Development of Porous Polyurethane Implants Manufactured via Hot-Melt Extrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Rheological Properties and Thermal Characterization
2.2.2. Manufacturing of the Porous Extrudates
2.2.3. Apparent Density and Ovality Measurements
2.2.4. True and Skeletal Density
2.2.5. Pore Morphology
2.2.6. Mechanical Properties
2.2.7. Liquid Uptake
3. Results and Discussion
3.1. Rheological Properties, Thermal Characterization and Manufacturing
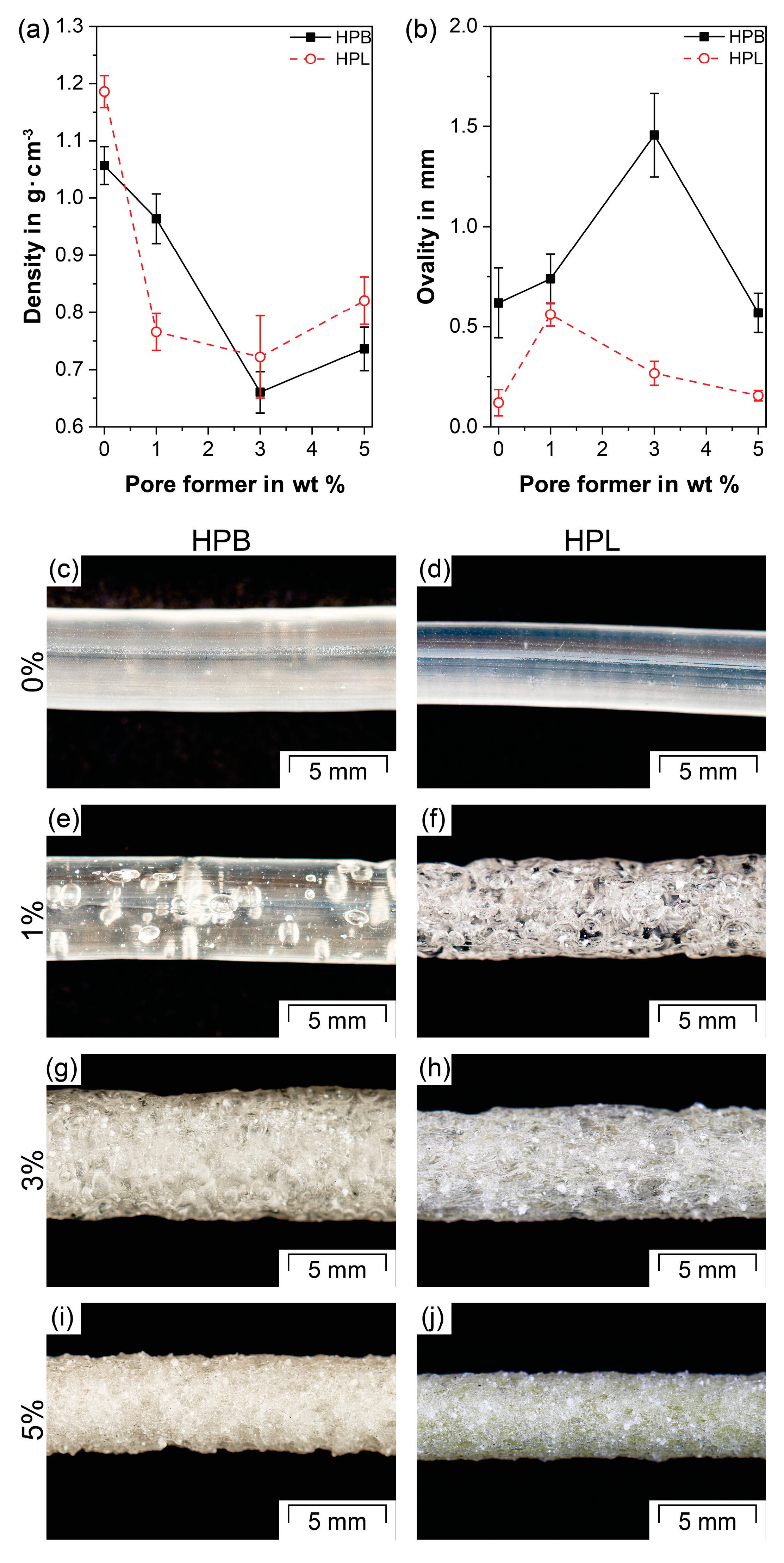
3.2. Apparent Density and Ovality Measurements
3.3. True and Skeletal Density
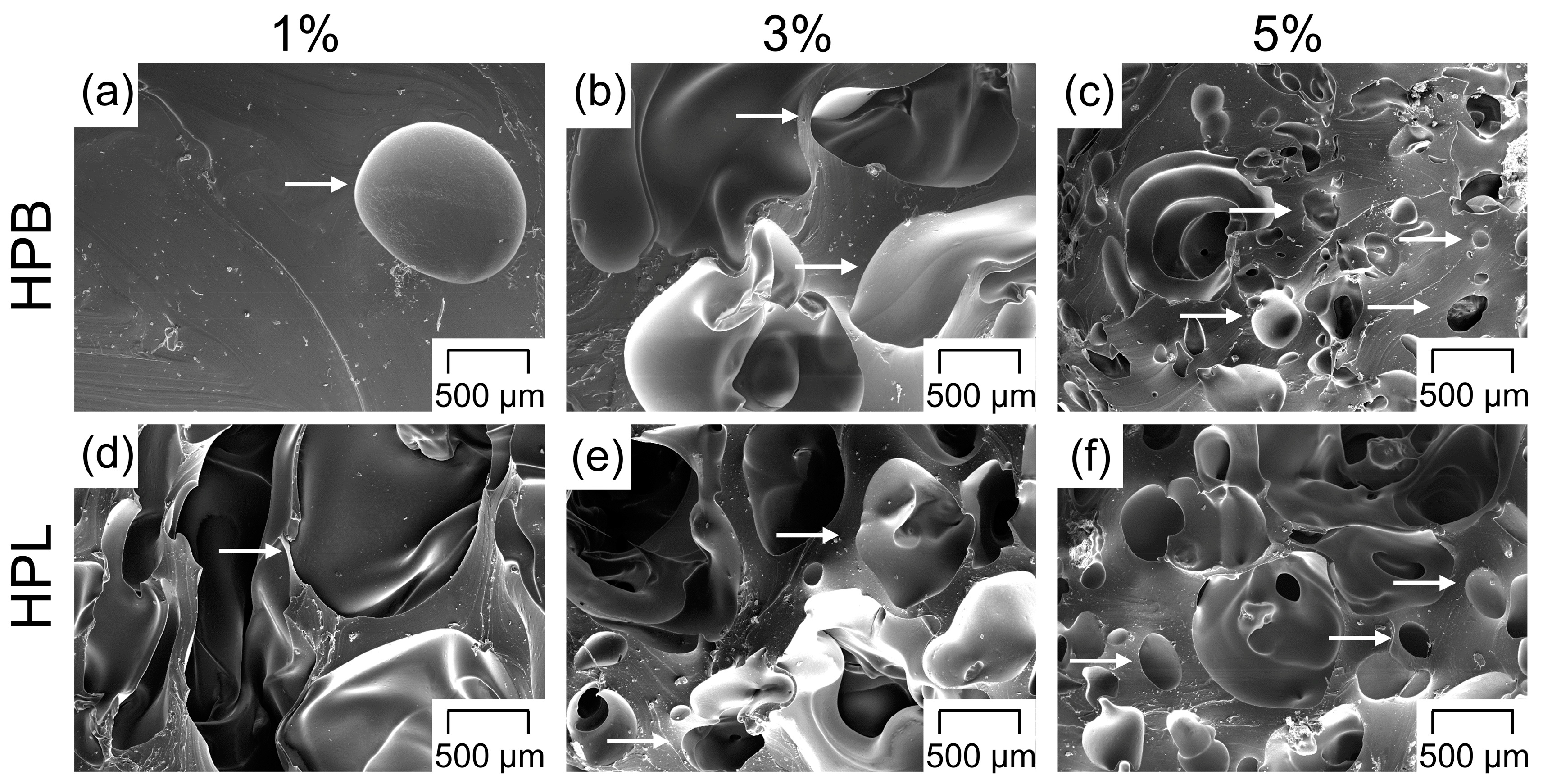
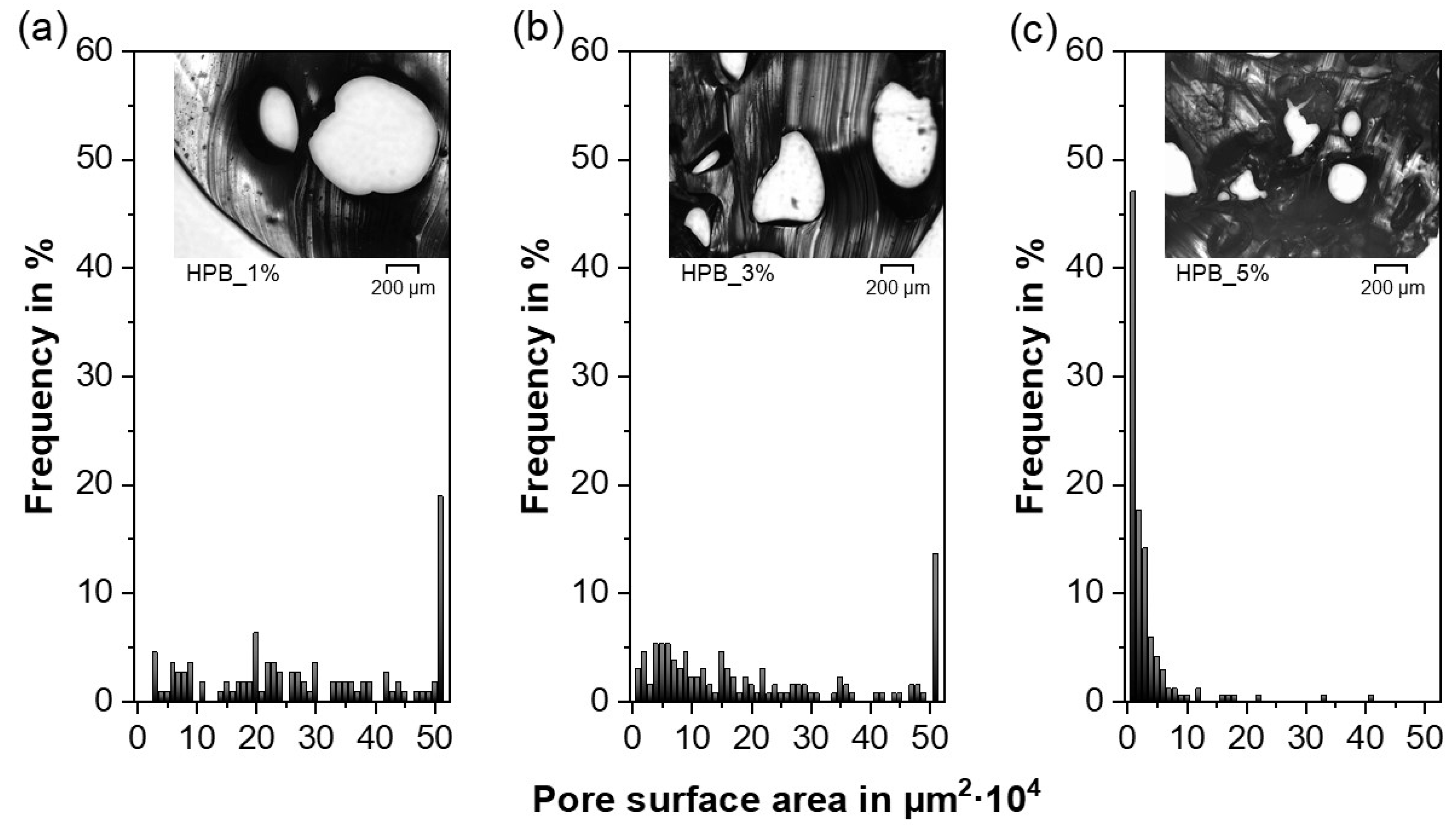
3.4. Pore Morphology
3.5. Mechanical Properties
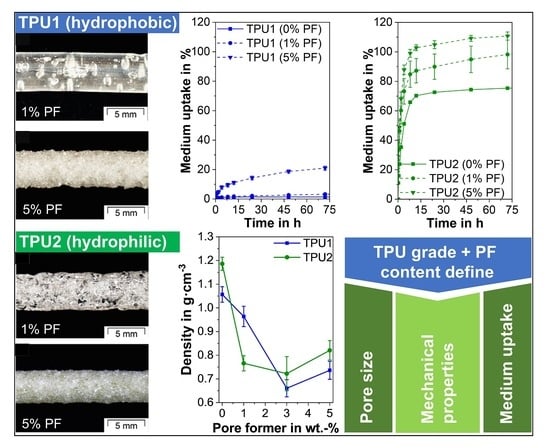
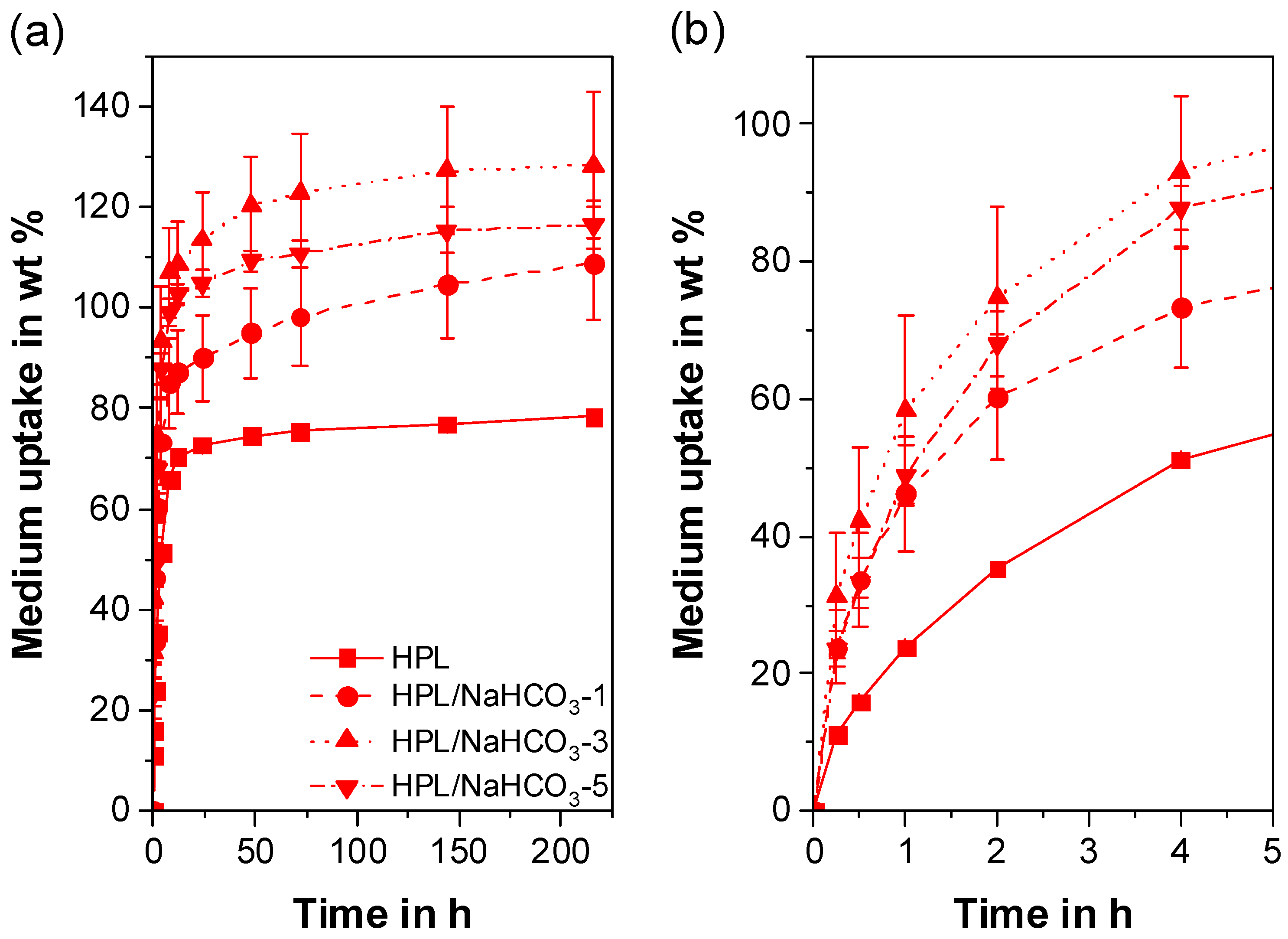
3.6. Liquid Uptake
4. Conclusions
- For the TPUs investigated, only PF with decomposition temperatures that overlap with the HME temperature, i.e., NaHCO3, led to a reproducible porous network.
