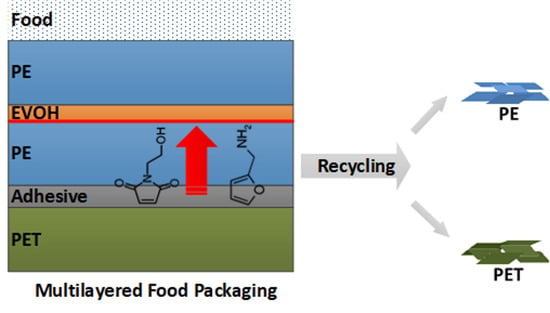
Recyclable Multilayer Packaging by Means of Thermoreversibly Crosslinking Adhesive in the Context of Food Law
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization by Infrared Spectroscopy
2.2. Determination of the Molecular Weight
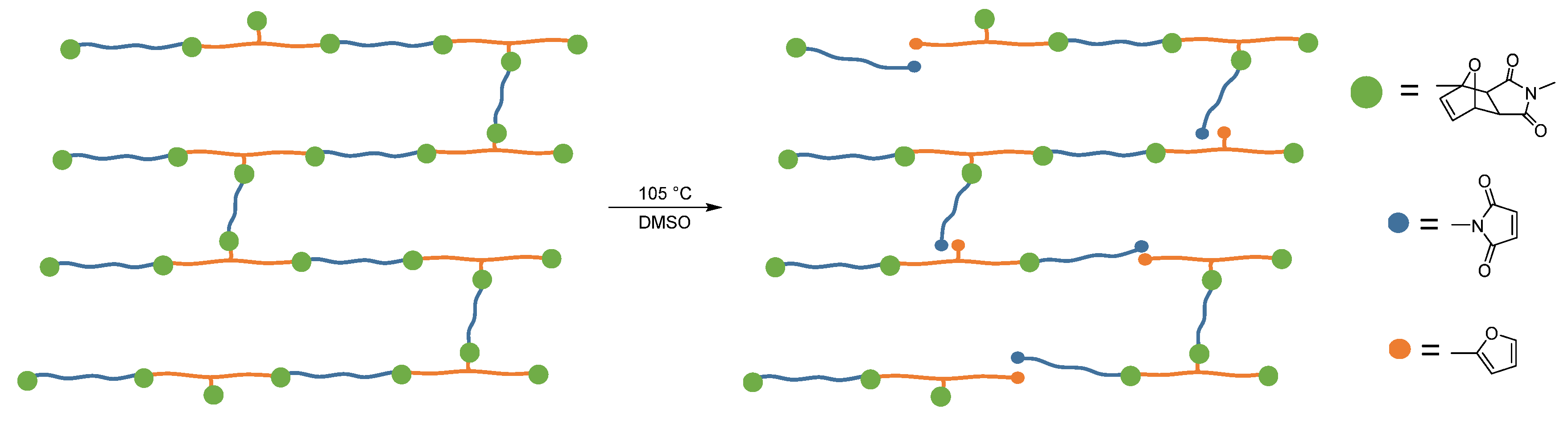
2.3. Preparation of the Adhesive
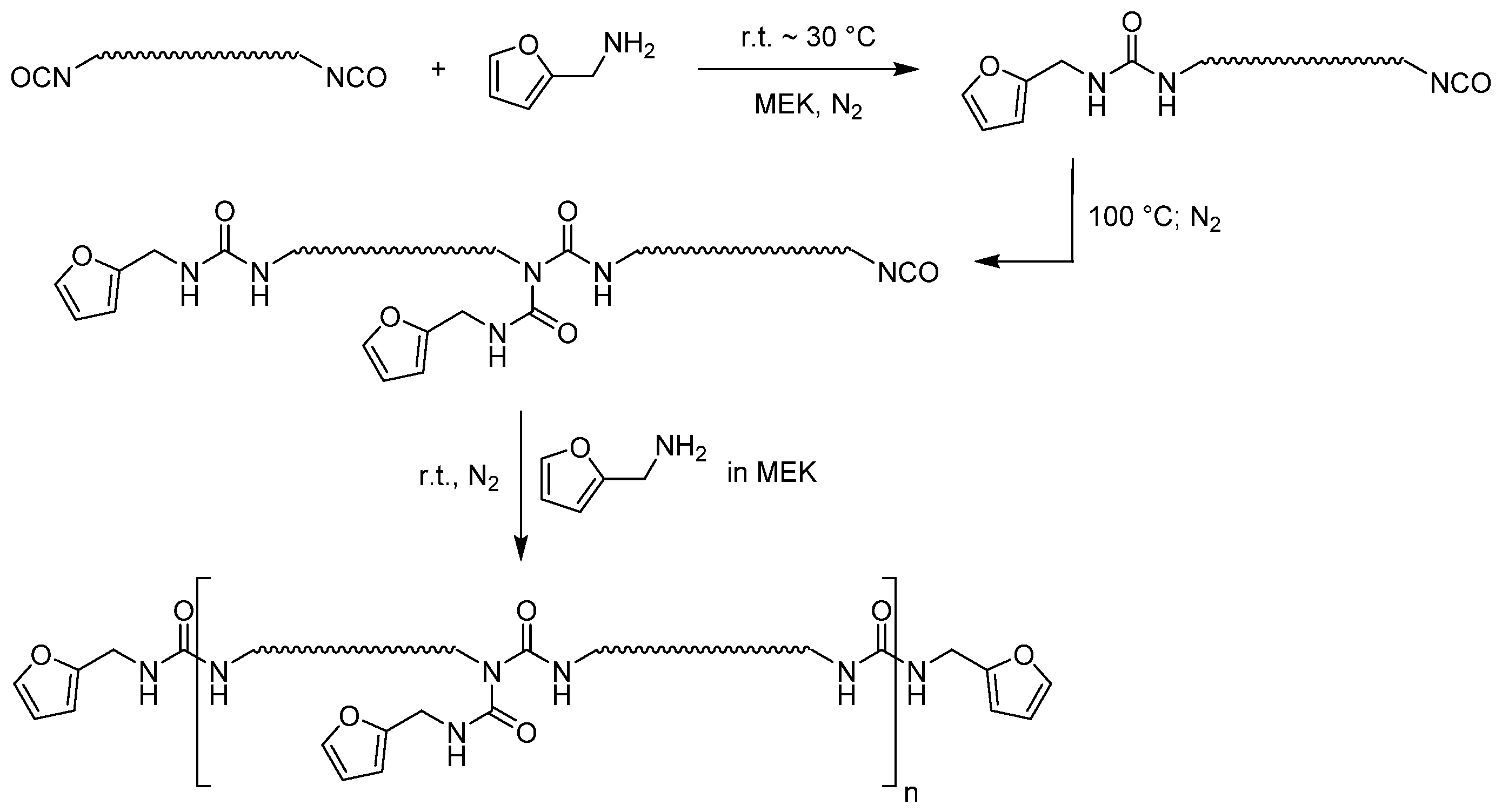
2.4. Furan-Functionalized Prepolymer
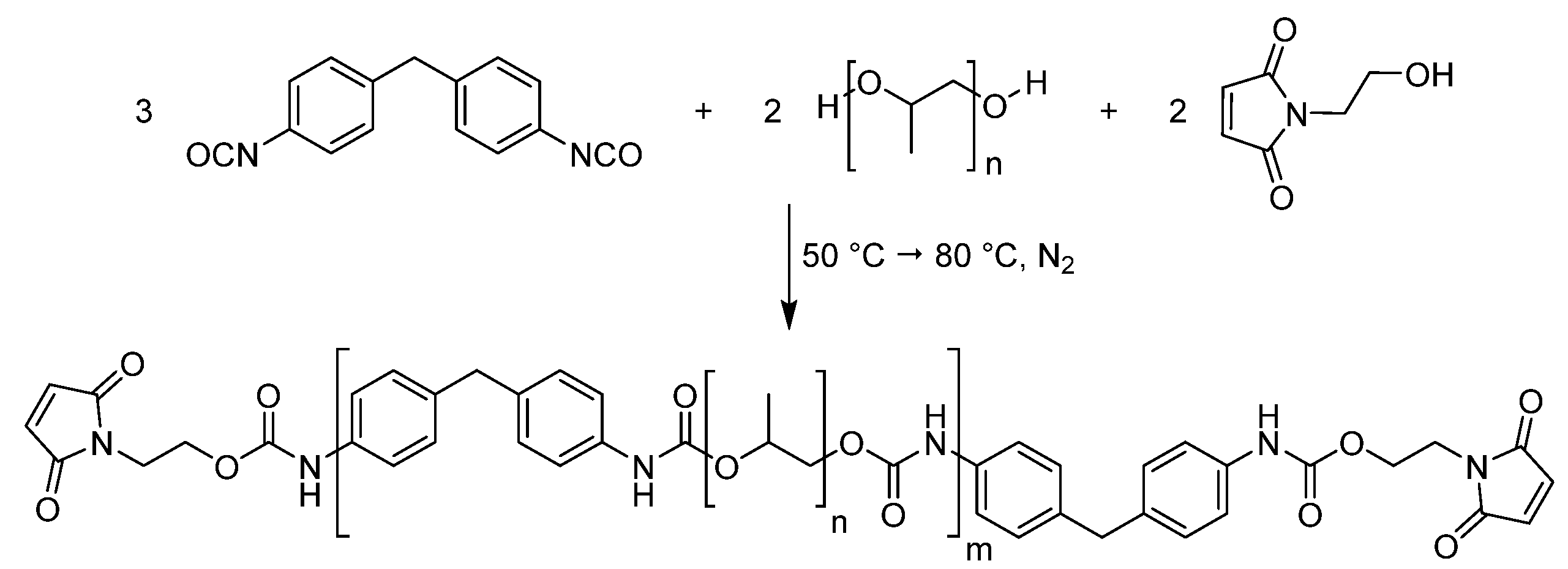
2.5. Maleimide-Functionalized Prepolymer
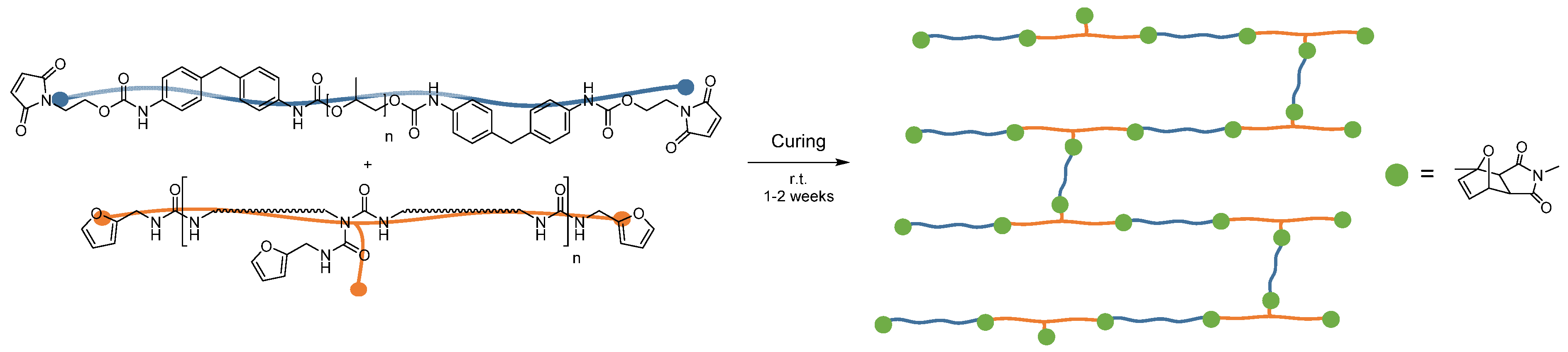
2.6. Preparation of the Adhesive Mixture
2.7. Production of Laminates
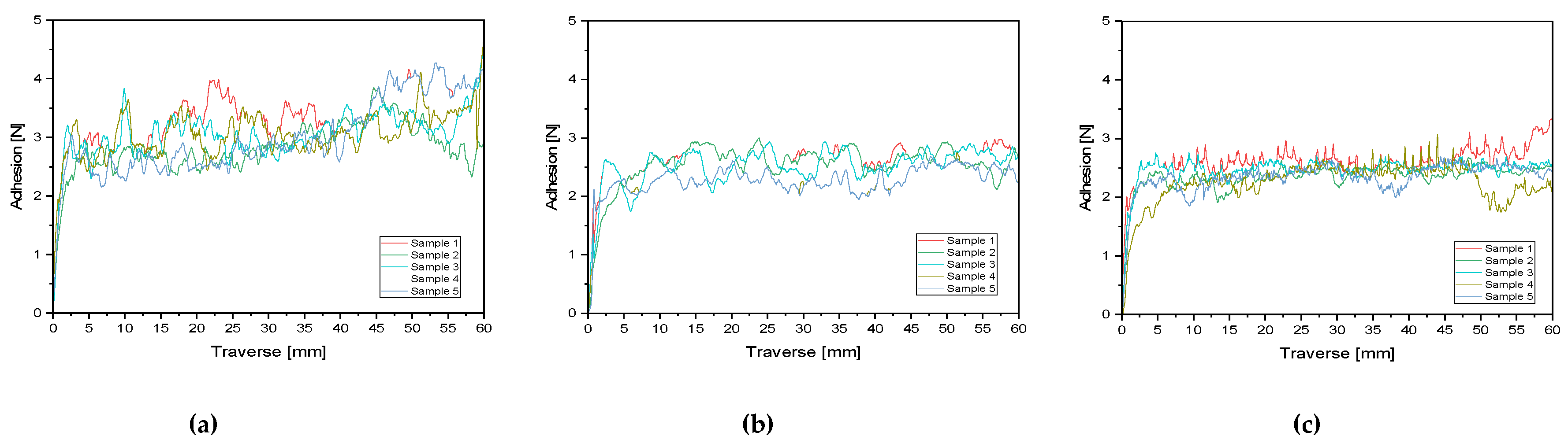
2.8. T-Peel Test
2.9. Statistical Hypothesis Testing
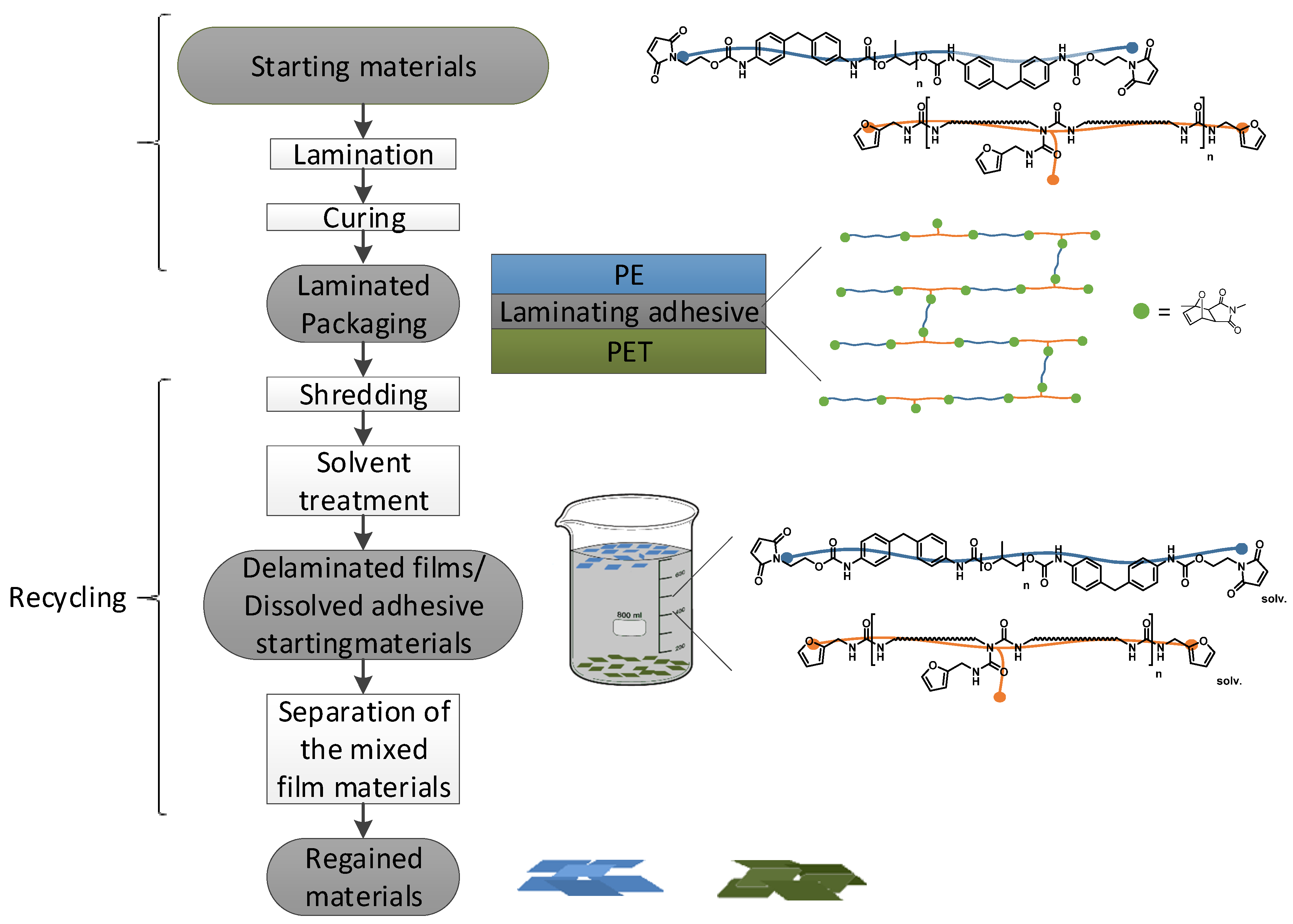
2.10. Recovery of the Materials
