Adsorption Thermodynamics and Kinetics of Resin for Metal Impurities in Bis(2-hydroxyethyl) Terephthalate
Abstract
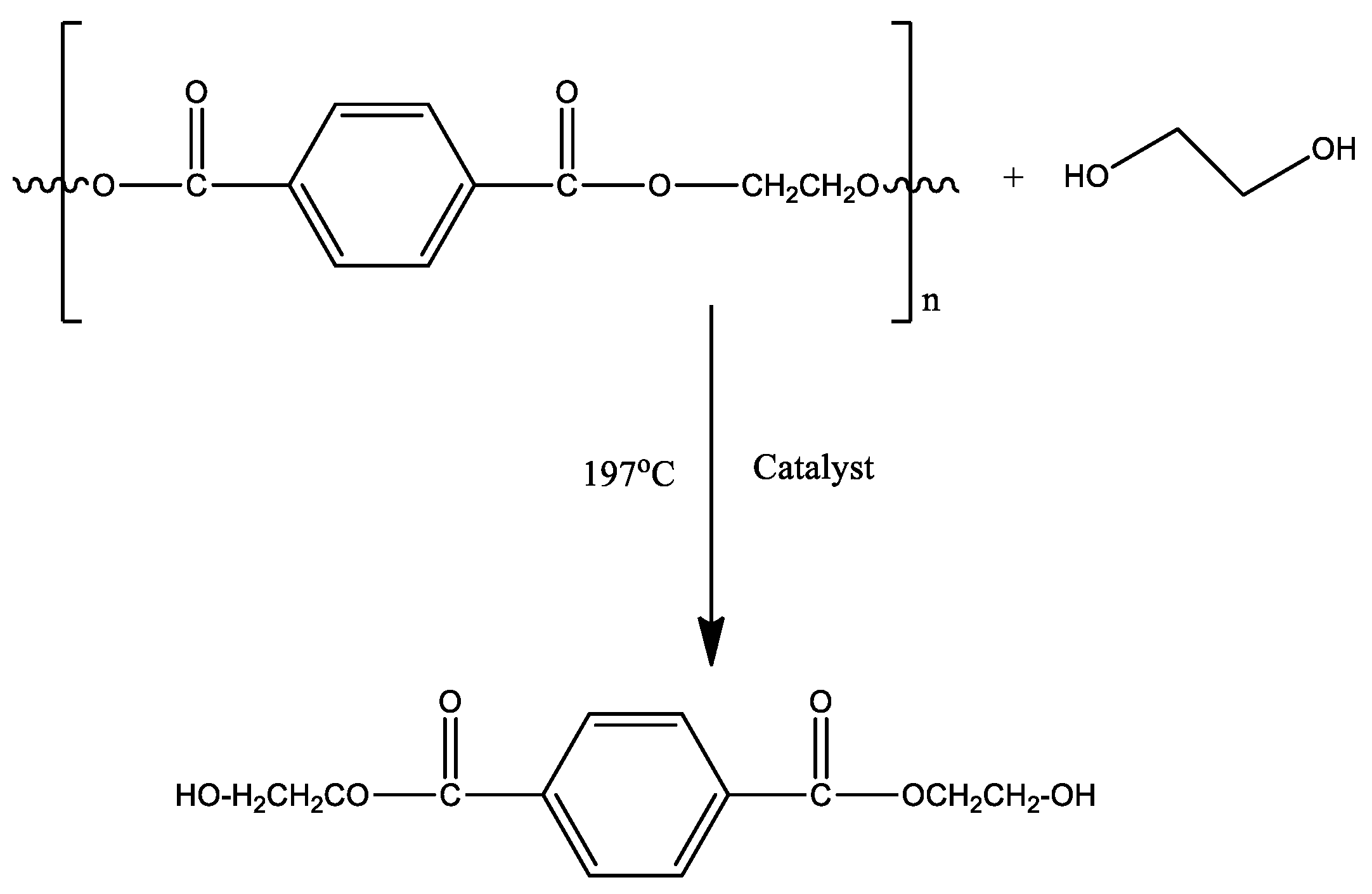
:1. Introduction
2. Materials and Methods
3. Results and Discussion
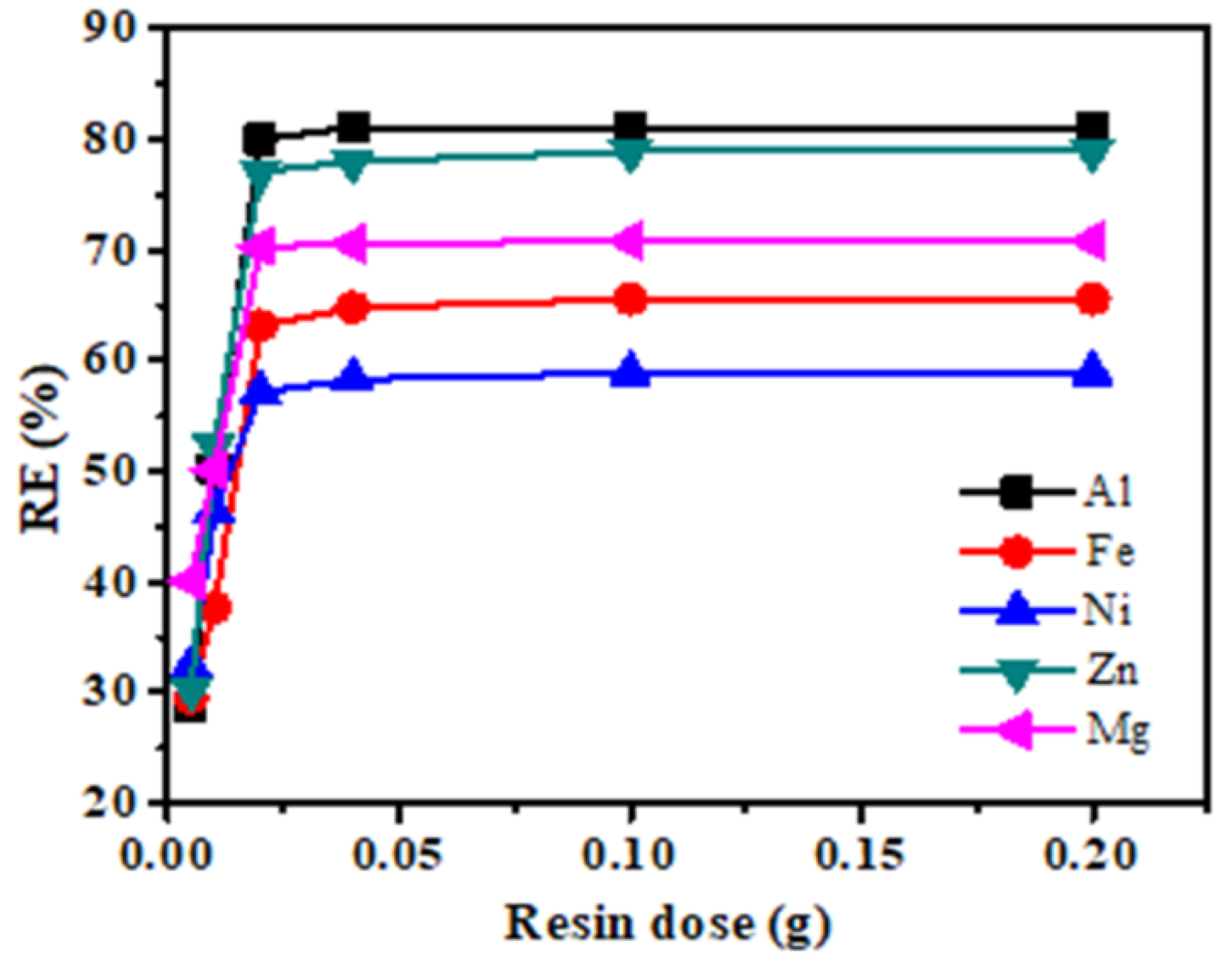