- For both TPU types and the employed experimental setup, the porosity increased with increasing PF concentrations and reached a maximum at 40%. However, the appropriate PF concentration to reach the porosity maximum depended on the TPU type (i.e., hydrophobic or hydrophilic), as the gases produced due to PF decomposition during HME played a major role in the pore nucleation kinetics, leading to faster pore formation for the hydrophilic TPU. The final pore size was not affected by the hydrophilicity of the TPUs, but rather by geometrical constraints. As a result, the average pore size decreased and the pore size distribution became narrower with increasing PF content. In addition, the porous network became more interconnected whereby the initially closed-cell pores gradually rearranged into open-cell pores with increasing PF concentration.
- The compression properties of the porous extrudates were strongly influenced by the porosity and the pore morphology. Due to the smaller pores, extrudates comprising 5 wt% of PF resulted in a comparable compression modulus and compressive stress as extrudates containing 1 wt%, despite their difference in porosity. Consequently, IDDS with a broad application portfolio in terms of porosity, but comparable and consistent mechanical properties were produced.
- Depending on the hydrophilicity of the TPU, a wide range of liquid uptake was obtained, which correlated directly with the extrudate porosity. For the hydrophobic TPU, a 20-fold increase in mass was found, since liquid uptake was determined by the number of open-cell pores. Due to the high liquid uptake of the pure hydrophilic TPU, only a 1.6-fold mass increase, which was dependent on the total porosity, was achieved for the porous hydrophilic extrudates.
