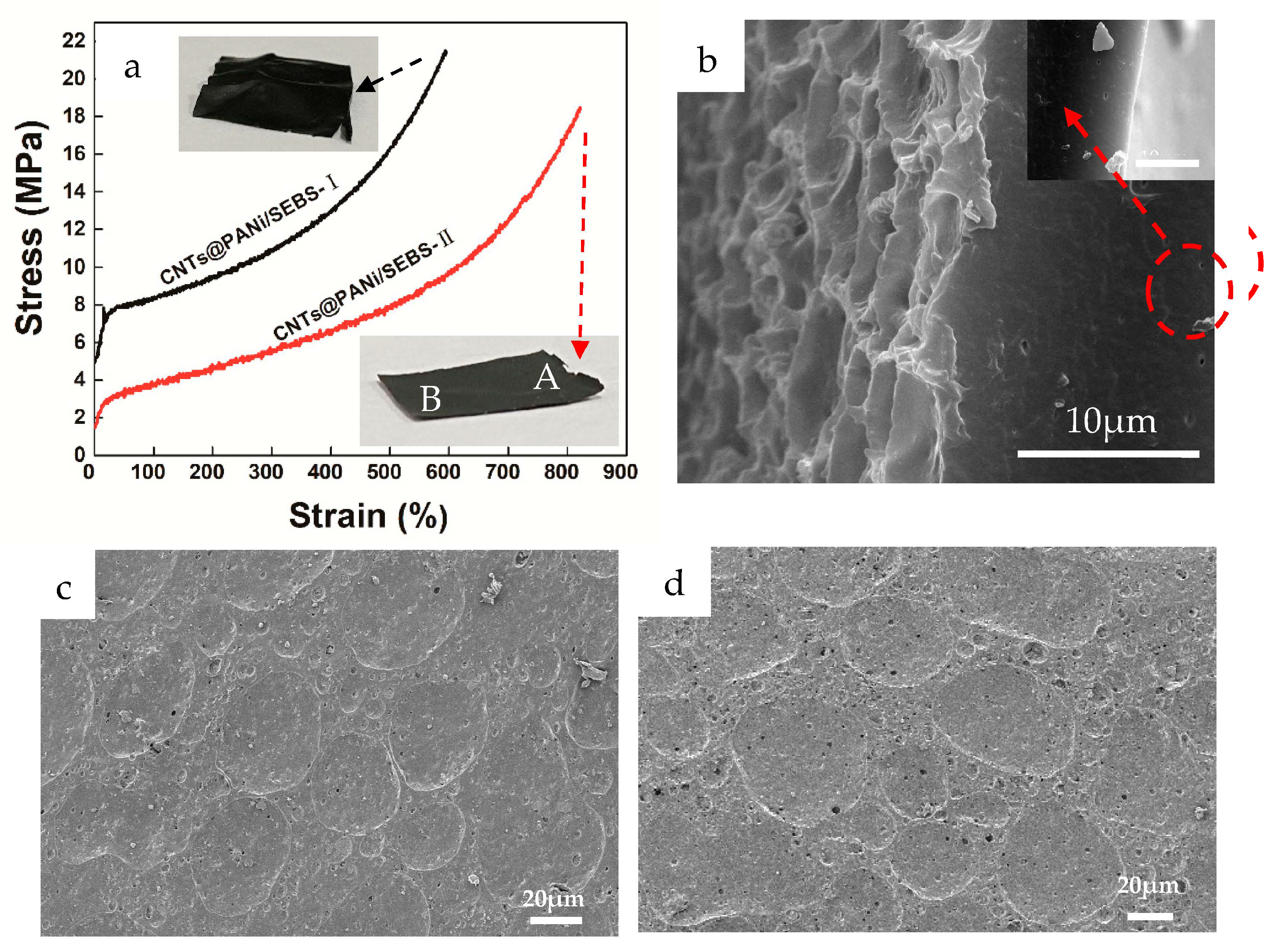
3.2. Morphology of CNTs@PANi/SEBS Film
3.3. Conductivity and Stretchability of CNTs@PANi/SEBS Film
3.4. Electrochemical Properties of CNTs@PANi/SEBS Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeng, J.; Dong, L.; Sha, W.; Wei, L.; Guo, X. Highly stretchable, compressible and arbitrarily deformable all-hydrogel soft supercapacitors. Chem. Eng. J. 2020, 383, 123098. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, N.; Wen, Z.; Cheng, P.; Zheng, H.; Shao, Y.; Xia, Y.; Chen, C.; Lan, H.; Xie, X.; et al. Liquid-metal-based super-stretchable and structure-designable triboelectric nanogenerator for wearable electronics. ACS Nano 2018, 12, 2027–2034. [Google Scholar] [CrossRef]
- Liu, Y.; He, K.; Chen, G.; Leow, W.; Chen, X. Nature-inspired structural materials for flexible electronic devices. Chem. Rev. 2017, 117, 12893–12941. [Google Scholar] [CrossRef]
- Son, D.; Bao, Z. Nanomaterials in skin-inspired electronics: Toward soft and robust skin-like electronic nanosystems. ACS Nano 2018, 12, 11731–11739. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, H.; Song, S.; Ma, Y.; Li, J.; Lee, G.; Han, Q.; Liu, L. Highly stretchable conductive fibers from few-walled carbon nanotubes coated on poly(m-phenylene isophthalamide) polymer core/shell structures. ACS Nano 2015, 9, 10252–10257. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, D.; Guo, P.; Liu, Y.; Zhu, B.; Yang, H.; Liu, Y.; Li, B.; Zhang, C.; Yu, J.; et al. Thickness-gradient films for high gauge-factor stretchable strain sensors. Adv. Mater. 2015, 27, 6230–6237. [Google Scholar] [CrossRef]
- Wu, X.; Han, Y.; Zhang, X.; Lu, C. Spirally structured conductive composites for highly stretchable, robust conductors and sensors. ACS Appl. Mater. Interfaces 2017, 9, 23007–23016. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Dou, Y.; Li, J.; Liu, Z. Buckled structures: Fabrication and applications in wearable electronics. Small 2019, 15, 1804805. [Google Scholar] [CrossRef] [PubMed]
- Al-Milaji, K.; Zhao, H. New Perspective of mitigating the coffee-ring effect: Interfacial assembly. J. Phys. Chem. C 2019, 123, 12029–12041. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Xin, Z.; Deng, M.; Wen, Y.; Song, Y. Controlled inkjetting of a conductive pattern of silver nanoparticles based on the coffee—ring effect. Adv. Mater. 2013, 25, 6714–6718. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Stassi, S.; Pisano, A.P.; Zohdi, T.I. Coffee-ring effect-based three dimensional patterning of micro/nanoparticle assembly with a single droplet. Langmuir 2010, 26, 11690–11698. [Google Scholar] [CrossRef]
