Study on Temperature-Dependent Properties and Fire Resistance of Metakaolin-Based Geopolymer Foams
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of BGFs
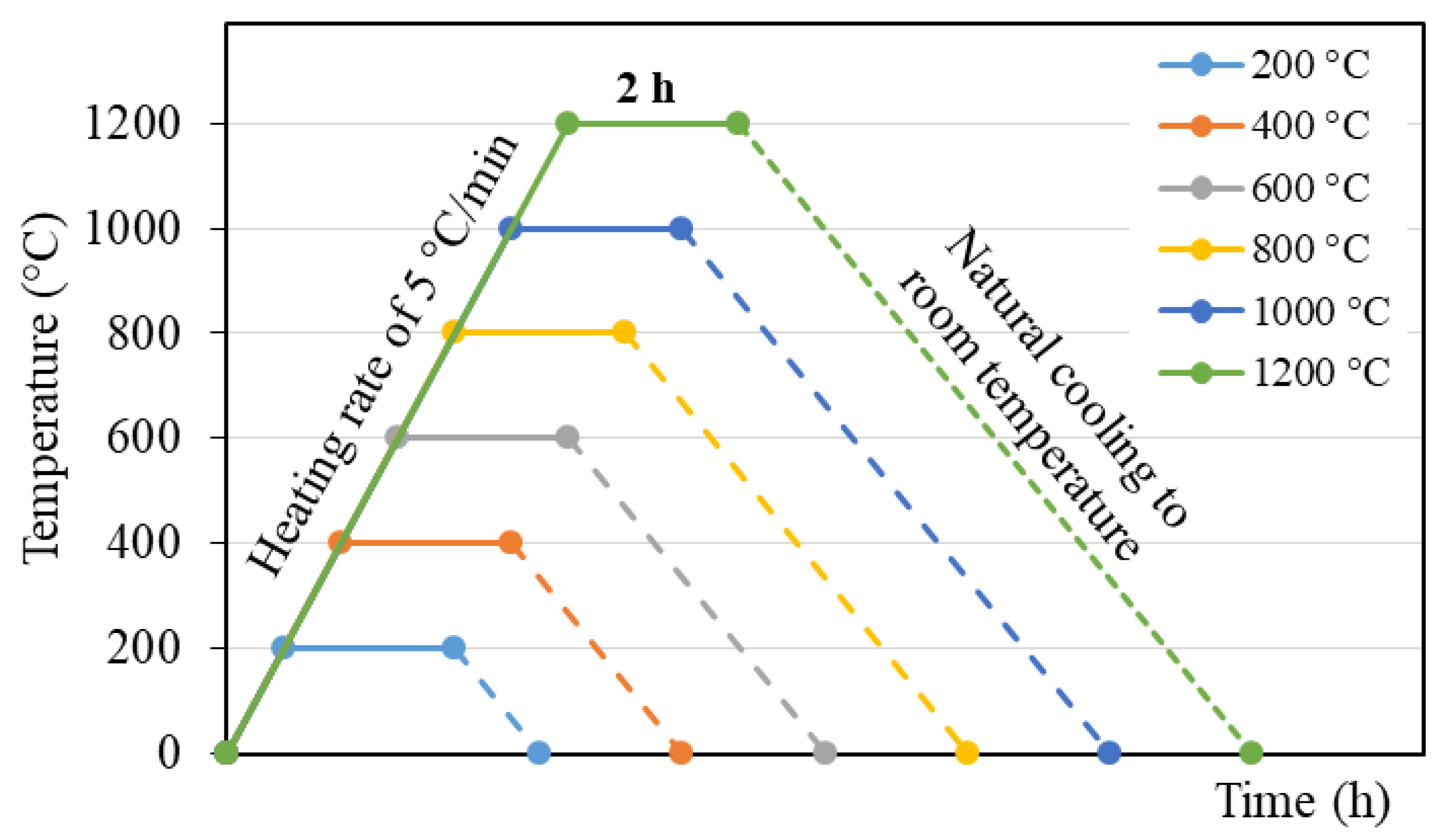
2.3. Heat Treatment of the BGFs
2.4. Characterizations
2.5. Mechanical Testing
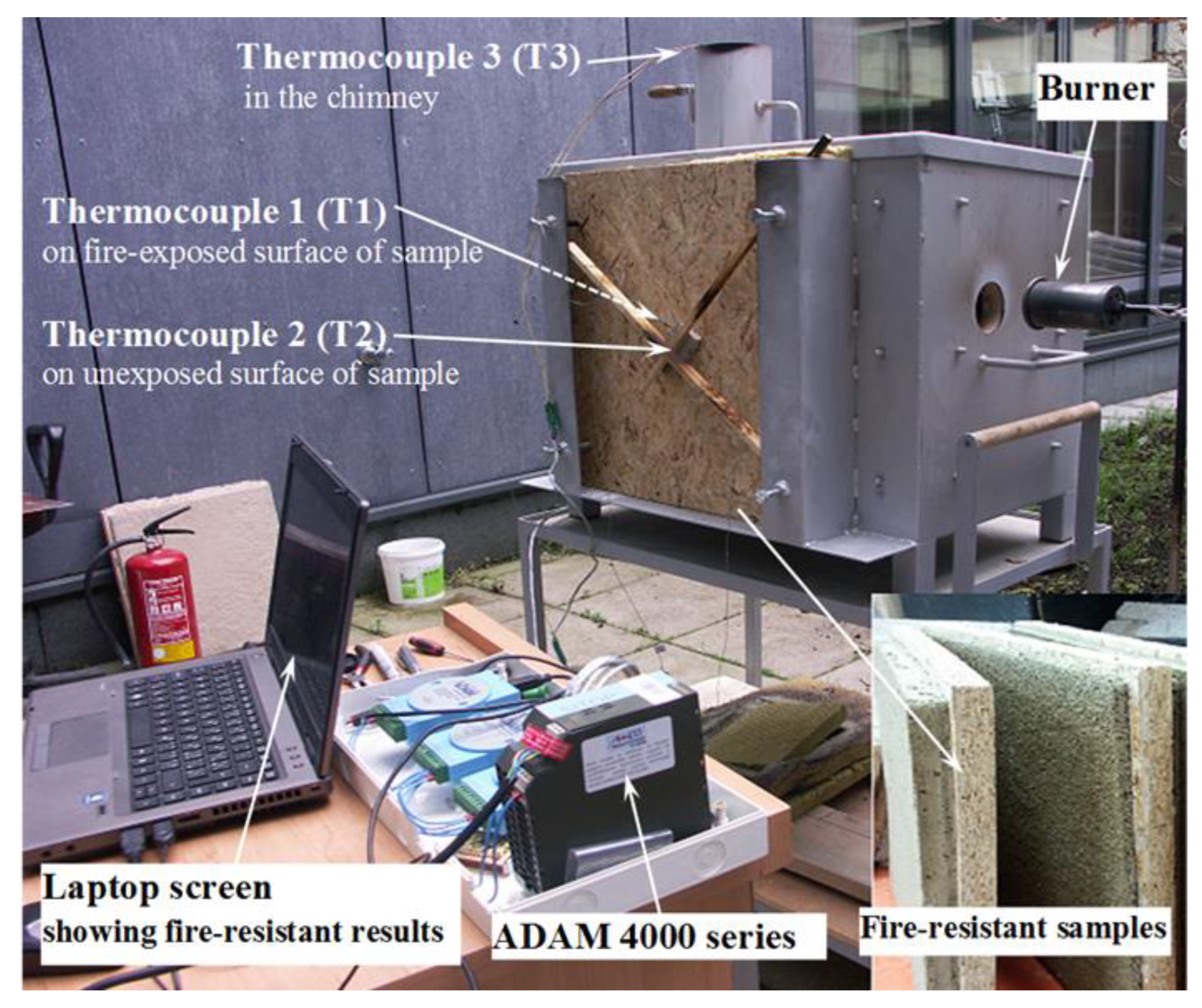
2.6. Fire Resistance Test
3. Results and Discussion
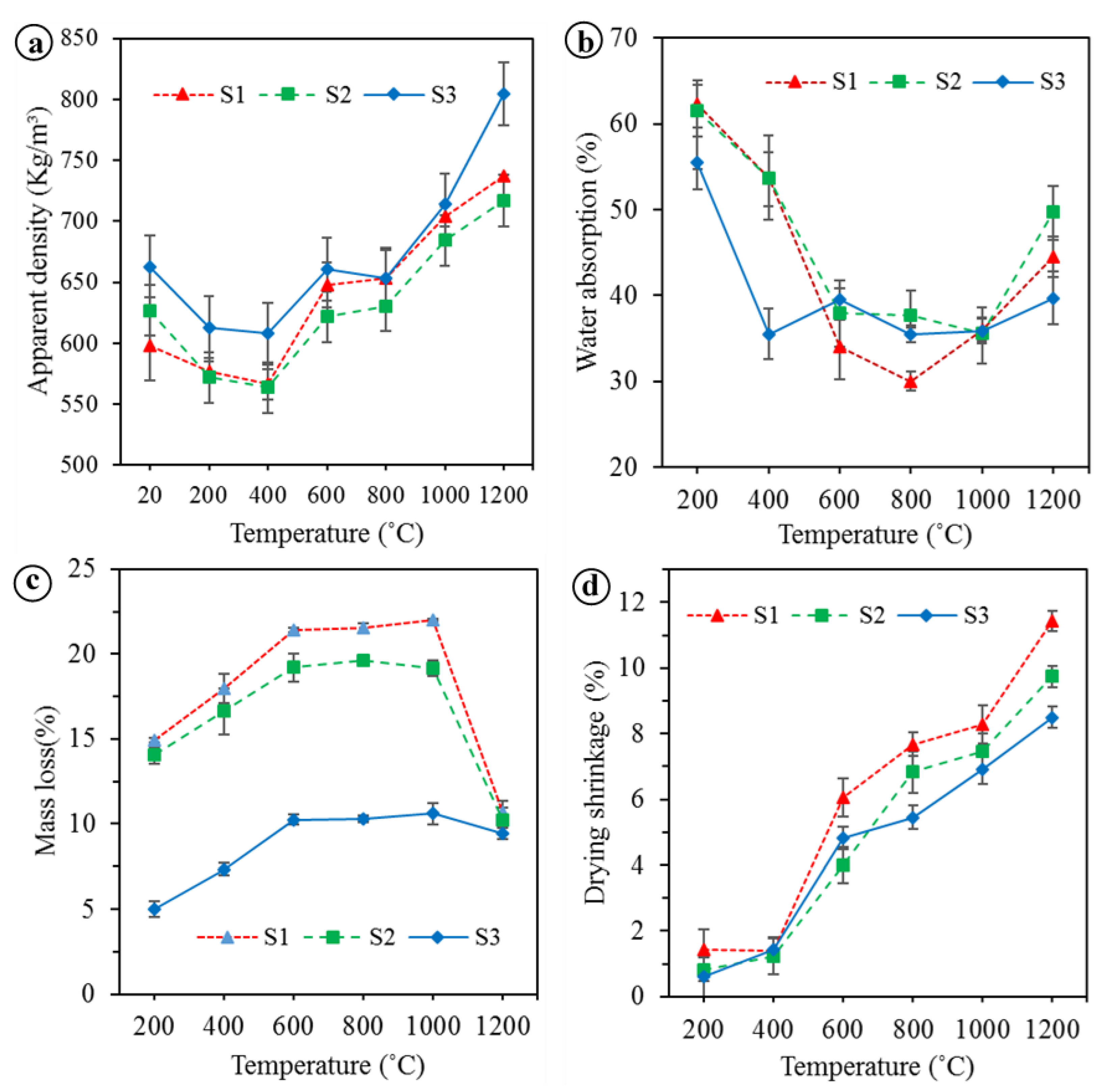
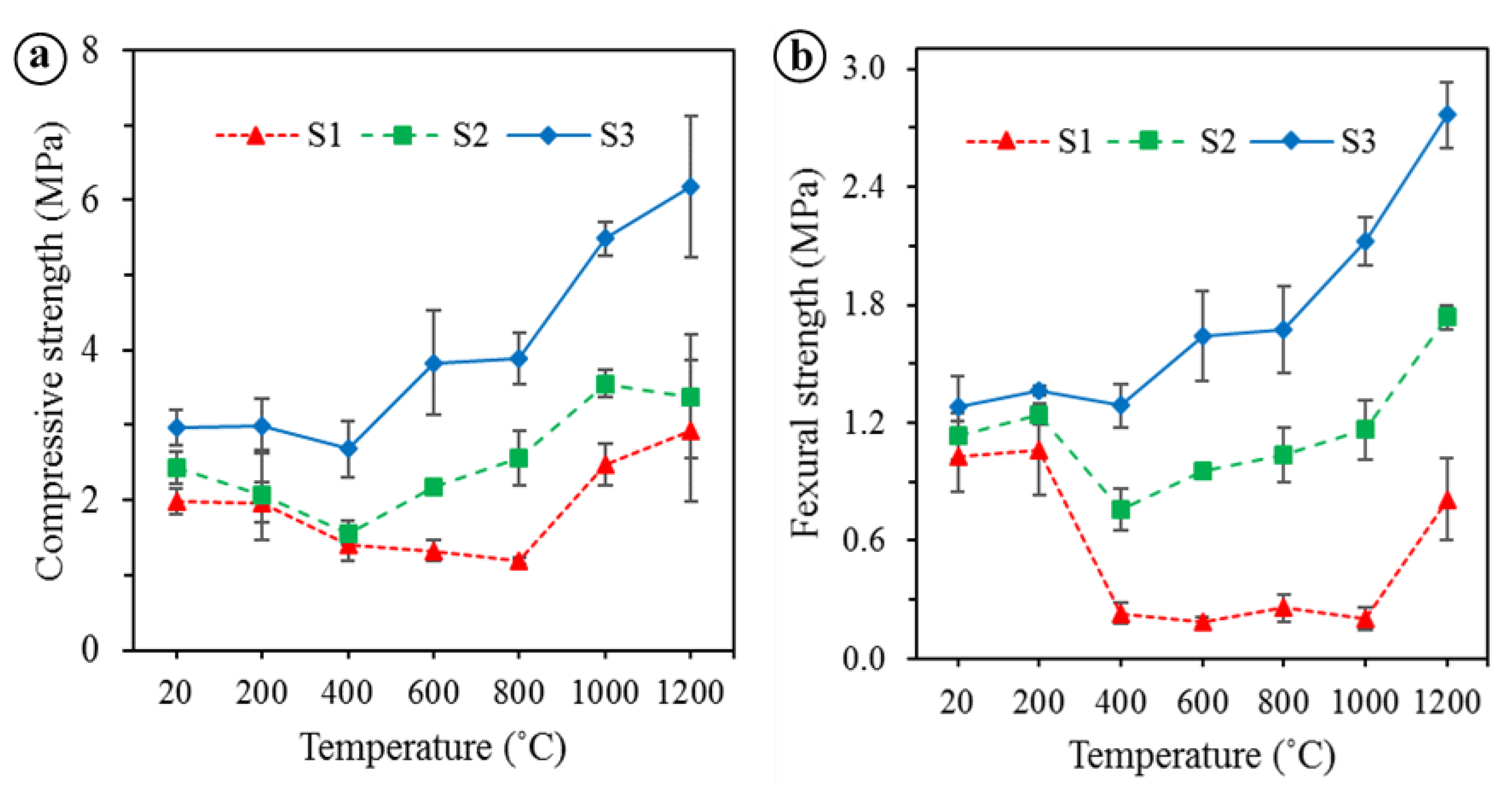
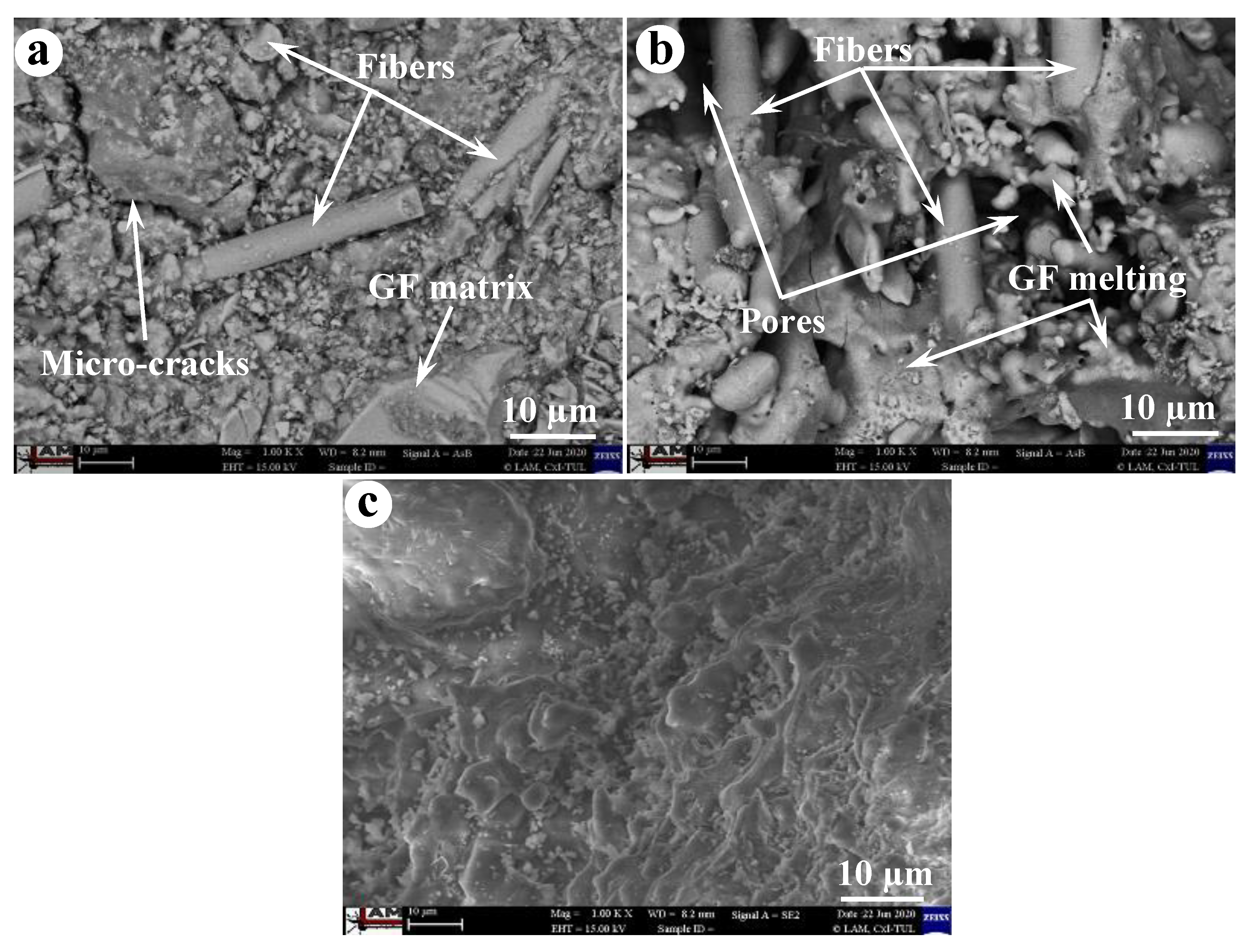
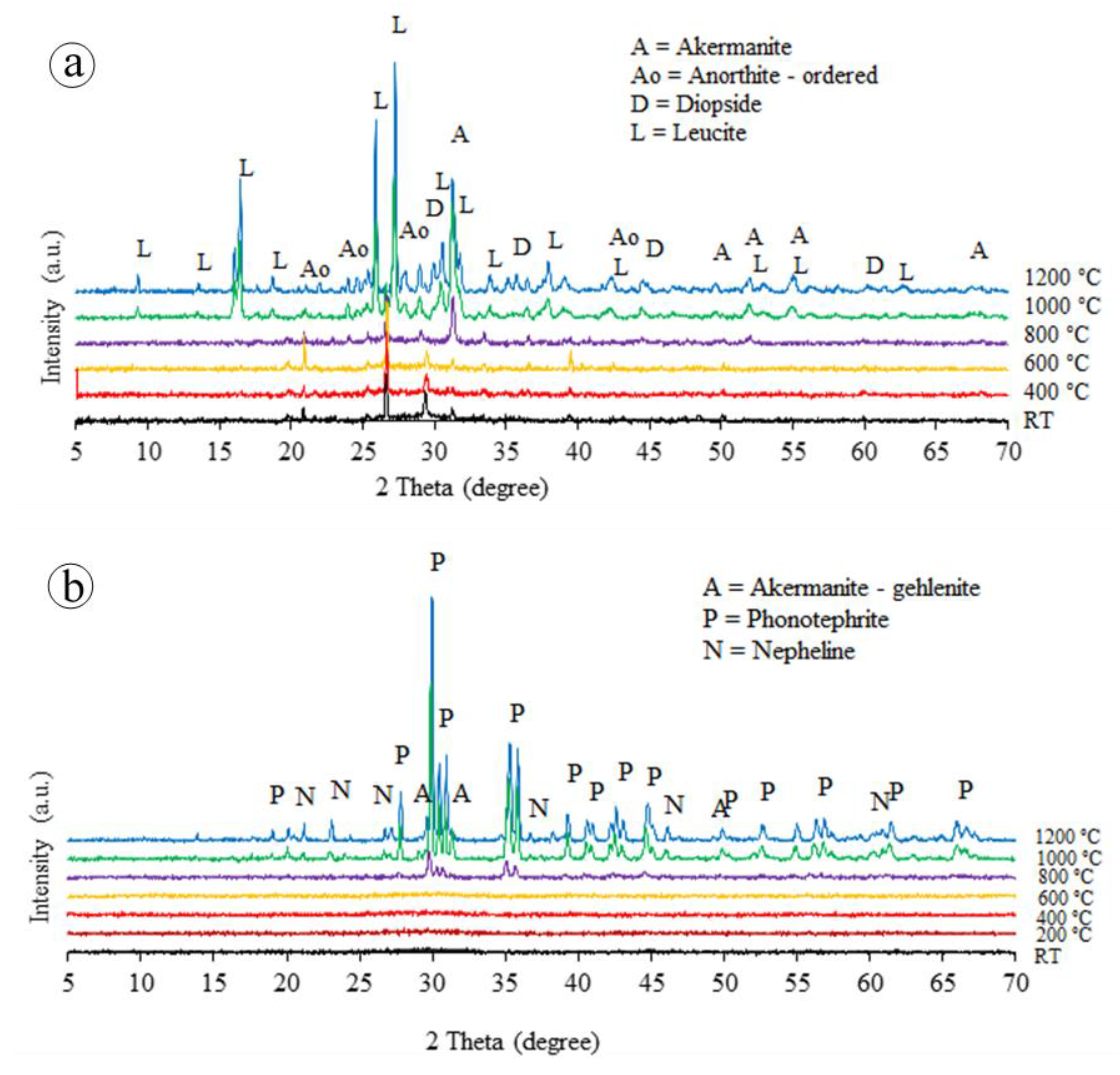
3.1. Temperature-Dependent Properties
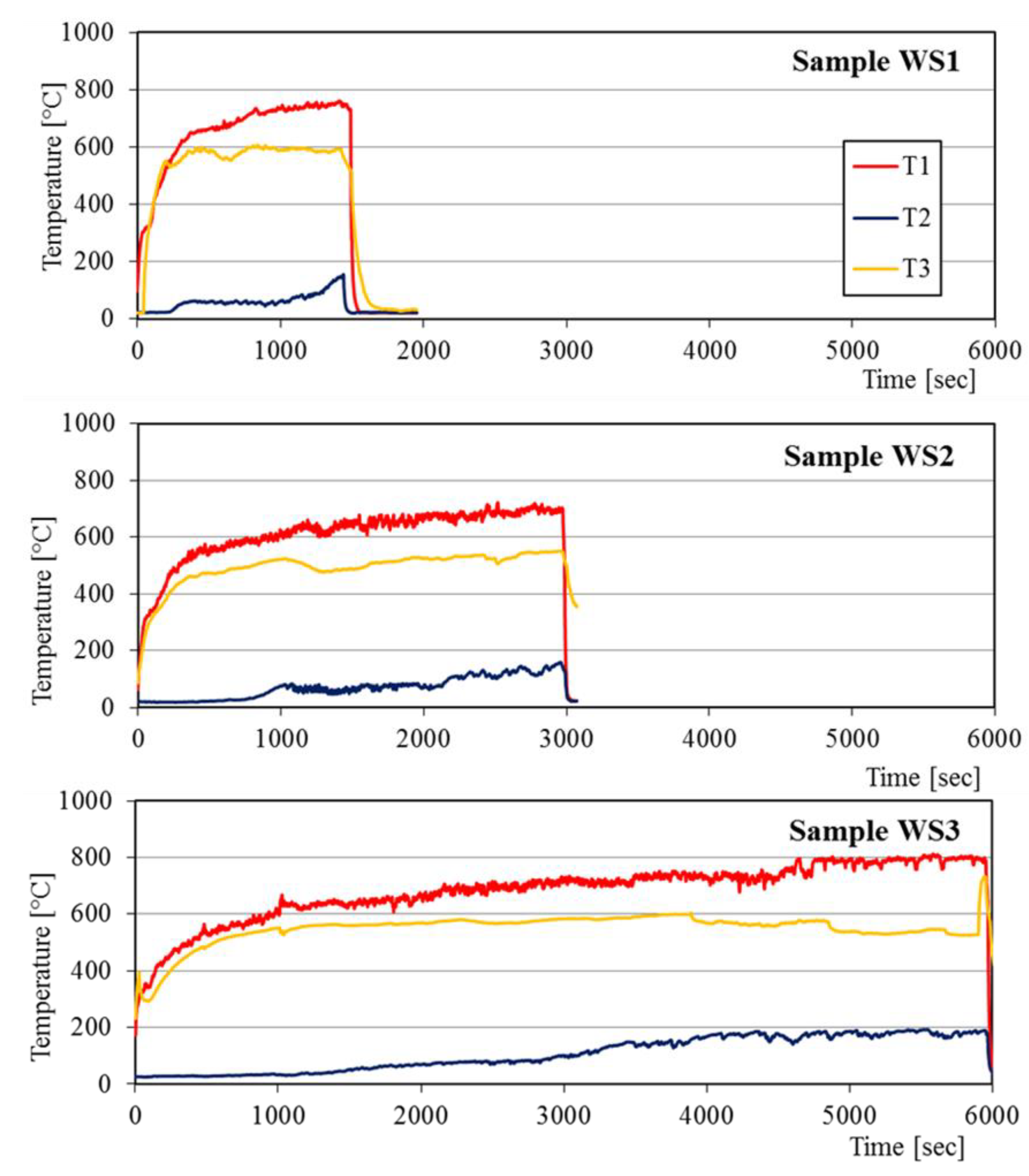
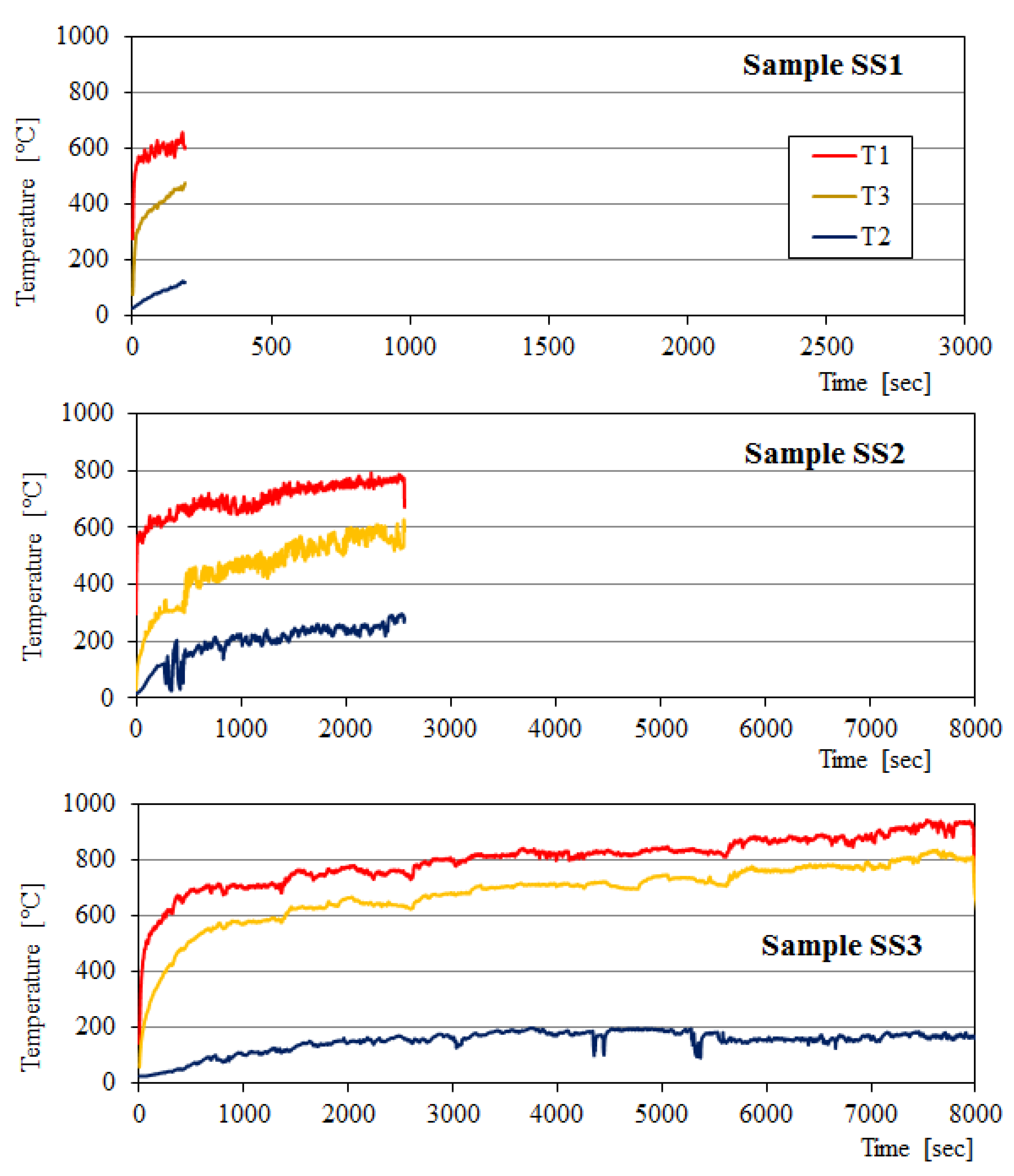
3.2. Fire Resistance of BGFs
4. Conclusions
- The apparent density and drying shrinkage of the BGFs increased with increasing the temperature from 400 to 1200 °C. Under 600 °C the mass loss was enhanced while the water absorption was reduced and they both changed slightly between 600 and 1000 °C. Over 1000 °C the mass loss was decreased considerably, whereas the water absorption was enhanced. The compressive and flexural strengths of the high fiber loading BGFs were improved significantly at temperatures above 600 °C and reached the maximum at 1200 °C.
- The BGFs exposed to high temperatures exhibited a smooth change from amorphous to crystalline phase. Moreover, the BGFs with high fiber content exposed to high temperatures resulted in the dense crystalline structure, thereby improving their mechanical strengths. Therefore, the high fiber content BGFs showed excellent mechanical stability at high temperatures.