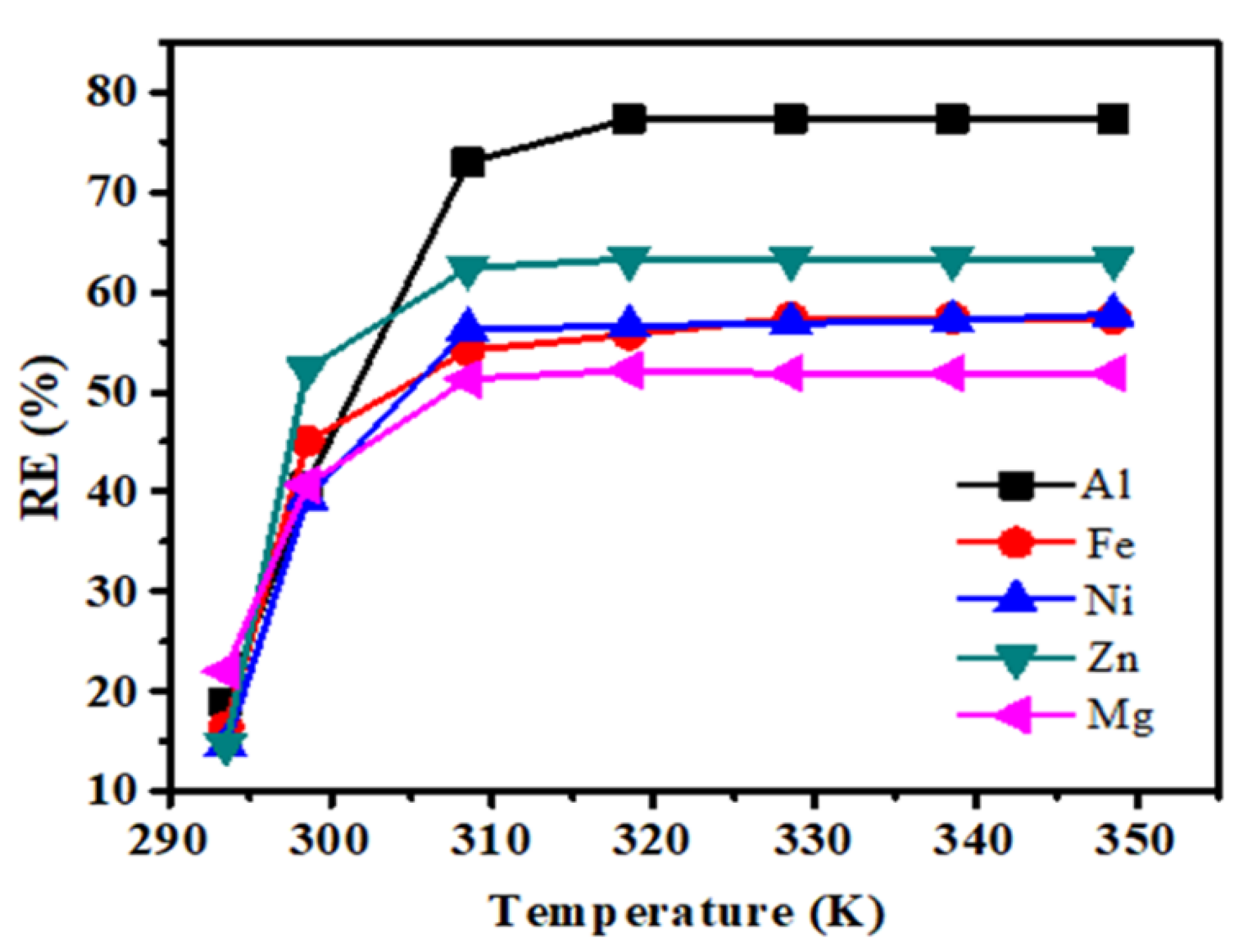
3.1. Metal Uptake Parameter Optimizations
3.2. Ion Exchange Isotherm
3.3. Ion Exchange Kinetic
3.4. Application of Purified BHET
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Welle, F. Twenty years of PET bottle to bottle recycling: An overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USEPA. Facts and Figures about Materials, Was Tend Recycling. 2017. Available online: https://www.epa.gov/facts-and-figures-about-materials-wasteand-recycling/plastics-material-specific-data (accessed on 1 November 2019).