- Mayarani, M.; Basavaraj, M.G.; Satapathy, D.K. Viscoelastic particle–laden interface inhibits coffee-ring formation. Langmuir 2018, 34, 14294–14301. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.L.; Saengchairat, N.; Agarwala, S.; Yeong, W.Y.; Tran, T. Sessile droplets containing carbon nanotubes: A study of evaporation dynamics and CNT alignment for printed electronics. Nanoscale 2019, 11, 10603–10614. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, A.; Azoubel, S.; Magdassi, S. Inkjet printing of flexible high-performance carbon nanotube transparent conductive films by “coffee ring effect”. Nanoscale 2014, 6, 11084–11089. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, S.; Li, A.; Tang, X.; Li, L.; Guo, L. Bioinspired interfacial chelating-like reinforcement strategy toward mechanically enhanced lamellar materials. ACS Nano 2018, 12, 4269–4279. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Ma, Y.; Song, Y.; Zhong, Q.; Chu, Y.; Karakurt, I.; Bogy, D.B.; Lin, L. A flexible piezoelectret actuator/sensor patch for mechanical human–machine interfaces. ACS Nano 2019, 13, 7107–7116. [Google Scholar] [CrossRef]
- Yin, B.; Wen, Y.; Hong, T.; Xie, Z.; Yuan, G.; Ji, Q.; Jia, H. Highly stretchable, ultrasensitive, and wearable strain sensors based on facilely prepared reduced graphene oxide woven fabrics in an ethanol flame. ACS Appl. Mater. Interfaces 2017, 9, 32054–32064. [Google Scholar] [CrossRef]
- Choi, S.; Han, S.; Kim, D.; Hyeon, T.; Kim, D.H. High-performance stretchable conductive nanocomposites: Materials, processes, and device applications. Chem. Soc. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef]
- Moon, G.; Joo, J.; Yin, Y. Stacked multilayers of alternating reduced graphene oxide and carbon nanotubes for planar supercapacitors. Nanoscale 2013, 5, 11577–11581. [Google Scholar] [CrossRef]
- Wang, X.X.; Yu, G.F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.Z. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Sun, Z.; Chang, H. Graphene and graphene-like two-dimensional materials in photodetection: Mechanisms and methodology. ACS Nano 2014, 8, 4133–4156. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, K.; Li, Y.; Zhuang, T.T.; Gao, H.L.; Liu, Y.Y.; He, C.X.; Yu, S.H. Regulating silver nanowire size enables efficient photoelectric conversion. Sci. China Chem. 2020, 63, 1046–1052. [Google Scholar] [CrossRef]