- The fire resistance of the wood and steel plates has been considerably improved after coating a BGF layer on their surface. The coated BGF kept its structural integrity without any substantial macroscopic fracture after fire resistance test. The longest fire-resistant times for the wood and steel boards were 99 and 134 min, respectively.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. Processing, properties and applications of highly porous geopolymers: A review. Ceram. Int. 2018, 44, 16103–16118. [Google Scholar] [CrossRef]
- Novais, R.M.; Pullar, R.C.; Labrincha, J.A. Geopolymer foams: An overview of recent advancements. Prog. Mater. Sci. 2020, 109, 100621. [Google Scholar] [CrossRef]
- Le, A.S.; Hájková, P.; Kovacic, V.; Bakalova, T.; Lukáš, V.; Le, C.H.; Seifert, K.C.; Peres, A.P.; Louda, P. Thermal conductivity of reinforced geopolymer foams. Ceramics-Silikáty 2019, 63, 365–373. [Google Scholar] [CrossRef]
- Ge, Y.; Cui, X.; Kong, Y.; Li, Z.; He, Y.; Zhou, Q. Porous geopolymeric spheres for removal of Cu (II) from aqueous solution: Synthesis and evaluation. J. Hazard. Mater. 2015, 283, 244–251. [Google Scholar] [CrossRef]
- Ducman, V.; Korat, L. Characterization of geopolymer fly-ash based foams obtained with the addition of Al powder or H2O2 as foaming agents. Mater. Charact. 2016, 113, 207–213. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Buruberri, L.H.; Senff, L.; Labrincha, J.A. Influence of blowing agent on the freshand hardened-state properties of lightweight geopolymers. Mater. Des. 2016, 108, 551–559. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. High-porosity geopolymer membrane supports by peroxide route with the addition of egg white as surfactant. Ceram. Int. 2017, 43, 2267–2273. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Pacheco-Torgal, F.; Félix, T.; Tahri, W.; Aguiar, J.B. Mix design, properties and cost analysis of fly ash-based geopolymer foam. Constr. Build. Mater. 2015, 80, 18–30. [Google Scholar] [CrossRef]
- Chiu, Y.P.; Lu, Y.M.; Shiau, Y.C. Applying inorganic geopolymers added with aluminium powder to fire-resistant fillers. Mater. Res. Innov. 2015, 19, S5–S642. [Google Scholar] [CrossRef]
- Sanjayan, J.G.; Nazari, A.; Chen, L.; Nguyen, G.H. Physical and mechanical properties of lightweight aerated geopolymer. Constr. Build. Mater. 2015, 79, 236–244. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Sanjayan, J. Regulating the chemical foaming reaction to control the porosity of geopolymer foams. Mater. Des. 2017, 120, 255–265. [Google Scholar] [CrossRef]
- Masi, G.; Rickard, W.D.; Bignozzi, M.C.; Van Riessen, A. The effect of organic and inorganic fibres on the mechanical and thermal properties of aluminate activated geopolymers. Compos. Part B Eng. 2015, 76, 218–228. [Google Scholar] [CrossRef]
- Delair, S.; Prud’homme, É.; Peyratout, C.; Smith, A.; Michaud, P.; Eloy, L.; Joussein, E.; Rossignol, S. Durability of inorganic foam in solution: The role of alkali elements in the geopolymer network. Corros. Sci. 2012, 59, 213–221. [Google Scholar] [CrossRef]