2.11. Determination of Residual N-(2-hydroxyethyl)maleimide and Furfurylamine
2.12. Determination of the Ethylene Content in the Measured EVOH Barrier
2.13. Determination of Diffusion Coefficients in EVOH
2.14. Diffusion Modelling
3. Results and Discussion
3.1. Adhesion and Recyclability
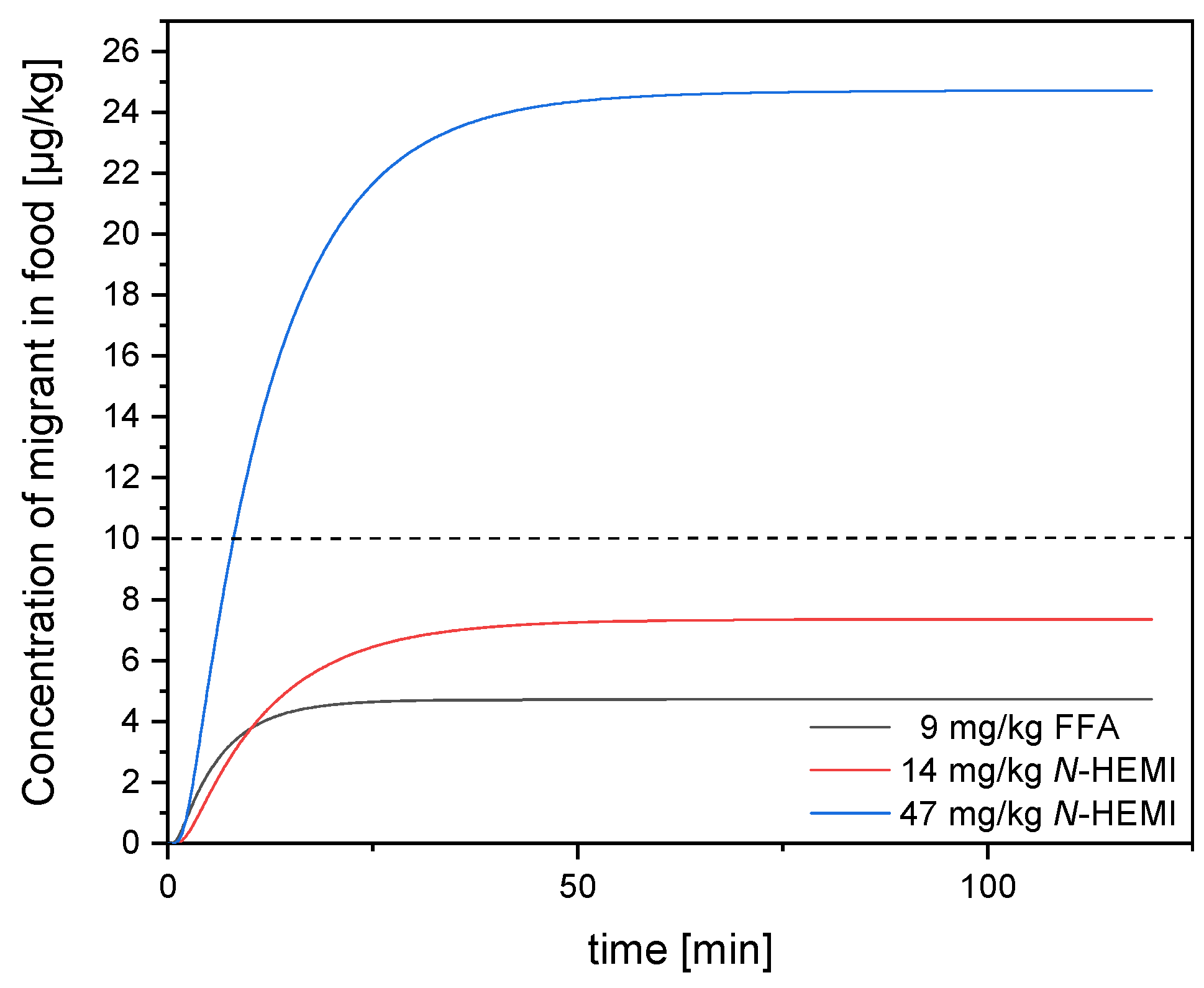
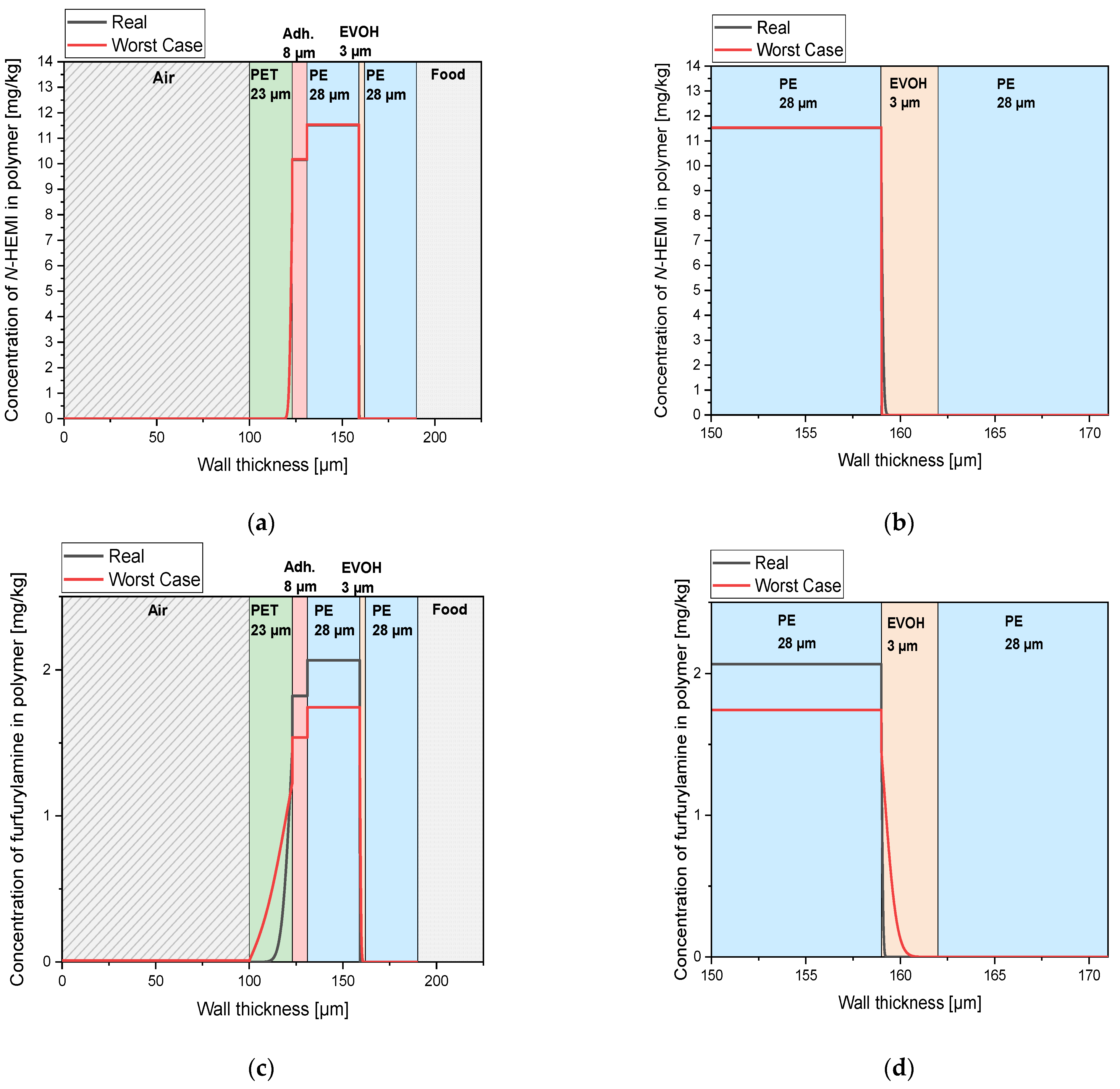
3.2. Migration Modelling of N-(2-hydroxyethyl)maleimide and Furfurylamine through PE According to Piringer
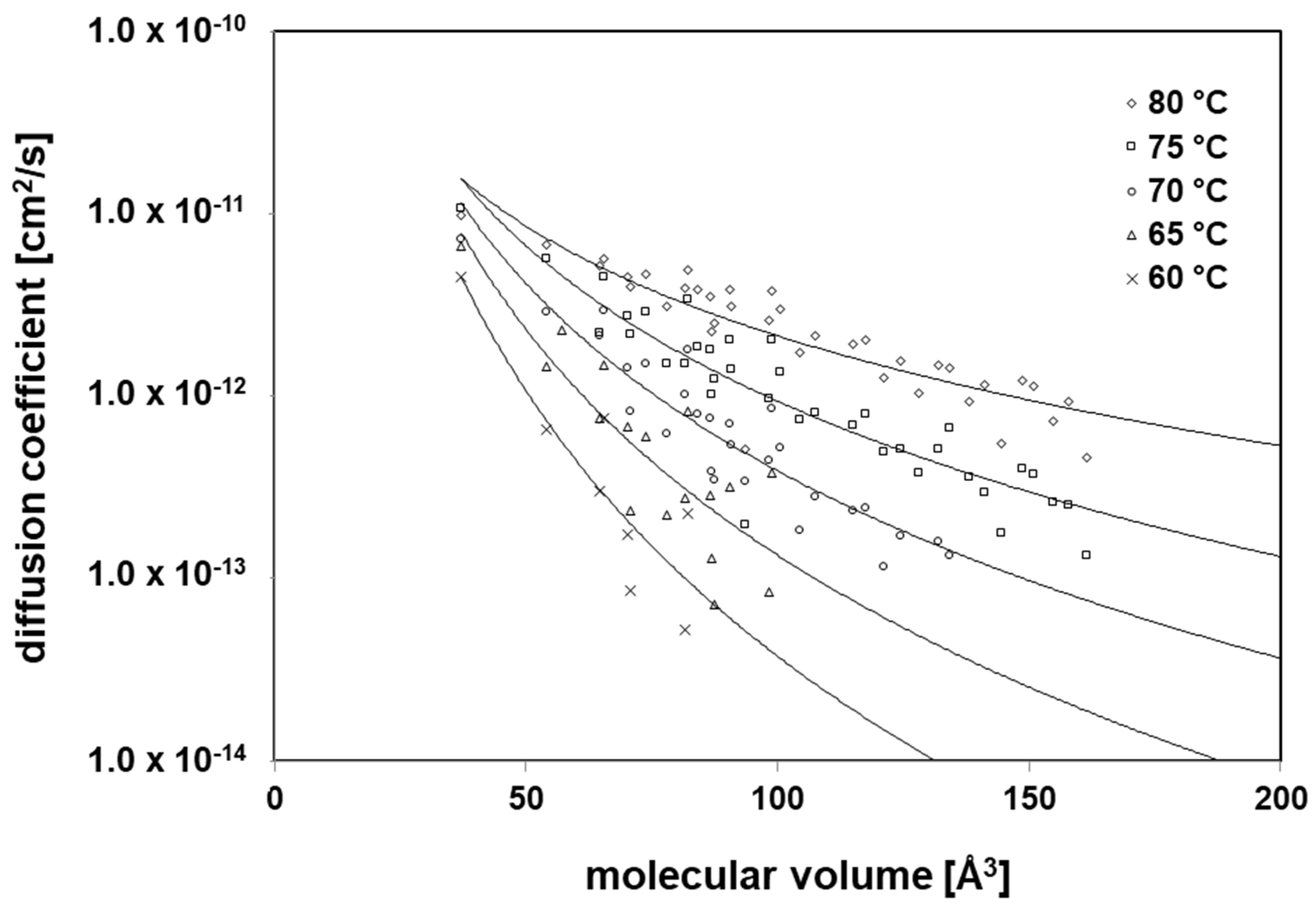
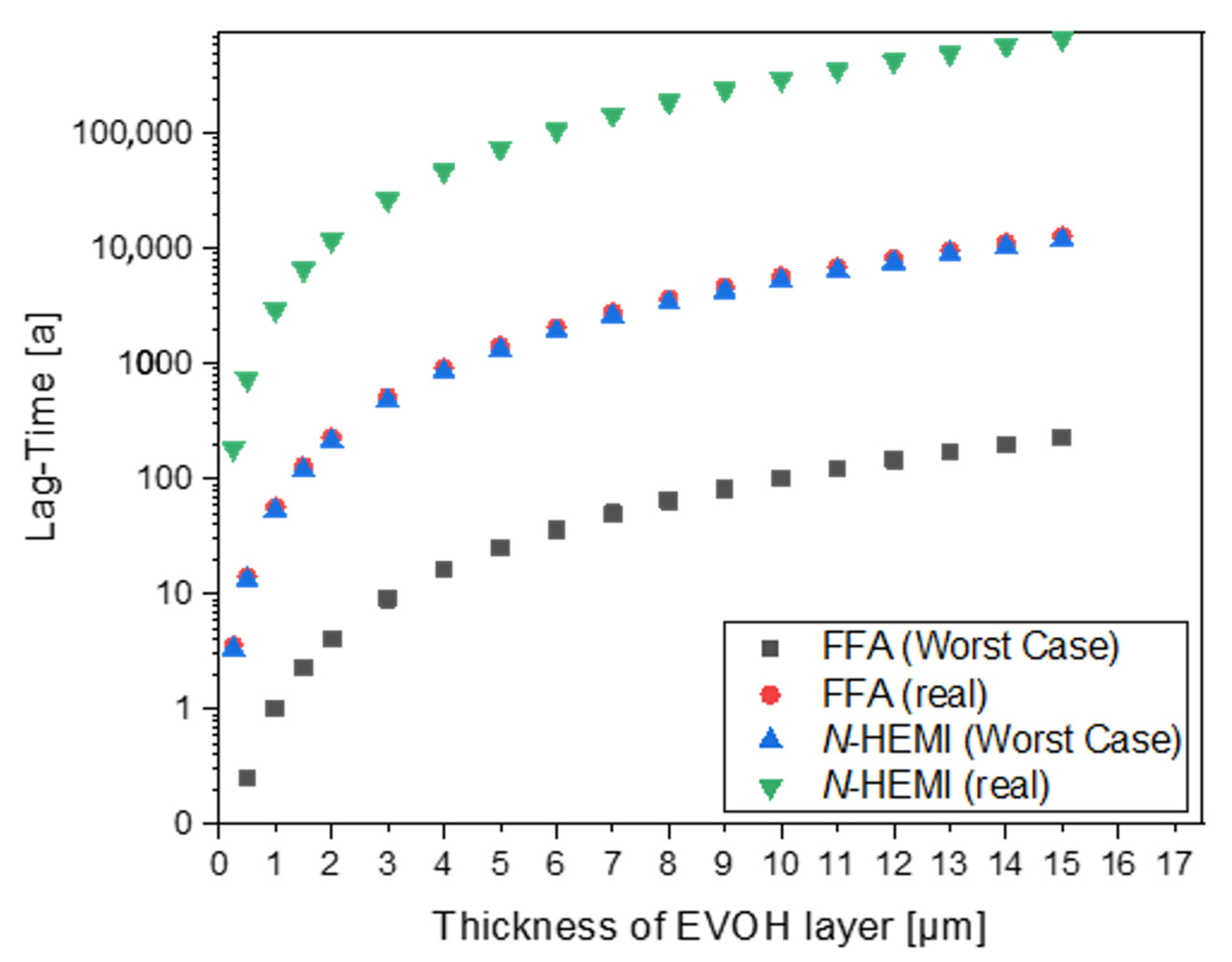
3.3. Diffusion Coefficients
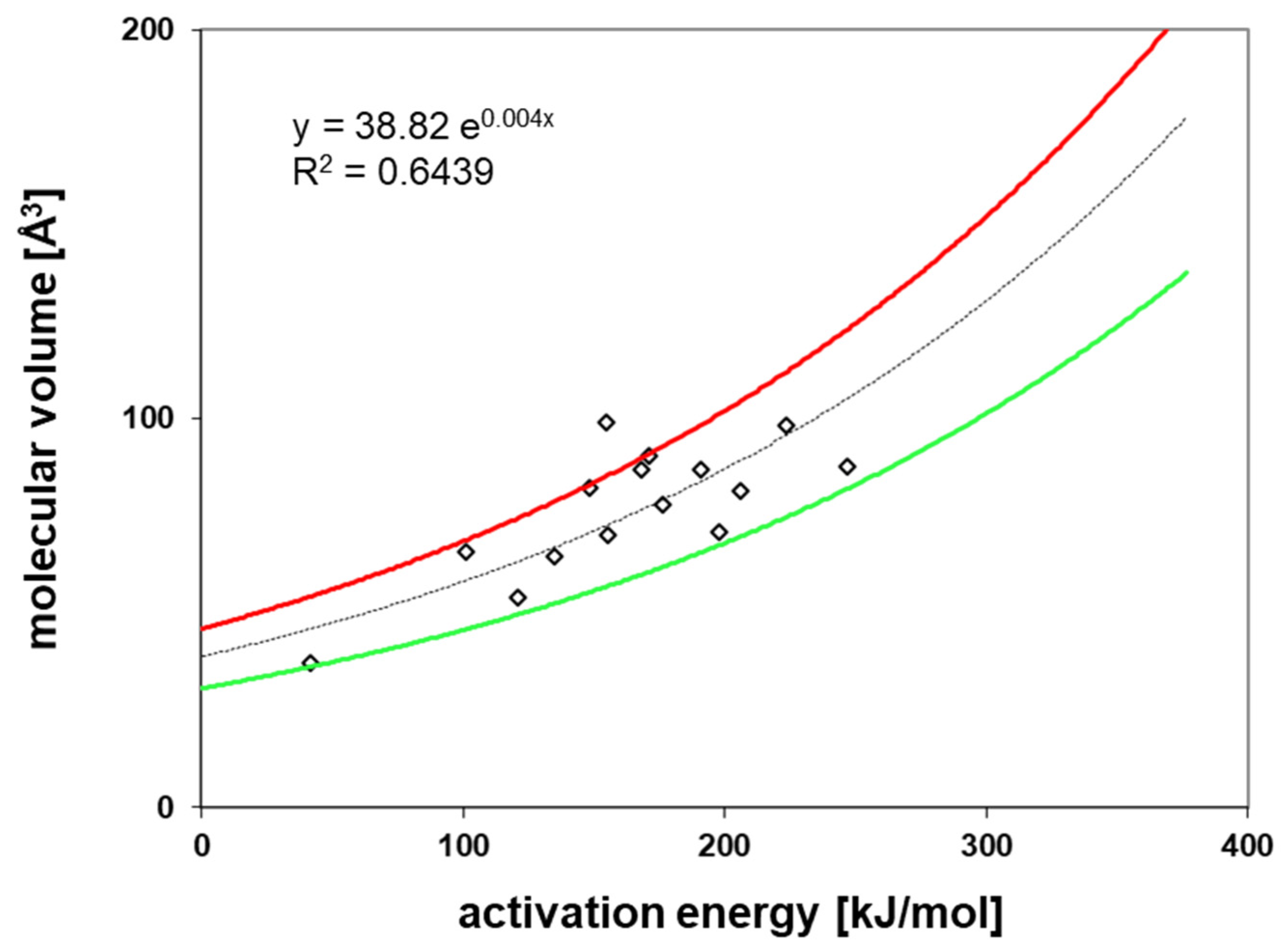
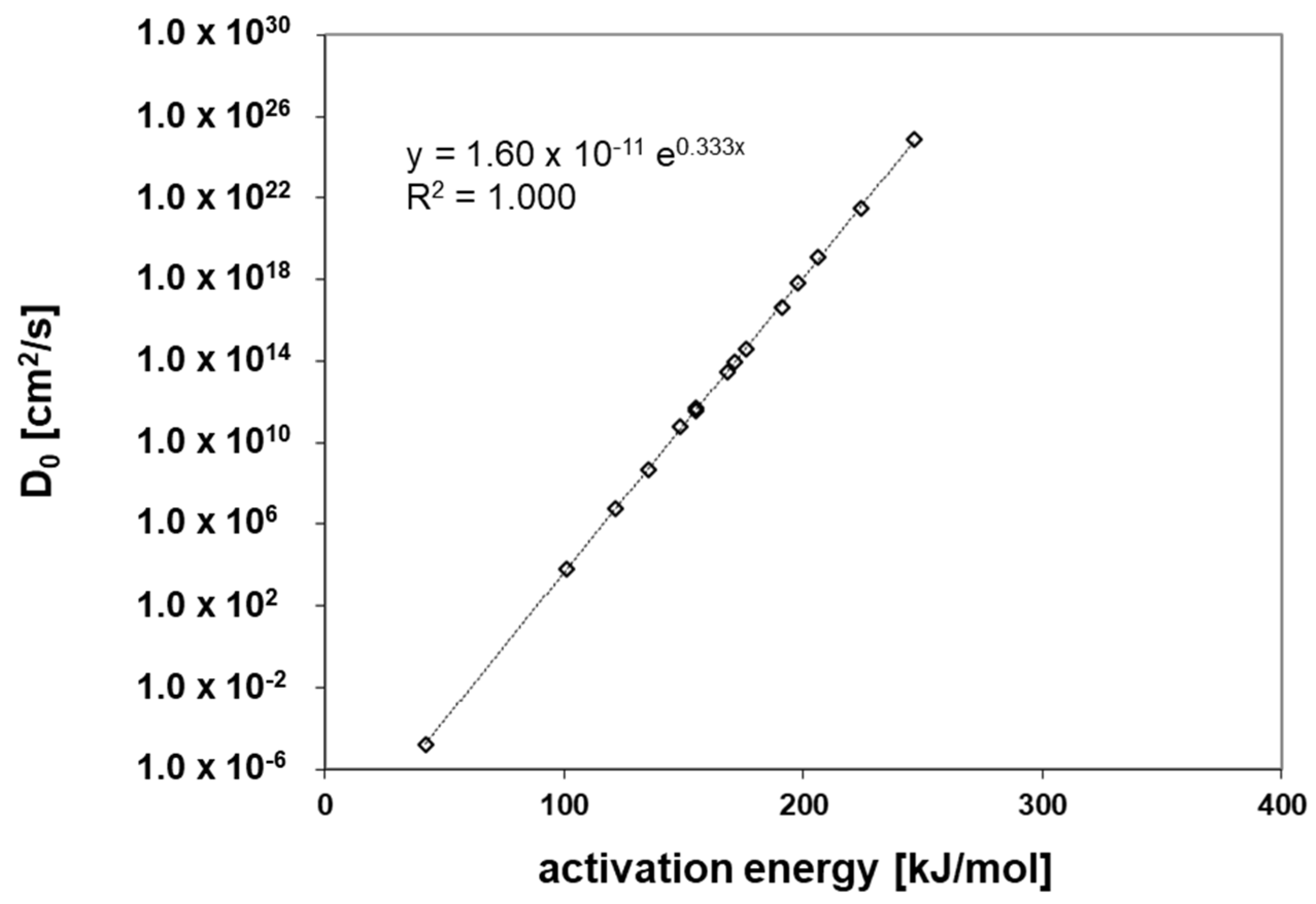
3.4. Activation Energies of Diffusion
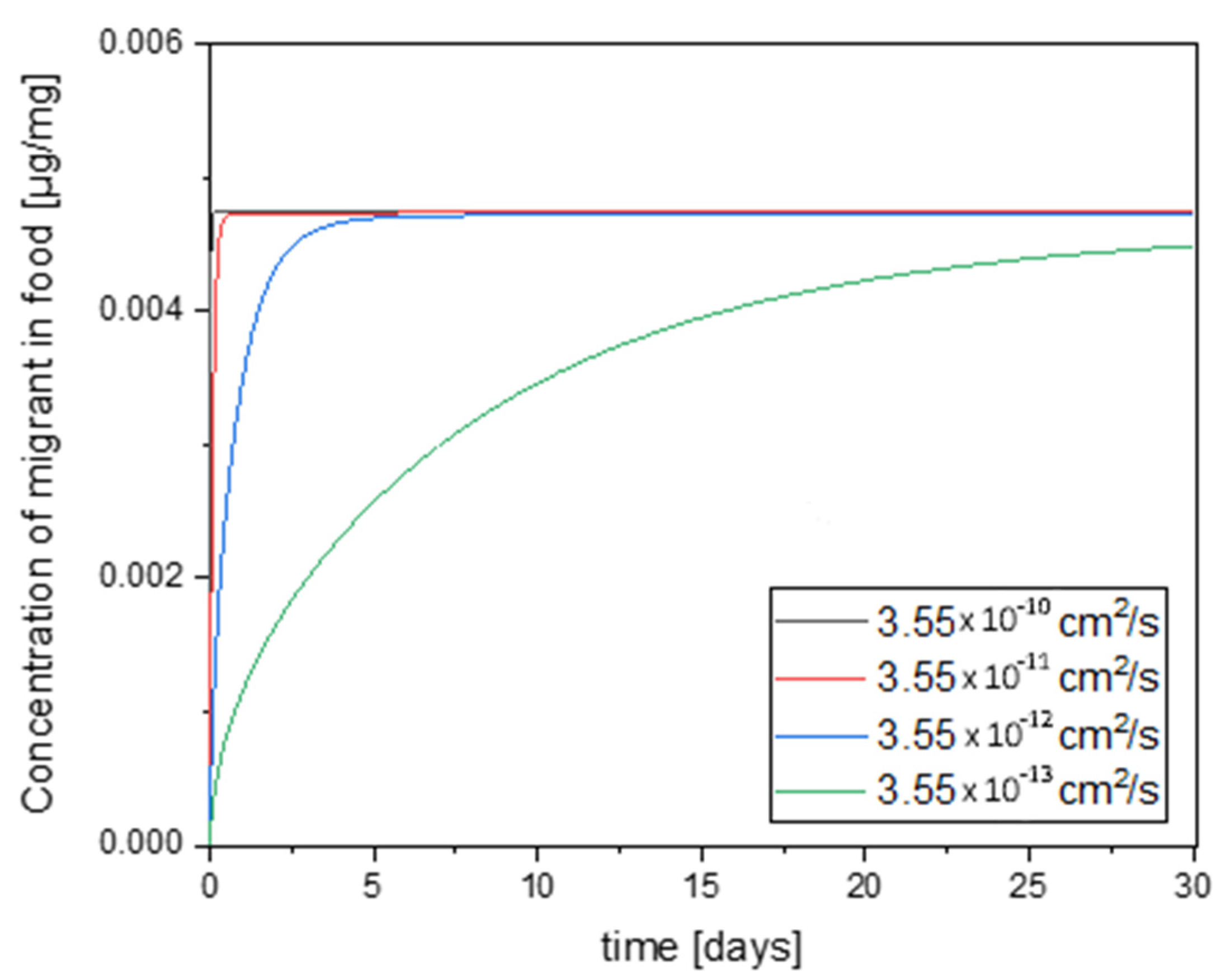