- Lei, Y.; Chen, F.; Luo, Y.; Zhang, L. Synthesis of three-dimensional graphene oxide foam for the removal of heavy metal ions. Chem. Phys. Lett. 2014, 593, 122–127. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Ion exchange of Pb2+, Cu2+, Fe3+, and Cr3+ on natural clinoptilolite: Selectivity determination and influence of acidity on metal uptake. J. Colloid Interface Sci. 2003, 261, 49–54. [Google Scholar] [CrossRef]
- Rivera, J.; Sanchez-Polo, M. Adsorption of Cr (III) on ozonised activated carbon. Importance of Cπ cation interactions. Water Res. 2003, 37, 3335–3340. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettier, I.P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. J. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Shukla, S.R.; Harad, A.M. Polyaminolysis of polyethylene terephthalate waste. Polymer Degradation Stability. J. Degrad. Stab. 2006, 91, 1850–1854. [Google Scholar] [CrossRef]
- Daniel, P.; Spychaj, T. Chemical recycling of poly (ethylene terephthalate). J. Ind. Eng. Chem. Res. 1997, 36, 1373–1383. [Google Scholar]
- Lorenzetti, C.; Manaresi, P.; Berti, C.; Barbiroli, G. Chemical recovery of useful chemicals from polyester (PET) waste for resource conservation: A survey of state of the art. J. Polym. Environ. 2006, 14, 89–101. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Achilias, D.S. Chemical recycling of poly (ethylene terephthalate). Macromol. Mater. Eng. 2007, 292, 128–146. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Arnaiz, S.; Gutiérrez-Ortiz, J.I. Chemical recycling of post-consumer PET wastes by glycolysis in the presence of metal salts. Polym. Degrad. Stab. 2010, 95, 1022–1028. [Google Scholar]
- Sinha, V.K.; Patel, M.R.; Patel, J.V. PET Waste Management by Chemical Recycling: A review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Vaidya, U.R.; Nadkarni, V.M. Polyester polyols for polyurethanes from pet waste: Kinetics of polycondensation. J. Appl. Polym. Sci. 1988, 35, 775–785. [Google Scholar] [CrossRef]
- Pingale, N.D.; Palekar, V.S.; Shukla, S.R. Glycolysis of postconsumer polyethylene terephthalate waste. J. Appl. Polym. Sci. 2010, 115, 249–254. [Google Scholar] [CrossRef]
- Panahi, H.A.; Abdouss, M.; Ghiabi, F.; Moniri, E.; Shoushtari, A.M. Modification and characterization of poly (ethylene terephthalate) grafted-acrylic acid/acryl amide fiber for removal of lead from human plasma and environmental samples. J. Appl. Polym. Sci. 2012, 124, 5236–5246. [Google Scholar]
- Karayannidis, G.P.; Nikolaidis, A.K.; Sideridou, I.D.; Bikiaris, D.N.; Achilias, D.S. Chemical recycling of PET by glycolysis: Polymerization and characterization of the dimethacrylated glycolysate. Macromol. Mater. Eng. 2006, 291, 1338–1347. [Google Scholar] [CrossRef]
- Li, M.J.; Huang, Y.H.; Ju, A.Q.; Yu, T.S.; Ge, M.Q. Synthesis and characterization of azo dyestuff based on bis (2-hydroxyethyl) terephthalate derived from depolymerized waste poly (ethylene terephthalate) fibers. Chin. Chem. Lett. 2014, 25, 1550–1554. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET: A review. Eur. J. Polym. 2005, 41, 1453. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Christopher, W.K.; Saint, C. Recent development in photocatalytic water treatment technology: A review. Water Resour. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Simon, J. The MBK Book, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2010; 536p. [Google Scholar]