- Cha, C.; Shin, S.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, X.; Liu, Y.; Fu, Q. Highly sensitive, ultrastretchable strain sensors prepared by pumping hybrid fillers of carbon nanotubes/cellulose nanocrystal into electrospun polyurethane membranes. ACS Appl. Mater. Interfaces 2019, 11, 12968–12977. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, Z.; Sun, H.; Li, Y. Composite films based on aligned carbon nanotube arrays and a poly(n−isopropyl acrylamide) hydrogel. Adv. Mater. 2008, 20, 2201–2205. [Google Scholar] [CrossRef]
- Vankalaya, R.; Lai, W.J.; Cheng, K.C.; Hwang, K. Enhanced electrical conductivity of nylon 6 composite using polyaniline-coated multi-walled carbon nanotubes as additives. Polymer 2011, 52, 3337–3343. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.; Kim, N.; Lee, J. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Wang, H.; Meng, F.; Huang, F.; Jing, C.; Li, Y.; Wei, W.; Zhou, Z. Interface modulating CNTs@PANi hybrids by controlled unzipping of the walls of CNTs to achieve tunable high-performance microwave absorption. ACS Appl. Mater. Interfaces 2019, 11, 12142–12153. [Google Scholar] [CrossRef]
- Hu, R.; Wang, Y.; Zhao, J.; Jiang, R.; Zheng, J. Fabrication of stretchable multi-element composite for flexible solid-state electrochemical capacitor application. Chem. Eng. J. 2019, 361, 109–116. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Wang, X.; Qiu, B.; Li, Z.; Ding, J. Preparation of vertically aligned carbon nanotube/polyaniline composite membranes and the flash welding effect on their supercapacitor properties. RSC Adv. 2016, 6, 98598–98605. [Google Scholar] [CrossRef]
- Xu, J.; Ding, J.; Zhou, X.; Zhang, Y.; Zhu, W.; Liu, Z.; Ge, S.; Yuan, N.; Fang, X.; Baughman, R.H. Enhanced rate performance of flexible and stretchable linear supercapacitors based on polyaniline@Au@carbon nanotube with ultrafast axial electron transport. J. Power Sources 2017, 340, 302–308. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wei, Q.; Yang, J.; Qiu, B.; Xu, J.; Wang, X. Synergistic effect of organophosphate functionalized montmorillonite on properties and water resistance of intumescent flame—retarded SEBS. Fire Mater. 2018, 43, 74–83. [Google Scholar] [CrossRef]
- Castañeda, S.; Ribadeneira, R. Description of hydroxide ion structural diffusion in a quaternized SEBS anion exchange membrane using Ab initio molecular dynamics. J. Phys. Chem. C 2020, 124, 9834–9851. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Kang, X.; Chen, G.; Zhu, M.; Xu, J. Effect of injection molding on structure and properties of poly(styrene—ethylene—butylene—styrene) and its nanocomposite with functionalized montmorillonite. J. Appl. Polym. Sci. 2020, 138, 49633. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Chen, G.; Zhang, R.; Li, Y.; Qiu, B.; Li, X. Effects of phosphate emulsion—based montmorillonite on structure and properties of poly(styrene-ethylene-butylene-styrene) triblock copolymer. Polym. Eng. Sci. 2020, 60, 1342–1352. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Zhou, X.; Wei, Q.; Li, J.; Qi, B.; Wunderlich, K.; Wang, X. Effect of compatibilizer on morphology, rheology and properties of SEBS/clay nanocomposites. Polym. Test. 2018, 67, 435–440. [Google Scholar] [CrossRef]
- Pantoja, M.; Jian, P.Z.; Cakmak, M.; Cavicchi, K.A. Shape memory properties of polystyrene-block-poly(ethylene-co-butylene)-block-polystyrene (SEBS) ABA triblock copolymer thermoplastic elastomers. ACS Appl. Polym. Mater. 2019, 1, 414–424. [Google Scholar] [CrossRef]
- Gennari, C.; Quaroni, G.; Creton, C.; Minghetti, P.; Cilurzo, F. SEBS block copolymers as novel materials to design transdermal patches. Int. J. Pharm. 2020, 575, 118975. [Google Scholar] [CrossRef]
- Kurusu, R.S.; Demarquette, N.R. Wetting of hydrophilic electrospun mats produced by blending SEBS with PEO–PPO–PEO copolymers of different molecular weight. Langmuir 2016, 32, 1846–1853. [Google Scholar] [CrossRef]
- Krishnan, S.; Ward, R.J.; Hexemer, A.; Sohn, K.E.; Lee, K.L.; Angert, E.R.; Fischer, D.R.; Kramer, E.J.; Ober, C.K. Surfaces of fluorinated pyridinium block copolymers with enhanced antibacterial activity. Langmuir 2006, 22, 11255–11266. [Google Scholar] [CrossRef]
- Yang, X.; Sun, L.; Zhang, C.; Huang, B.; Chu, Y.; Zhao, B. Modulating the sensing behaviors of poly(styrene-ethylene-butylenestyrene)/carbon nanotubes with low-dimensional fillers for large deformation sensors. Compos. Part B 2019, 160, 605–614. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Wang, G.J.N.; Zhu, C.; Luo, S.; Jin, L.; Gu, X.; Chen, S.; Feig, V.R.; To, J.W.; et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 2017, 355, 59. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.; Kumar, S.; Sarkar, J.; Singh, J.P. Mechanical strain induced tunable anisotropic wetting on buckled PDMS silver nanorods arrays. ACS Appl. Mater. Interfaces 2015, 7, 8419–8426. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.W.; Chou, Y.C.; Wang, P.Y. Integration of ultrathin MoS2/PANI/CNT composite paper in producing All-Solid-State flexible supercapacitors with exceptional volumetric energy density. J. Phys. Chem. C 2019, 123, 17864–17872. [Google Scholar] [CrossRef]
- Purkayastha, D.; Madhurima, V. Interactions in water–THF binary mixture by contact angle, FTIR and dielectric studies. J. Mol. Liq. 2013, 187, 54–57. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, K.; Zhu, M.; Chen, G.; Tang, Z.; Li, Y.; Yu, H.; Qiu, B.; Li, X. Corrosion resistance of stretchable electrospun SEBS/PANi micro-nano fiber membrane. Eur. Polym. J. 2019, 123, 109394. [Google Scholar] [CrossRef]