- Henon, J.; Alzina, A.; Absi, J.; Smith, D.S.; Rossignol, S. Potassium geopolymer foams made with silica fume pore forming agent for thermal insulation. J. Porous Mater. 2013, 20, 37–46. [Google Scholar] [CrossRef]
- Böke, N.; Birch, G.D.; Nyale, S.M.; Petrik, L.F. New synthesis method for the production of coal fly ash-based foamed geopolymers. Constr. Build. Mater. 2015, 75, 189–199. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Mechanical, thermal insulation, thermal resistance and acoustic absorption properties of geopolymer foam concrete. Cem. Concr. Compos. 2015, 62, 97–105. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Kpogbemabou, D.; Morinière, V.; Laumonier, J.; Vaccari, A.; Rossignol, S. Porosity and insulating properties of silica-fume based foams. Energy Build. 2016, 131, 223–232. [Google Scholar] [CrossRef]
- Minelli, M.; Medri, V.; Papa, E.; Miccio, F.; Landi, E.; Doghieri, F. Geopolymers as solid adsorbent for CO2 capture. Chem. Eng. Sci. 2016, 148, 267–274. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Seabra, M.P.; Labrincha, J.A. Novel porous fly-ash containing geopolymer monoliths for lead adsorption from wastewaters. J. Hazard. Mater. 2016, 318, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Yuan, Y.; Wang, K.; He, Y.; Cui, X. Preparation of geopolymer-based inorganic membrane for removing Ni2+ from wastewater. J. Hazard. Mater. 2015, 299, 711–718. [Google Scholar] [CrossRef] [PubMed]