- Rengaraj, S.; Yeon, J.-W.; Kim, Y.; Jung, Y.; Ha, Y.K.; Kim, W.H. Adsorption characteristics of Cu (II) onto ion exchange resins 252H and 1500H: Kinetics, isotherms and error analysis. J. Hazard. Mater 2007, 143, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Morcali, M.H.; Zeytuncu, B.; Baysal, A.; Akman, S.; Yucel, O. Adsorption of copper and zinc from sulfate media on a commercial sorbent. J. Environ. Chem. Eng. 2014, 2, 1655–1662. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, G. Study on the macromolecular coagulant PEX which traps heavy metals. Chem. Eng. Sci. 2007, 62, 4636–4643. [Google Scholar] [CrossRef]
- Imam, E.A.; Sayed, I.E.; Mahfouz, M.G.; Tolba, A.A.; Akashi, T.; Galhoum, A.A.; Guibal, E. Synthesis of α-aminophosphonate functionalized chitosan sorbents: Effect of methyl vs. phenyl group on uranium sorption. J. Chem. Eng. 2018, 352, 1022–1034. [Google Scholar] [CrossRef]
- Jha, M.K.; Nguyen, N.V.; Lee, J.C.; Jeong, J.; Yoo, J.-M. Adsorption of copper from the sulphate solution of low copper contents using the cationic resin Amberlite IR 120. J. Hazard. Mater. 2009, 164, 948–953. [Google Scholar] [CrossRef]
- Yu, Z.; Qi, T.; Qu, J.; Wang, L.; Chu, J. Removal of Ca (II) and Mg (II) from potassium chromate solution on Amberlite IRC 748 synthetic resin by ion exchange. J. Hazard. Mater. 2009, 167, 406–412. [Google Scholar] [CrossRef]
- Goher, M.E.; Hassan, A.M.; Abdel, I.A.; Fahmy, H.A.; Abdo, M.H.; El-sayed, M.S. Removal of aluminum, iron and manganese ions from industrial wastes using granular activated carbon and Amberlite IR-120H. Egypt. J. Aquat. Res. 2015, 41, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Izadi, A.; Mohebbi, A.; Amiri, M.; Izadi, N. Removal of iron ions from industrial copper raffinate and electro winning electrolyte solutions by chemical precipitation and ion exchange. Miner. Eng. 2017, 113, 23–35. [Google Scholar] [CrossRef]
- Ates, N.; Basak, A. Selective removal of aluminum, nickel and chromium ions by polymeric resins and natural zeolite from anodic plating wastewater. Int. J. Environ. Health Res. 2019, 1–18. [Google Scholar] [CrossRef]
- Gode, F.; Pehlivan, E. Removal of chromium (III) from aqueous solutions using Lewatit S 100: The effect of pH, time, metal concentration and temperature. J. Hazard. Mater. 2006, 136, 330–337. [Google Scholar] [CrossRef]
- Bo, L.; Fu, W.; Lu, X.; Zhou, Q.; Zhan, S. Lewis Acid–Base Synergistic Catalysis for Polyethylene Terephthalate Degradation by 1, 3-Dimethylurea/Zn (OAc) 2 Deep Eutectic Solvent. ACS Sust. Chem. Eng. 2018, 7, 3292–3300. [Google Scholar]
- Zhang, Z.; Li, H.; Li, J.; Li, X.; Wang, Z.; Liu, X.; Zhang, L. A novel adsorbent of core-shell construction of chitosin-cellulose magnetic carbon foam: Synthesis, characterization and application to remove copper in waste water. Chem. Phys. Lett. 2019, 731, 136573. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Application of bio char derived from date palm biomass for removal of lead and copper ions in a batch reactor: Kinetics and isotherm scrutiny. Chem. Phys. Lett. 2019, 722, 64–73. [Google Scholar] [CrossRef]
- Singanan, M. Removal of lead (II) and cadmium (II) ions from wastewater using activated biocarbon. Sci. Asia 2011, 37, 115–119. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; El-Desoky, H.S.; El-Moselhy, K.M.; Amer, A.; El-Naga, E.H.A.; Mohamedein, L.I.; Al-Prol, A.E. Removal of cadmium from aqueous solution using marine green algae, Ulva lactuca. Egypt. J. Aquat. Res. 2014, 40, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ye, Z.; Wang, L.; Yang, L.; Li, Y. Removal of Cd (II) by polystyrene-base chelating resins: Adsorption properties and experiences of industrial wastewater treatment. Desalin. Water Treat. 2014, 52, 6481–6491. [Google Scholar] [CrossRef]