3.5. Migration Modelling of N-(2-hydroxyethyl)maleimide and Furfurylamine through EVOH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wagner, J.R. (Ed.) Multilayer Flexible Packaging, 2nd ed.; Elsevier: Oxford, UK, 2016; ISBN 9780323371001. [Google Scholar]
- Morris, B.A. The Science and Technology of Flexible Packaging. In Multilayer Films from Resin and Process to End Use; Elsevier William Andrew: Amsterdam, The Netherlands, 2017; ISBN 0323242731. [Google Scholar]
- Nonclercq, A. Mapping Flexible Packaging in a Circular Economy. Final Report. 2016. Available online: https://ceflex.eu/resources/ (accessed on 12 February 2020).
- Singh, P.; Wani, A.; Langowski, H.-C. Food Packaging Materials; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4665-5994-3. [Google Scholar]
- Ragaert, K.; Delva, L.; van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2018, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Dixon, J. Packaging Materials; ILSI: Brussels, Belgium, 2011; ISBN 9789078637264. [Google Scholar]
- A European Strategy for Plastics in a Circular Economy; European Commisson: Brussels, Belgium, 2018.
- Kaiser, K.M.A. Recycling of multilayer packaging using a reversible cross—Linking adhesive. J. Appl. Polym. Sci. 2020, 137, 49230. [Google Scholar] [CrossRef] [Green Version]
- Banea, M.D. Debonding on Demand of Adhesively Bonded Joints: A Critical Review. Rev. Adhes. Adhes. 2019, 7, 33–50. [Google Scholar] [CrossRef]
- Woidasky, J.; Schmidt, J.; Auer, M.; Sander, I.; Schau, A.; Moesslein, J.; Wendler, P.; Kirchenbauer, D.; Wacker, D.; Gao, G.; et al. Photoluminescent Tracer Effects on Thermoplastic Polymer Recycling. In Advances in Polymer Processing 2020, Proceedings