- López, F.J.; Sugita, S.; Kobayashi, T. Cesium-adsorbent geopolymer foams based on silica from rice husk and metakaolin. Chem. Lett. 2014, 43, 128–130. [Google Scholar] [CrossRef]
- Sakkas, K.; Panias, D.; Nomikos, P.; Sofianos, A. Potassium based geopolymer for passive fire protection of concrete tunnels linings. Tunn. Undergr. Space Technol. 2014, 43, 148–156. [Google Scholar] [CrossRef]
- Sarker, P.K.; Mcbeath, S. Fire endurance of steel reinforced fly ash geopolymer concrete elements. Constr. Build. Mater. 2015, 90, 91–98. [Google Scholar] [CrossRef]
- Peng, X.; Li, H.; Shuai, Q.; Wang, L. Fire Resistance of Alkali Activated Geopolymer Foams Produced from Metakaolin and Na2O2. Materials 2020, 13, 535. [Google Scholar] [CrossRef]
- Shuai, Q.; Xu, Z.; Yao, Z.; Chen, X.; Jiang, Z.; Peng, X.; An, R.; Li, Y.; Jiang, X.; Li, H. Fire resistance of phosphoric acid-based geopolymer foams fabricated from metakaolin and hydrogen peroxide. Mater. Lett. 2020, 263, 127228. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, W.; Shi, D. Effect of elevated temperature on the properties of geopolymer synthesized from calcined ore-dressing tailing of bauxite and ground-granulated blast furnace slag. Constr. Build. Mater. 2014, 69, 41–48. [Google Scholar] [CrossRef]
- Chithambaram, S.J.; Kumar, S.; Prasad, M.M. Thermo-mechanical characteristics of geopolymer mortar. Constr. Build. Mater. 2019, 213, 100–108. [Google Scholar] [CrossRef]
- Yang, Z.; Mocadlo, R.; Zhao, M.; Sisson, R.D., Jr.; Tao, M.; Liang, J. Preparation of a geopolymer from red mud slurry and class F fly ash and its behavior at elevated temperatures. Constr. Build. Mater. 2019, 221, 308–317. [Google Scholar] [CrossRef]