- Based on the results of the extrudate characterization, both hydrophobic and hydrophilic drugs can be considered as candidates. The release of a hydrophobic API will be increased due to the larger surface area provided by the open-cell pores of HPB and by the higher total porosity of HPL. However, due to the rapid liquid uptake of the porous HPL extrudates, PF concentrations lower than 1 wt% should be investigated to reduce the uptake of large medium amounts and avoid the immediate release of the API. Due to its high solubility in aqueous media, a hydrophilic API might be mainly considered in combination with the porous HPB carrier for a delayed, extended-release formulation.
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Ren, Y.; Mei, L.; Zhou, L.; Guo, G. Recent perspectives in hot melt extrusion-based polymeric formulations for drug delivery: Applications and innovations. AAPS PharmSciTech 2019, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Kohlgrüber, K.; Ullrich, D.M.; Werner, C.; Heidemeyer, P.; Lechner, D.F.; Sämann, D.H. Co-Rotating Twin-Screw Extruders; Hanser Fachbuchverlag: Munich, Germany, 2008; ISBN 9781569904220. [Google Scholar]
- Simões, M.F.; Pinto, R.M.A.; Simões, S. Hot-melt extrusion in the pharmaceutical industry: Toward filing a new drug application. Drug Discov. Today 2019, 24, 1749–1768. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; Bandari, S.; Kallakunta, V.R.; Vo, A.Q.; McFall, H.; Pimparade, M.B.; Bhagurkar, A.M. Melt extrusion with poorly soluble drugs–An integrated review. Int. J. Pharm. 2018, 535, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.; Pillai, A.; Ashour, E.A.; Repka, M.A. Systematic screening of pharmaceutical polymers for hot melt extrusion processing: A comprehensive review. Int. J. Pharm. 2020, 576, 118989. [Google Scholar] [CrossRef] [PubMed]
- Roblegg, E.; Jäger, E.; Hodzic, A.; Koscher, G.; Mohr, S.; Zimmer, A.; Khinast, J. Development of sustained-release lipophilic calcium stearate pellets via hot melt extrusion. Eur. J. Pharm. Biopharm. 2011, 79, 635–645. [Google Scholar] [CrossRef]
- Dumpa, N.R.; Sarabu, S.; Bandari, S.; Zhang, F.; Repka, M.A. Chronotherapeutic drug delivery of ketoprofen and ibuprofen for improved treatment of early morning stiffness in arthritis using hot-melt extrusion technology. AAPS PharmSciTech 2018, 19, 2700–2709. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt extrusion: From theory to application in pharmaceutical formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef]
- Vo, A.Q.; Feng, X.; Pimparade, M.; Ye, X.; Kim, D.W.; Martin, S.T.; Repka, M.A. Dual-mechanism gastroretentive drug delivery system loaded with an amorphous solid dispersion prepared by hot-melt extrusion. Eur. J. Pharm. Sci. 2017, 102, 71–84. [Google Scholar] [CrossRef]
- Baumgartner, R.; Eitzlmayr, A.; Matsko, N.; Tetyczka, C.; Khinast, J.; Roblegg, E. Nano-extrusion: A promising tool for continuous manufacturing of solid nano-formulations. Int. J. Pharm. 2014, 477, 1–11. [Google Scholar] [CrossRef]
- Baumgartner, R.; Matić, J.; Schrank, S.; Laske, S.; Khinast, J.; Roblegg, E. NANEX: Process design and optimization. Int. J. Pharm. 2016, 506, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Cossé, A.; König, C.; Lamprecht, A.; Wagner, K.G. Hot melt extrusion for sustained protein release: Matrix erosion and in vitro release of PLGA-based implants. AAPS PharmSciTech 2017, 18, 15–26. [Google Scholar] [CrossRef]
- Pattani, A.; Lowry, D.; Curran, R.M.; McGrath, S.; Kett, V.L.; Andrews, G.P.; Malcolm, R.K. Characterisation of protein stability in rod-insert vaginal rings. Int. J. Pharm. 2012, 430, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Morrow, R.J.; Woolfson, A.D.; Donnelly, L.; Curran, R.; Andrews, G.; Katinger, D.; Malcolm, R.K. Sustained release of proteins from a modified vaginal ring device. Eur. J. Pharm. Biopharm. 2011, 77, 3–10. [Google Scholar] [CrossRef]
- Regev, G.; Patel, S.K.; Moncla, B.J.; Twist, J.; Devlin, B.; Rohan, L.C. Novel application of hot melt extrusion for the manufacturing of vaginal films containing microbicide candidate dapivirine. AAPS PharmSciTech 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, R.K.; Boyd, P.J.; McCoy, C.F.; Murphy, D.J. Microbicide vaginal rings: Technological challenges and clinical development. Adv. Drug Deliv. Rev. 2016, 103, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Van Laarhoven, J.A.H.; Kruft, M.A.B.; Vromans, H. In vitro release properties of etonogestrel and ethinyl estradiol from a contraceptive vaginal ring. Int. J. Pharm. 2002, 232, 163–173. [Google Scholar] [CrossRef]
- Koutsamanis, I.; Eder, S.; Beretta, M.; Witschnigg, A.; Paudel, A.; Nickisch, K.; Friedrich, M.; Eggenreich, K.; Roblegg, E. Formulation and processability screening for the rational design of ethylene-vinyl acetate based intra-vaginal rings. Int. J. Pharm. 2019, 564, 90–97. [Google Scholar] [CrossRef]
- Vynckier, A.K.; Dierickx, L.; Voorspoels, J.; Gonnissen, Y.; Remon, J.P.; Vervaet, C. Hot-melt co-extrusion: Requirements, challenges and opportunities for pharmaceutical applications. J. Pharm. Pharmacol. 2014, 66, 167–179. [Google Scholar] [CrossRef]
- Costantini, L.C.; Kleppner, S.R.; McDonough, J.; Azar, M.R.; Patel, R. Implantable technology for long-term delivery of nalmefene for treatment of alcoholism. Int. J. Pharm. 2004, 283, 35–44. [Google Scholar] [CrossRef]