of the International Symposium on Plastics Technology, Berlin, Heidelberg, 10 March 2020; Hopmann, C., Dahlmann, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–13. ISBN 978-3-662-60809-8. [Google Scholar]
- Woidasky, J.; Moesslein, J.; Wendler, P.; Kirchenbauer, D.; Wacker, D.; Gao, G.; Lang-Koetz, C. Kunststoffidentifikation und-sortierung in der Circular Economy durch Fluoreszenzmarker. Chemie Ingenieur Technik 2020, 92, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Kosior, E.; Mitchell, J. Chapter 6—Current industry position on plastic production and recycling. In Plastic Waste and Recycling: Environmental Impact, Societal Issues, Prevention, and Solutions; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 133–162. ISBN 978-0-12-817880-5. [Google Scholar]
- Brunner, S.; Fomin, P.; Kargel, C. Automated sorting of polymer flakes: Fluorescence labeling and development of a measurement system prototype. Waste Manag. 2015, 38, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.R. A new technology for automatic identification and sorting of plastics for recycling. Environ. Technol. 2004, 25, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Pla.to GmbH. Die Pla.to GmbH und die Hochschule Zittau/Görlitz entwickeln ein innovatives Recyclingverfahren für die Trennung von Folien aus Altverpackungen. 2019. Available online: https://www.plato-technology.de/downloads/ (accessed on 12 February 2020).
- National Center for Biotechnology Information—PubChem. Furfuryl Alcohol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Furfuryl-alcohol (accessed on 9 November 2020).
- The European Commission, COMMISSION REGULATION (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. OJ 2011, L12, 1–89.
- Stepanski, H.; Leimenstoll, M.C. Polyurethan-Klebstoffe. Unterschiede und Gemeinsamkeiten, 1. Ed. 2016; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783658122706. [Google Scholar]
- National Center for Biotechnology Information—PubChem. 1-(2-Hydroxyethyl)-1H-pyrrole-2,5-dione. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1-_2-Hydroxyethyl_-1H-pyrrole-2_5-dione (accessed on 19 November 2020).
- Institute for Occupational Safety and Health of the German Social Accident Insurance. GESTIS Substance Database—Furfurylamine. Available online: http://gestis.itrust.de/nxt/gateway.dll/gestis_en/000000.xml?f=templates&fn=default.htm&vid=gestiseng:sdbeng (accessed on 23 October 2020).