- Kürklü, G. The effect of high temperature on the design of blast furnace slag and coarse fly ash-based geopolymer mortar. Compos. Part B Eng. 2016, 92, 9–18. [Google Scholar] [CrossRef]
- František, Š.; Rostislav, Š.; Zdeněk, T.; Petr, S.; Vít, Š.; Zuzana, Z.C. Preparation and properties of fly ashbased geopolymer foams. Ceramics-Silikáty 2014, 58, 188–197. [Google Scholar]
- Hlaváček, P.; Šmilauer, V.; Škvára, F.; Kopecký, L.; Šulc, R. Inorganic foams made from alkali-activated fly ash: Mechanical, chemical and physical properties. J. Eur. Ceram. Soc. 2015, 35, 703–709. [Google Scholar] [CrossRef]
- Cilla, M.S.; de Mello Innocentini, M.D.; Morelli, M.R.; Colombo, P. Geopolymer foams obtained by the saponification/peroxide/gelcasting combined route using different soap foam precursors. J. Am. Ceram. Soc. 2017, 100, 3440–3450. [Google Scholar] [CrossRef]
- Liu, L.P.; Cui, X.M.; Yan, H.; Liu, S.D.; Gong, S.Y. The phase evolution of phosphoric acid-based geopolymers at elevated temperatures. Mater. Lett. 2012, 66, 10–12. [Google Scholar] [CrossRef]
- Liu, L.P.; Cui, X.M.; Qui, S.H.; Yu, J.L.; Zhang, L. Preparation of phosphoric acid-based porous geopolymers. Appl. Clay Sci. 2010, 50, 600–603. [Google Scholar]
- Łach, M.; Mikuła, J.; Lin, W.; Bazan, P.; Figiela, B.; Korniejenko, K. Development and characterization of thermal insulation geopolymer foams based on fly ash. Proc. Eng. Technol. Innov. 2020, 16, 23–29. [Google Scholar] [CrossRef]