- Ghalanbor, Z.; Körber, M.; Bodmeier, R. Improved lysozyme stability and release properties of Poly(lactide-co-glycolide) implants prepared by hot-melt extrusion. Pharm. Res. 2010, 27, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Friend, D.R. Development of controlled release systems over the past 50 years in the area of contraception. J. Control. Release 2016, 240, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, M.; Engert, J.; Winter, G. European Journal of Pharmaceutics and Biopharmaceutics New insights into process understanding of solid lipid extrusion (SLE) of extruded lipid implants for sustained protein delivery. Eur. J. Pharm. Biopharm. 2018, 130, 11–21. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Fivez, A.; Siepmann, F.; Siepmann, J. Often neglected: PLGA/PLA swelling orchestrates drug release: HME implants. J. Control. Release 2019, 306, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. The origins and evolution of “controlled” drug delivery systems. J. Control. Release 2008, 132, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Mohtashami, Z.; Esmaili, Z.; Vakilinezhad, M.A.; Seyedjafari, E.; Akbari Javar, H. Pharmaceutical implants: Classification, limitations and therapeutic applications. Pharm. Dev. Technol. 2020, 25, 116–132. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, F. Twin-screw extrusion of sustained-release oral dosage forms and medical implants. Drug Deliv. Transl. Res. 2018, 8, 1694–1713. [Google Scholar] [CrossRef]
- Pons-Faudoa, F.P.; Ballerini, A.; Sakamoto, J.; Grattoni, A. Advanced implantable drug delivery technologies: Transforming the clinical landscape of therapeutics for chronic diseases. Biomed. Microdevices 2019, 21, 47. [Google Scholar] [CrossRef]
- Kumar, A.; Pillai, J. Implantable Drug Delivery Systems: An Overview. In Nanostructures for the Engineering of Cells, Tissues and Organs; Grumezescu, A.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 473–511. ISBN 9780128136669. [Google Scholar]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable polymers–A review on recent trends and emerging perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Mascarenhas, L. Insertion and removal of Implanon: Practical considerations. Eur. J. Contracept. Reprod. Health Care 2000, 5, 29–34. [Google Scholar] [PubMed]
- Kleiner, L.W.; Wright, J.C.; Wang, Y. Evolution of implantable and insertable drug delivery systems. J. Control. Release 2014, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D. Biomedical applications of synthetic polymers. In Comprehensive Polymer Science; Allen, G., Bevington, J.C., Eds.; Pergamon Press: Oxford, UK, 1989; Volume 1, pp. 201–247. [Google Scholar]
- Stanković, M.; Frijlink, H.W.; Hinrichs, W.L.J. Polymeric formulations for drug release prepared by hot melt extrusion: Application and characterization. Drug Discov. Today 2015, 20, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Lostritto, R.T. Diffusion of benzocaine in poly(ethylene-vinyl acetate) membranes: Effects of vehicle ethanol concentration and membrane vinyl acetate content. J. Control. Release 1996, 38, 185–191. [Google Scholar] [CrossRef]
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. J. Control. Release 2017, 262, 284–295. [Google Scholar] [CrossRef]
- Koutsamanis, I.; Paudel, A.; Nickisch, K.; Eggenreich, K.; Roblegg, E.; Eder, S. Controlled-Release from high-loaded reservoir type systems—A case study of ethylene-vinyl acetate and progesterone. Pharmaceutics 2020, 12, 103. [Google Scholar] [CrossRef]
- Almeida, A.; Brabant, L.; Siepmann, F.; De Beer, T.; Bouquet, W.; Van Hoorebeke, L.; Siepmann, J.; Remon, J.P.; Vervaet, C. Sustained release from hot-melt extruded matrices based on ethylene vinyl acetate and polyethylene oxide. Eur. J. Pharm. Biopharm. 2012, 82, 526–533. [Google Scholar] [CrossRef]
- Almeida, A.; Possemiers, S.; Boone, M.N.; De Beer, T.; Quinten, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur. J. Pharm. Biopharm. 2011, 77, 297–305. [Google Scholar] [CrossRef]
- Suzuki, T.; Kopia, G.; Hayashi, S.I.; Bailey, L.R.; Llanos, G.; Wilensky, R.; Klugherz, B.D.; Papandreou, G.; Narayan, P.; Leon, M.B.; et al. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation 2001, 104, 1188–1193. [Google Scholar] [CrossRef]
- Helbling, I.M.; Ibarra, J.C.D.; Luna, J.A. The optimization of an intravaginal ring releasing progesterone using a mathematical model. Pharm. Res. 2014, 31, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Ugaonkar, S.R.; Wesenberg, A.; Wilk, J.; Seidor, S.; Mizenina, O.; Kizima, L.; Rodriguez, A.; Zhang, S.; Levendosky, K.; Kenney, J.; et al. A novel intravaginal ring to prevent HIV-1, HSV-2, HPV, and unintended pregnancy. J. Control. Release 2015, 213, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Loxley, A.; Mitchnick, M.; Okoh, O.; McConnell, J.; Goldman, L.; Morgan, C.; Clark, M.; Friend, D.R. Ethylene vinyl acetate intravaginal rings for the simultaneous delivery of the antiretroviral UC781 and contraceptive levonogestrel. Drug Deliv. Transl. Res. 2011, 1, 247–255. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.W.; Boyd, P.; Dias, N.; Cameron, D.; Offord, R.E.; Hartley, O.; Kett, V.L.; Malcolm, R.K. Vaginal rings with exposed cores for sustained delivery of the HIV CCR5 inhibitor 5P12-RANTES. J. Control. Release 2019, 298, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lowinger, M.B.; Barrett, S.E.; Zhang, F.; Williams, R.O. Sustained release drug delivery applications of polyurethanes. Pharmaceutics 2018, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, G.; Mertens, P.; Grymonpré, W.; Van Bockstal, P.J.; De Beer, T.; Boone, M.N.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. A comparative study between melt granulation/compression and hot melt extrusion/injection molding for the manufacturing of oral sustained release thermoplastic polyurethane matrices. Int. J. Pharm. 2016, 513, 602–611. [Google Scholar] [CrossRef]
- Basu, A.; Farah, S.; Kunduru, K.R.; Doppalapudi, S.; Khan, W.; Domb, A.J. Polyurethanes for Controlled Drug Delivery. In Advances in Polyurethane Biomaterials; Cooper, S.L., Guan, J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 217–246. ISBN 9780081006221. [Google Scholar]
- Siepmann, J.; Siepmann, F. Swelling controlled drug delivery systems. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R.A., Rathbone, M.J., Eds.; Springer: Boston, MA, USA, 2012; pp. 153–170. [Google Scholar] [CrossRef]
- Johnson, T.J.; Clark, M.R.; Albright, T.H.; Nebeker, J.S.; Tuitupou, A.L.; Clark, J.T.; Fabian, J.; McCabe, R.T.; Chandra, N.; Doncel, G.F.; et al. A 90-day tenofovir reservoir intravaginal ring for mucosal HIV prophylaxis. Antimicrob. Agents Chemother. 2012, 56, 6272–6283. [Google Scholar] [CrossRef]