- Costa, P.; Oliveira, J.; Horta-Romarís, L.; Abad, M.-J.; Moreira, J.; Zapiráin, I.; Aguado, M.; Galván, S.; Lanceros-Mendez, S. Piezoresistive polymer blends for electromechanical sensor applications. Compos. Sci. Technol. 2018, 168, 353–362. [Google Scholar] [CrossRef]
- Ribeiro, S.; Costa, P.; Ribeiro, C.; Sencadas, V.; Botelho, G.; Lanceros-Méndez, S. Electrospun styrene–butadiene–styrene elastomer copolymers for tissue engineering applications: Effect of butadiene/styrene ratio, block structure, hydrogenation and carbon nanotube loading on physical properties and cytotoxicity. Compos. Part B Eng. 2014, 67, 30–38. [Google Scholar] [CrossRef]




| Sample | Rs (Ω·cm2) | Rc (Ω·cm2) | Cc (F·cm−2) |
|---|---|---|---|
| CNTs@PANi/SEBS-I | 3810 | 32,083 | 6.93 × 10−8 |
| CNTs@PANi/SEBS-IIA | 241 | 18,080 | 1.36 × 10−8 |
| CNTs@PANi/SEBS-IIB | 160 | 4080 | 3.41 × 10−7 |
| Sample | Ecorr (V) | Icorr (A·cm−2) | Rp (Ω·cm2) | Vcorr (mm/year) |
|---|---|---|---|---|
| 304SS | −0.490 | 1.943 × 10−6 | 9798 | 0.0270 |
| SEBS | −0.481 | 5.374 × 10−7 | 54,409 | 0.0060 |
| CNTs@PANi/SEBS-I | −0.456 | 5.445 × 10−9 | 5,400,049 | 0.0006 |
| CNTs@PANi/SEBS-IIA | −0.237 | 3.760 × 10−9 | 3,252,258 | 0.0003 |
| CNTs@PANi/SEBS-IIB | −0.481 | 9.753 × 10−7 | 46,119 | 0.0012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Zhang, R.; Chen, G.; He, W.; Chen, Y.; Yu, D.-G.; Li, X. Self-Assembly CNTs@PANi Coffee Rings on Poly(styrene-ethylene-butylene-styrene) Triblock Copolymer for Largely Stretchable Electronics. Polymers 2020, 12, 2847. https://doi.org/10.3390/polym12122847
Zhu M, Zhang R, Chen G, He W, Chen Y, Yu D-G, Li X. Self-Assembly CNTs@PANi Coffee Rings on Poly(styrene-ethylene-butylene-styrene) Triblock Copolymer for Largely Stretchable Electronics. Polymers. 2020; 12(12):2847. https://doi.org/10.3390/polym12122847
Chicago/Turabian StyleZhu, Ming, Ruifeng Zhang, Gang Chen, Wenjun He, Yaowei Chen, Deng-Guang Yu, and Xiaoyan Li. 2020. "Self-Assembly CNTs@PANi Coffee Rings on Poly(styrene-ethylene-butylene-styrene) Triblock Copolymer for Largely Stretchable Electronics" Polymers 12, no. 12: 2847. https://doi.org/10.3390/polym12122847
APA StyleZhu, M., Zhang, R., Chen, G., He, W., Chen, Y., Yu, D.-G., & Li, X. (2020). Self-Assembly CNTs@PANi Coffee Rings on Poly(styrene-ethylene-butylene-styrene) Triblock Copolymer for Largely Stretchable Electronics. Polymers, 12(12), 2847. https://doi.org/10.3390/polym12122847