- Badmus, M.O.A.; Audu, T.O.K.; Anyata, B. Removal of copper from industrial wastewaters by activated carbon prepared from periwinkle shells. Korean J. Chem. Eng. 2007, 24, 246–252. [Google Scholar] [CrossRef]
- Irving, L. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar]
- Abo-Farha, S.A.; Abdel-Aal, A.Y.; Ashour, I.A.; Garamon, S.E. Removal of some heavy metal cations by synthetic resin purolite C100. J. Hazard. Mater. 2009, 169, 190–194. [Google Scholar] [CrossRef]
- Herbert, F. Über die adsorption in lösungen. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Bishop, L.P. Pollution Prevention: Fundamentals and Practice; Wave land Press: Long Grove, IL, USA, 2000; Volume 1, p. 699. [Google Scholar]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead (II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Wołowicz, A.; Hubicki, Z. Comparison of strongly basic anion exchange resins applicability for the removal of palladium (II) ions from acidic solutions. Chem. Eng. J. 2011, 171, 206–215. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Transit. Met. Chem. 1998, 76, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Sahmoune, M.N.; Louhab, K.; Boukhiar, A.; Addad, J.; Barr, S. Kinetic and equilibrium models for the biosorption of Cr (III) on Streptomyces rimosus. Toxicol. Environ. Chem. 2009, 91, 1291–1303. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Z.; Li, R.; Hu, D.; Lu, Y.; Luo, H.; Yan, K. Biomass-derived porous carbon highly efficient for removal of Pb (II) and Cd (II). Green Energy Environ. 2019, 4, 414–423. [Google Scholar] [CrossRef]
- Giannotta, G.; Po, R.; Cardi, N.; Tampellini, E.; Occhiello, E.; Garbassi, F.; Nicolais, L. Processing effects of poly (ethylene terephthalate) from bottle scrape. Polym. Eng. Sci. 1994, 34, 1219–1223. [Google Scholar] [CrossRef]



| Metals | Langmuir Isotherm | R2 | |
|---|---|---|---|
| b | Qo | ||
| Al | 0.019 | 90.9 | 0.997 |
| Fe | 0.050 | 83.3 | 0.997 |
| Ni | 0.010 | 16.6 | 0.990 |
| Mg | 0.095 | 20.0 | 0.994 |
| Zn | 0.023 | 13.8 | 0.993 |
| Freundlich Isotherm | |||
|---|---|---|---|
| Metal | kf | 1/n | R2 |
| Al | 0.66 | 0.53 | 0.987 |
| Fe | 0.49 | 0.62 | 0.984 |
| Ni | 0.70 | 0.86 | 0.982 |
| Mg | 0.68 | 0.98 | 0.993 |
| Zn | 0.76 | 0.44 | 0.990 |
| Metals | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| k1 (L min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | R2 | |
| Al | 0.165 | 0.227 | 0.973 | 1.08 × 10−3 | 200 | 0.997 |
| Fe | 0.179 | 0.266 | 0.939 | 1.63 × 10−3 | 142 | 0.998 |
| Ni | 0.131 | 0.378 | 0.957 | 1.6 × 10−4 | 500 | 0.990 |
| Zn | 0.244 | 0.280 | 0.803 | 1.51 × 10−3 | 166 | 0.999 |
| Mg | 0.082 | 0.330 | 0.944 | 2.10 × 10−4 | 500 | 0.990 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, N.; Zhang, Q.; Chen, R.; Yan, D.; Zhou, Q.; Lu, X.; Xin, J. Adsorption Thermodynamics and Kinetics of Resin for Metal Impurities in Bis(2-hydroxyethyl) Terephthalate. Polymers 2020, 12, 2866. https://doi.org/10.3390/polym12122866
Fatima N, Zhang Q, Chen R, Yan D, Zhou Q, Lu X, Xin J. Adsorption Thermodynamics and Kinetics of Resin for Metal Impurities in Bis(2-hydroxyethyl) Terephthalate. Polymers. 2020; 12(12):2866. https://doi.org/10.3390/polym12122866
Chicago/Turabian StyleFatima, Noor, Qi Zhang, Ruru Chen, Dongxia Yan, Qing Zhou, Xingmei Lu, and Jiayu Xin. 2020. "Adsorption Thermodynamics and Kinetics of Resin for Metal Impurities in Bis(2-hydroxyethyl) Terephthalate" Polymers 12, no. 12: 2866. https://doi.org/10.3390/polym12122866
APA StyleFatima, N., Zhang, Q., Chen, R., Yan, D., Zhou, Q., Lu, X., & Xin, J. (2020). Adsorption Thermodynamics and Kinetics of Resin for Metal Impurities in Bis(2-hydroxyethyl) Terephthalate. Polymers, 12(12), 2866. https://doi.org/10.3390/polym12122866