- Petersmann, S.; Spoerk, M.; Huber, P.; Lang, M.; Pinter, G.; Arbeiter, F. Impact optimization of 3D-printed poly(methyl methacrylate) for cranial implants. Macromol. Mater. Eng. 2019, 304, 1–10. [Google Scholar] [CrossRef]
- Simpson, S.M.; Widanapathirana, L.; Su, J.T.; Sung, S.; Watrous, D.; Qiu, J.; Pearson, E.; Evanoff, A.; Karunakaran, D.; Chacon, J.E.; et al. Design of a drug-eluting subcutaneous implant of the antiretroviral tenofovir alafenamide fumarate. Pharm. Res. 2020, 37, 16. [Google Scholar] [CrossRef]
- Lowinger, M.B.; Ormes, J.D.; Su, Y.; Small, J.H.; Williams, R.O.; Zhang, F. How broadly can poly(urethane)-based implants be applied to drugs of varied properties? Int. J. Pharm. 2019, 568, 118550. [Google Scholar] [CrossRef]
- Lowinger, M.B.; Su, Y.; Lu, X.; Williams, R.O.; Zhang, F. Can Drug Release Rate from Implants Be Tailored Using Poly(urethane) Mixtures? Int. J. Pharm. 2019, 557, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.M.; Pearce, S.M.; Poursaid, A.E.; Aliyar, H.A.; Tresco, P.A.; Mitchnick, M.A.; Kiser, P.F. Polyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1. J. Pharm. Sci. 2008, 97, 4228–4239. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.T.; Clark, M.R.; Shelke, N.B.; Johnson, T.J.; Smith, E.M.; Andreasen, A.K.; Nebeker, J.S.; Fabian, J.; Friend, D.R.; Kiser, P.F. Engineering a segmented dual-reservoir polyurethane intravaginal ring for simultaneous prevention of HIV transmission and unwanted pregnancy. PLoS ONE 2014, 9, e88509. [Google Scholar] [CrossRef]
- Johnson, T.J.; Gupta, K.M.; Fabian, J.; Albright, T.H.; Kiser, P.F. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur. J. Pharm. Sci. 2010, 39, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, G.; Vandenbussche, L.; Kasmi, S.; Nuhn, L.; Brouckaert, D.; Van Renterghem, J.; Grymonpré, W.; Vanhoorne, V.; Coenye, T.; De Geest, B.G.; et al. Thermoplastic polyurethane-based intravaginal rings for prophylaxis and treatment of (recurrent) bacterial vaginosis. Int. J. Pharm. 2017, 529, 218–226. [Google Scholar] [CrossRef]
- Claeys, B.; De Bruyn, S.; Hansen, L.; De Beer, T.; Remon, J.P.; Vervaet, C. Release characteristics of polyurethane tablets containing dicarboxylic acids as release modifiers–A case study with diprophylline. Int. J. Pharm. 2014, 477, 244–250. [Google Scholar] [CrossRef]
- Claeys, B.; Vervaeck, A.; Hillewaere, X.K.D.; Possemiers, S.; Hansen, L.; De Beer, T.; Remon, J.P.; Vervaet, C. Thermoplastic polyurethanes for the manufacturing of highly dosed oral sustained release matrices via hot melt extrusion and injection molding. Eur. J. Pharm. Biopharm. 2015, 90, 44–52. [Google Scholar] [CrossRef]
- Oh, S.T.; Kim, W.R.; Kim, S.H.; Chung, Y.C.; Park, J.S. The preparation of polyurethane foam combined with pH-sensitive alginate/bentonite hydrogel for wound dressings. Fibers Polym. 2011, 12, 159–165. [Google Scholar] [CrossRef]
- Sin, D.C.; Miao, X.; Liu, G.; Wei, F.; Chadwick, G.; Yan, C.; Friis, T. Polyurethane (PU) scaffolds prepared by solvent casting/particulate leaching (SCPL) combined with centrifugation. Mater. Sci. Eng. C 2010, 30, 78–85. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef]
- Sivak, W.N.; Zhang, J.; Petoud, S.; Beckman, E.J. Simultaneous drug release at different rates from biodegradable polyurethane foams. Acta Biomater. 2009, 5, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, S.R.; Lee, S.H.; Lee, C.H.; Kim, D.D. The effect of pore formers on the controlled release of cefadroxil from a polyurethane matrix. Int. J. Pharm. 2000, 201, 29–36. [Google Scholar] [CrossRef]
- Donelli, G.; Francolini, I.; Ruggeri, V.; Guaglianone, E.; D’Ilario, L.; Piozzi, A. Pore formers promoted release of an antifungal drug from functionalized polyurethanes to inhibit Candida colonization. J. Appl. Microbiol. 2006, 100, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Simha, R.; Moulinie, P.; Gendron, R.; Daigneault, L.E.; Ramesh, N.S.; Ohsima, M.; Sansone, L.F.; Thiele, W.C.; Yang, C.-T.; et al. Foam Extrusion Principles and Practice; Lee, S.T., Ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 1-56676-879-9. [Google Scholar]
- Di Maio, E.; Mensitieri, G.; Iannace, S.; Nicolais, L.; Li, W.; Flumerfelt, R.W. Structure optimization of polycaprolactone foams by using mixtures of CO2 and N2 as blowing agents. Polym. Eng. Sci. 2005, 45, 432–441. [Google Scholar] [CrossRef]
- Kmetty, Á.; Litauszki, K.; Réti, D. Characterization of different chemical blowing agents and their applicability to produce poly(lactic acid) foams by extrusion. Appl. Sci. 2018, 8, 1960. [Google Scholar] [CrossRef]
- Guo, Q.; Kenny, A.; Oshawa; Ahmed, S.; Rizvi, G. Thermoplastic polyurethane foaming through extrusion using a blowing agent. In Proceedings of the SPEC ANTEC 2017, Anaheim, CA, USA, 8–10 May 2017; pp. 1175–1179. [Google Scholar]
- Nakamichi, K.; Yasuura, H.; Fukui, H.; Oka, M.; Izumi, S. Evaluation of a floating dosage form of nicardipine hydrochloride and hydroxypropylmethylcellulose acetate succinate prepared using a twin-screw extruder. Int. J. Pharm. 2001, 218, 103–112. [Google Scholar] [CrossRef]
- Verreck, G.; Decorte, A.; Li, H.; Tomasko, D.; Arien, A.; Peeters, J.; Rombaut, P.; Van den Mooter, G.; Brewster, M.E. The effect of pressurized carbon dioxide as a plasticizer and foaming agent on the hot melt extrusion process and extrudate properties of pharmaceutical polymers. J. Supercrit. Fluids 2006, 38, 383–391. [Google Scholar] [CrossRef]
- Vo, A.Q.; Feng, X.; Morott, J.T.; Pimparade, M.B.; Tiwari, R.V.; Zhang, F.; Repka, M.A. A novel floating controlled release drug delivery system prepared by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2016, 98, 108–121. [Google Scholar] [CrossRef]
- LLS Health Medical Polymers Comparison Table. Available online: https://www.lubrizol.com/en/Health/Medical/Polymers (accessed on 17 March 2020).