- International Standards Organisation. Fire Resistance Tests, Elements of Building Construction (ISO 834); ISO: Geneva, Switzerland, 1980. [Google Scholar]
- Tang, C.; Xu, F.X.; Li, G. Combustion performance and thermal stability of basalt fiber-reinforced polypropylene composites. Polymers 2019, 11, 1826. [Google Scholar] [CrossRef]
- Mierzwiński, D.; Łach, M.; Hebda, M.; Walter, J.; Hebda, M.; Mikuła, J. Thermal phenomena of alkali-activated metakaolin studied with a negative temperature coefficient system. J. Therm. Anal. Calorim. 2019, 138, 4167–4175. [Google Scholar] [CrossRef]
- Bayrak, G.; Yilmaz, S. Granite based glass-ceramic materials. Acta Phys. Pol. A 2014, 125, 623–625. [Google Scholar] [CrossRef]



| Constituents | SiO2 | Al2O3 | CaO | MgO | TiO2 | Fe2O3 | K2O | SO3 | MnO | Na2O | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geopolymer | 44.5 | 28.9 | 17.6 | 2.23 | 1.31 | 0.82 | 0.75 | 0.46 | 0.28 | 0.25 | − | 2.56 |
| Basalt fiber | 33.6 | 14.4 | 26.1 | 8.26 | 1.98 | 6.61 | 1.21 | 0.29 | 0.76 | 1.38 | 0.14 | 2.05 |
| BGF Sample | Binder | Aluminum Powder/Binder | Basalt Fiber/Binder | |
|---|---|---|---|---|
| Baucis lk | Activator | |||
| S1 | 1 | 0.9 | 0.008 | 0.05 |
| S2 | 0.008 | 0.16 | ||
| S3 | 0.008 | 0.26 | ||
| Base Material | Wood | Steel | ||||
|---|---|---|---|---|---|---|
| Sample ID | WS1 | WS2 | WS3 | SS1 | SS2 | SS3 |
| Thickness of the coated BGF layer (mm) | 0 | 10 | 20 | 0 | 5 | 10 |