- Heo, Y.; Sodano, H.A. Self-Healing Polyurethanes with Shape Recovery. Adv. Funct. Mater. 2014, 24, 5261–5268. [Google Scholar] [CrossRef]
- Duan, H.-Y.; Wang, Y.-X.; Wang, L.-J.; Min, Y.-Q.; Zhang, X.-H.; Du, B.-Y. An Investigation of the Selective Chain Scission at Centered Diels–Alder Mechanophore under Ultrasonication. Macromolecules 2017, 50, 1353–1361. [Google Scholar] [CrossRef]
- Du, P.; Wu, M.; Liu, X.; Zheng, Z.; Wang, X.; Sun, P.; Joncheray, T.; Zhang, Y. Synthesis of linear polyurethane bearing pendant furan and cross-linked healable polyurethane containing Diels–Alder bonds. New J. Chem. 2014, 38, 770–776. [Google Scholar] [CrossRef]
- Ewender, J.; Welle, F. Determination and Prediction of the Lag Times of Hydrocarbons through a Polyethylene Terephthalate Film. Packag. Technol. Sci. 2014, 27, 963–974. [Google Scholar] [CrossRef]
- Ewender, J.; Welle, F. Functional Barrier Performance of a Polyamide-6 Membrane towards n -Alkanes and 1-Alcohols. Packag. Technol. Sci. 2016, 29, 277–287. [Google Scholar] [CrossRef]
- Ewender, J.; Welle, F. Diffusion Coefficients of n-Alkanes and 1-Alcohols in Polyethylene Naphthalate (PEN). Int. J. Polym. Sci. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roduit, B.; Borgeat, C.H.; Cavin, S.; Fragnière, C.; Dudler, V. Application of Finite Element Analysis (FEA) for the simulation of release of additives from multilayer polymeric packaging structures. Food Addit. Contam. 2005, 22, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Polyurethane; mit 126 Tabellen, 3rd ed.; Oertel, G.; Becker, G.W.; Braun, D. (Eds.) Hanser: Munich, Germany, 1993; ISBN 3446162631. [Google Scholar]
- Begley, T.; Castle, L.; Feigenbaum, A.; Franz, R.; Hinrichs, K.; Lickly, T.; Mercea, P.; Milana, M.; O’Brien, A.; Rebre, S.; et al. Evaluation of migration models that might be used in support of regulations for food-contact plastics. Food Addit. Contam. 2005, 22, 73–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welle, F. A new method for the prediction of diffusion coefficients in poly(ethylene terephthalate). J. Appl. Polym. Sci. 2013, 129, 1845–1851. [Google Scholar] [CrossRef]
- Kaushiva, B.D. Structure-Property Relationships of Flexible Polyurethane Foams; Virginia Tech: Blacksburg, VA, USA, 1999. [Google Scholar]
- The Dow Chemical Company. Laminating Adhesives for Flexible Packaging. In Science that Connects; The Dow Chemical Company: Midland, MI, USA, 2016. [Google Scholar]
- Da Silva, L.F.; Öchsner, A.; Adams, R.D. Handbook of Adhesion Technology; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9783642011689. [Google Scholar]
- Kinloch, A.J. Adhesion and Adhesives. In Science and Technology; Springer: Berlin/Heidelberg, Germany, 1987; ISBN 9789048140039. [Google Scholar]
- Dole, P.; Feigenbaum, A.E.; La Cruz, C.d.; Pastorelli, S.; Paseiro, P.; Hankemeier, T.; Voulzatis, Y.; Aucejo, S.; Saillard, P.; Papaspyrides, C. Typical diffusion behaviour in packaging polymers—Application to functional barriers. Food Addit. Contam. 2006, 23, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Rafailovich, M.H.; Sokolov, J. Networks: Diffusion. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 6111–6113. ISBN 9780080431529. [Google Scholar]
- Dunn, T. Manufacturing Flexible Packaging. In Materials, Machinery, and Techniques; William Andrew: Oxford, UK; Waltham, MA, USA, 2015; ISBN 0323265057. [Google Scholar]
- Huang, C.-H.; Wu, J.-S.; Huang, C.-C.; Lin, L.-S. Morphological, Thermal, Barrier and Mechanical Properties of LDPE/EVOH Blends in Extruded Blown Films. J. Polym. Res. 2004, 11, 75–83. [Google Scholar] [CrossRef]
- PlastEurope. New Guidelines for Barrier Polytheylene Film/“RecyClass” Tests Show EVOH Concentrations Compatible for Recycling. Available online: https://www.plasteurope.com/news/RECYCLING_t243864/ (accessed on 3 September 2020).
- Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C. Multilayer packaging: Advances in preparation techniques and emerging food applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1156–1186. [Google Scholar] [CrossRef] [Green Version]
| Measurement 1 | Measurement 2 | ||
|---|---|---|---|
| Residue of furfurylamine | |||
| Furan-functionalized prepolymer 1 | mg/kg | 15.9 | 15.0 |
| Furan-functionalized prepolymer 2 | mg/kg | 15.3 | 13.5 |
| Residue of N-(2-hydroxyethyl)maleimide | |||
| Maleimide-functionalized prepolymer 1 | mg/kg | 36.0 | 35.5 |
| Maleimide-functionalized prepolymer 2 | mg/kg | 120.6 | 122.0 |
| Air | PET | Adhesive | PE | ||
|---|---|---|---|---|---|
| Thickness | µm | 100 | 23.0 | 8.00 | 45.0 |
| Density | g/cm3 | 1.20 × 10−3 | 1.38 | 1.10 | 0.97 |
| Partition coefficient | 1.00 | 1.00 | 1.00 | 1.00 |
| Air | PET | Adhesive | PE | EVOH | PE | ||
|---|---|---|---|---|---|---|---|
| Thickness | µm | 100 | 23.0 | 8.00 | 28.0 | 3.00 | 28.0 |
| Density | g/cm3 | 1.20 × 10−3 | 1.38 | 1.10 | 0.97 | 1.16 | 0.97 |
| Partition coefficient | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Migrant | Layer | Scenario | Diffusion Coefficient 23 °C | Source |
|---|---|---|---|---|
| Air | / | 0.02 cm2/s (assumed as worst case) | ||
| Furfurylamine | Adhesive | / | 3.55 × 10−8 cm2/s | Diffusion coefficient assumed as for PE |
| PE | / | 3.55 × 10−8 cm2/s | Piringer [30] | |
| PET | real | 2.78 × 10−15 cm2/s | [31] | |
| worst case | 2.39 × 10−14 cm2/s | [31] | ||
| EVOH | real | 9.26 × 10−19 cm2/s | this study | |
| worst case | 5.21 × 10−17 cm2/s | this study | ||
| N-(2-Hydroxyethyl) maleimide | Adhesive | / | 1.81 × 10−8 cm2/s | Diffusion coefficient assumed as for PE |
| PE | / | 1.81 × 10−8 cm2/s | Piringer [30] | |
| PET | real | 2.25 × 10−16 cm2/s | [31] | |
| worst case | 1.94 × 10−15 cm2/s | [31] | ||
| EVOH | real | 1.79 × 10−20 cm2/s | this study | |
| worst case | 1.00 × 10−18 cm2/s | this study |
| Laminate | Mean Value | |
|---|---|---|
| PET‑PE | N/15 mm | 3.07 ± 0.11 |
| PET‑aluminum | N/15 mm | 2.30 ± 0.41 |
| PE‑aluminum | N/15 mm | 2.39 ± 0.33 |
| Laminate | PET-PE | PE-Aluminum | PET-Aluminum |
|---|---|---|---|
| Start of delamination | 27 min | 29 min | 35 min |
| End of delamination | 33 min | 36 min | 40 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaiser, K.M.A.; Ewender, J.; Welle, F. Recyclable Multilayer Packaging by Means of Thermoreversibly Crosslinking Adhesive in the Context of Food Law. Polymers 2020, 12, 2988. https://doi.org/10.3390/polym12122988
Kaiser KMA, Ewender J, Welle F. Recyclable Multilayer Packaging by Means of Thermoreversibly Crosslinking Adhesive in the Context of Food Law. Polymers. 2020; 12(12):2988. https://doi.org/10.3390/polym12122988
Chicago/Turabian StyleKaiser, Katharina M. A., Johann Ewender, and Frank Welle. 2020. "Recyclable Multilayer Packaging by Means of Thermoreversibly Crosslinking Adhesive in the Context of Food Law" Polymers 12, no. 12: 2988. https://doi.org/10.3390/polym12122988
APA StyleKaiser, K. M. A., Ewender, J., & Welle, F. (2020). Recyclable Multilayer Packaging by Means of Thermoreversibly Crosslinking Adhesive in the Context of Food Law. Polymers, 12(12), 2988. https://doi.org/10.3390/polym12122988