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size, and Density; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2004; Volume 42, ISBN 978-1-4020-2303-3. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Khare, H.S.; Burris, D.L. A quantitative method for measuring nanocomposite dispersion. Polymer 2010, 51, 719–729. [Google Scholar] [CrossRef]
- Verstraete, G.; Van Renterghem, J.; Van Bockstal, P.J.; Kasmi, S.; De Geest, B.G.; De Beer, T.; Remon, J.P.; Vervaet, C. Hydrophilic thermoplastic polyurethanes for the manufacturing of highly dosed oral sustained release matrices via hot melt extrusion and injection molding. Int. J. Pharm. 2016, 506, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.Y.; Jing, X.; Salick, M.R.; Crone, W.C.; Peng, X.F.; Turng, L.S. Approach to fabricating thermoplastic polyurethane blends and foams with tunable properties by twin-screw extrusion and microcellular injection molding. Adv. Polym. Technol. 2014, 33, 1–11. [Google Scholar] [CrossRef]
- Ge, C.; Wang, S.; Zheng, W.; Zhai, W. Preparation of microcellular thermoplastic polyurethane (TPU) foam and its tensile property. Polym. Eng. Sci. 2018, 58, E158–E166. [Google Scholar] [CrossRef]
- Hiltz, J. Characterization of poly(ether)urethane thermoplastic elastomers. 1998. Available online: https://www.researchgate.net/profile/John_Hiltz/publication/235021049_Characterization_of_Polyetherurethane_Thermoplastic_Elastomers/links/0fcfd5111451d7d851000000.pdf (accessed on 10 December 2020).
- Gupta, S.S.; Solanki, N.; Serajuddin, A.T.M. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion, IV: AffinisolTM HPMC HME polymers. AAPS PharmSciTech 2016, 17, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Nema, A.K.; Deshmukh, A.V.; Palanivelu, K.; Sharma, S.K.; Malik, T. Effect of exo-and endothermic blowing and wetting agents on morphology, density and hardness of thermoplastic polyurethanes foams. J. Cell. Plast. 2008, 44, 277–292. [Google Scholar] [CrossRef]
- Sadik, T.; Pillon, C.; Carrot, C.; Reglero Ruiz, J.A. Dsc studies on the decomposition of chemical blowing agents based on citric acid and sodium bicarbonate. Thermochim. Acta 2018, 659, 74–81. [Google Scholar] [CrossRef]
- Rashmi, B.J.; Rusu, D.; Prashantha, K.; Lacrampe, M.F.; Krawczak, P. Development of water-blown bio-based thermoplastic polyurethane foams using bio-derived chain extender. J. Appl. Polym. Sci. 2013, 128, 292–303. [Google Scholar] [CrossRef]
- Thomas, N.L.; Eastup, R.P. Rigid PVC foam: A study on blowing agents and processign aids. In Proceedings of the Blowing Agent Systems: Formulations and Processing; Rapra Technology Limited: Shawbury, UK, 1998; pp. 1–8. [Google Scholar]
- Helbling, I.M.; Cabrera, M.I.; Luna, J.A. Mathematical modeling of drug delivery from one-layer and two-layer torus-shaped devices with external mass transfer resistance. Eur. J. Pharm. Sci. 2011, 44, 288–298. [Google Scholar] [CrossRef]
- Ito, S.; Matsunaga, K.; Tajima, M.; Yoshida, Y. Generation of microcellular polyurethane with supercritical carbon dioxide. J. Appl. Polym. Sci. 2007, 106, 3581–3586. [Google Scholar] [CrossRef]
- Siegel, R.A. Porous systems. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R.A., Rathbone, M.J., Eds.; Springer: New York, NY, USA, 2012; pp. 229–251. [Google Scholar]
- Abu-Diak, O.A.; Andrews, G.P.; Jones, D.S. Hydrophobic polymers of pharmaceutical significance. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R.A., Rathbone, M.J., Eds.; Springer: New York, NY, USA, 2012; pp. 47–74. [Google Scholar]
- Altan, M. Thermoplastic foams: Processing, manufacturing, and characterization. In Recent Research in Polymerization; Cankaya, N., Ed.; IntechOpen: London, UK, 2018; pp. 117–137. [Google Scholar]
- Kraschuster, A.; Turng, L.S. An injection molding process for manufacturing highly porous and interconnected biodegradable polymer matrices for use as tissue engineering scaffolds. J. Biomed. Mater. Res.-Part. B Appl. Biomater. 2010, 92, 366–376. [Google Scholar]
- Dai, C.; Zhang, C.; Huang, W.; Chang, K.-C.; Lee, L.J. Thermoplastic polyurethane microcellular fibers via supercritical carbon dioxide based extrusion foaming. Polym. Eng. Sci. 2013, 53, 2360–2369. [Google Scholar] [CrossRef]
- Mi, Y.; Jing, X.; Salick, M.R.; Peng, X.F.; Turng, L.S. A novel thermoplastic polyurethane scaffold fabrication method based on injection foaming with water and supercritical carbon dioxide as coblowing agents. Polym. Eng. Sci. 2014, 54, 2947–2957. [Google Scholar] [CrossRef]