| Constituents | C | O | Na | Mg | Al | Si | P | S | Cl | K | Ca | Ti | Mn | Fe | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | 5.59 | 45.76 | 0.20 | 1.05 | 10.23 | 17.91 | 0.00 | 0.06 | 0.11 | 10.0 | 7.91 | 0.42 | 0.21 | 0.56 | 100 |
| 1000 °C | 4.07 | 43.53 | 0.29 | 2.46 | 9.60 | 18.60 | 0.07 | 0.12 | 0.00 | 6.72 | 11.65 | 0.66 | 0.50 | 1.72 | 100 |
| 1200 °C | 4.77 | 43.96 | 0.29 | 1.55 | 10.52 | 21.48 | 0.03 | 0.05 | 0.00 | 7.82 | 8.24 | 0.40 | 0.19 | 0.71 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, V.S.; Louda, P.; Tran, H.N.; Nguyen, P.D.; Bakalova, T.; Ewa Buczkowska, K.; Dufkova, I. Study on Temperature-Dependent Properties and Fire Resistance of Metakaolin-Based Geopolymer Foams. Polymers 2020, 12, 2994. https://doi.org/10.3390/polym12122994
Le VS, Louda P, Tran HN, Nguyen PD, Bakalova T, Ewa Buczkowska K, Dufkova I. Study on Temperature-Dependent Properties and Fire Resistance of Metakaolin-Based Geopolymer Foams. Polymers. 2020; 12(12):2994. https://doi.org/10.3390/polym12122994
Chicago/Turabian StyleLe, Van Su, Petr Louda, Huu Nam Tran, Phu Dong Nguyen, Totka Bakalova, Katarzyna Ewa Buczkowska, and Iva Dufkova. 2020. "Study on Temperature-Dependent Properties and Fire Resistance of Metakaolin-Based Geopolymer Foams" Polymers 12, no. 12: 2994. https://doi.org/10.3390/polym12122994
APA StyleLe, V. S., Louda, P., Tran, H. N., Nguyen, P. D., Bakalova, T., Ewa Buczkowska, K., & Dufkova, I. (2020). Study on Temperature-Dependent Properties and Fire Resistance of Metakaolin-Based Geopolymer Foams. Polymers, 12(12), 2994. https://doi.org/10.3390/polym12122994