- Safina, L.; Mantalaris, A.; Bismarck, A. Nondestructive technique for the characterization of the pore size distribution of soft porous constructs for tissue engineering. Langmuir 2006, 22, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Roquerol, J.; Baron, G.V.; Denoyel, R.; Giesche, H.; Groen, J.; Klobes, P.; Levitz, P.; Neimark, A.V.; Rigby, S.; Skudas, R.; et al. The characterization of macroporous solids: An overview of the methodology. Microporous Mesoporous Mater. 2012, 154, 2–6. [Google Scholar] [CrossRef]
- Repel, T.; Korzeniowski, T.F.; Weinberg, K. Stereological transformation of pore size distributions with application to soft polymer and FDM-printed specimens. Zamm Z. Fur Angew. Math. Und Mech. 2019, 99, 1–15. [Google Scholar] [CrossRef]
- Loca, D.; Locs, J.; Salma, K.; Gulbis, J.; Salma, I.; Berzina-Cimdina, L. Porous hydroxyapatite bioceramic scaffolds for drug delivery and bone regeneration. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 192019. [Google Scholar] [CrossRef]
- Fukda, M.; Peppas, N.A.; McGinity, J.W. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. J. Control. Release 2006, 115, 121–129. [Google Scholar] [CrossRef]
- Bezai, A.; Scarpa, F. Mechanical behaviour of conventional and negative Poisson’s ratio thermoplastic polyurethane foams under compressive cyclic loading. Int. J. Fatigue 2007, 29, 922–930. [Google Scholar] [CrossRef]
- Ellitt, J.A.; Windle, A.H.; Hobdell, J.R.; Eeckhaut, G.; Oldman, R.J.; Ludwig, W.; Boller, E.; Cloetens, P.; Baruchel, J. In-situ deformation of an open-cell flexible polyurethane foam characterised by 3D computed microtomography. J. Mater. Sci. 2002, 37, 1547–1555. [Google Scholar] [CrossRef]
- Gibon, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997; ISBN 9781139878326. [Google Scholar]
- Yeh, S.K.; Liu, Y.C.; Chu, C.C.; Chang, K.C.; Wang, S.F. Mechanical properties of microcellular and nanocellular thermoplastic polyurethane nanocomposite foams created using supercritical carbon dioxide. Ind. Eng. Chem. Res. 2017, 56, 8499–8507. [Google Scholar] [CrossRef]
- Unema, T.; Yamazaki, T.; Igarashi, T.; Nitta, K. hei Effect of pore size distribution on compressive behavior of moderately expanded low-density polyethylene foams. Polym. Eng. Sci. 2019, 59, 510–518. [Google Scholar] [CrossRef]
- Wag, G.; Zhao, J.; Wang, G.; Mark, L.H.; Park, C.B.; Zhao, G. Low-density and structure-tunable microcellular PMMA foams with improved thermal-insulation and compressive mechanical properties. Eur. Polym. J. 2017, 95, 382–393. [Google Scholar]
- Dabn, G.; Bayram, C.; Bozdoğan, B.; Denkbaş, E.B. Porous polyurethane film fabricated via the breath figure approach for sustained drug release. J. Appl. Polym. Sci. 2019, 136, 1–9. [Google Scholar] [CrossRef]





| Sample Designation | Polymer Type | NaHCO3 (wt%) |
|---|---|---|
| HPB | Hydrophobic TPU | - |
| HPB/NaHCO3-1 | 1 | |
| HPB/NaHCO3-3 | 3 | |
| HPB/NaHCO3-5 | 5 | |
| HPL | Hydrophilic TPU | - |
| HPL/NaHCO3-1 | 1 | |
| HPL/NaHCO3-3 | 3 | |
| HPL/NaHCO3-5 | 5 |
| Sample Designation | Da (µm) |
|---|---|
| HPB/NaHCO3-1 | 596 ± 240 |
| HPB/NaHCO3-3 | 475 ± 247 |
| HPB/NaHCO3-5 | 146 ± 108 |
| HPL/NaHCO3-1 | 523 ± 281 |
| HPL/NaHCO3-3 | 405 ± 290 |
| HPL/NaHCO3-5 | 137 ± 100 |
| Sample Designation | EC (MPa) | σ50 (MPa) |
|---|---|---|
| HPB | 5.34 ± 0.98 | 7.75 ± 0.37 |
| HPB/NaHCO3-1 | 2.51 ± 0.68 | 5.67 ± 1.19 |
| HPB/NaHCO3-3 | 1.71 ± 0.51 | 1.58 ± 0.08 |
| HPB/NaHCO3-5 | 2.41 ± 0.65 | 3.76 ± 0.65 |
| HPL | 7.40 ± 0.69 | 12.16 ± 1.37 |
| HPL/NaHCO3-1 | 3.75 ± 1.25 | 4.48 ± 0.72 |
| HPL/NaHCO3-3 | 1.97 ± 0.79 | 1.53 ± 0.42 |
| HPL/NaHCO3-5 | 4.38 ± 1.52 | 6.26 ± 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutsamanis, I.; Spoerk, M.; Arbeiter, F.; Eder, S.; Roblegg, E. Development of Porous Polyurethane Implants Manufactured via Hot-Melt Extrusion. Polymers 2020, 12, 2950. https://doi.org/10.3390/polym12122950
Koutsamanis I, Spoerk M, Arbeiter F, Eder S, Roblegg E. Development of Porous Polyurethane Implants Manufactured via Hot-Melt Extrusion. Polymers. 2020; 12(12):2950. https://doi.org/10.3390/polym12122950
Chicago/Turabian StyleKoutsamanis, Ioannis, Martin Spoerk, Florian Arbeiter, Simone Eder, and Eva Roblegg. 2020. "Development of Porous Polyurethane Implants Manufactured via Hot-Melt Extrusion" Polymers 12, no. 12: 2950. https://doi.org/10.3390/polym12122950
APA StyleKoutsamanis, I., Spoerk, M., Arbeiter, F., Eder, S., & Roblegg, E. (2020). Development of Porous Polyurethane Implants Manufactured via Hot-Melt Extrusion. Polymers, 12(12), 2950. https://doi.org/10.3390/polym12122950