Bioactive Protecting Coating of Guar Gum with Thyme Oil to Extend Shelf Life of Tilapia (Oreoschromis niloticus) Fillets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
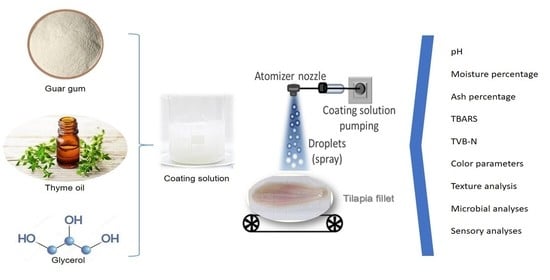
2.2. Preparation of Fish for Bioactive GG Coating Solution
2.3. Treatment and Storage of Fish Samples
2.4. Physicochemical Analyses
2.4.1. pH
2.4.2. Proximate Composition
2.4.3. Determination of Thiobarbituric Acid Reactive Substances (TBARS) (Lipid Oxidation)
2.4.4. Total Volatile Basic Nitrogen Analyses (TVB-N)
2.4.5. Color Measurements
2.4.6. Texture Analyses
2.5. Microbiological Analyses
2.6. Sensory Evaluation
2.7. Statistical Analyses
3. Results and Discussion
3.1. Effect of the Bioactive Coating Incorporated with Thyme Essential Oil on the pH Values of Fish Fillets
3.2. Proximate Composition
3.3. Color Measurements
3.4. Texture Analysis
3.5. Impact of Coating on the Microbiological Quality of Tilapia Fillets
3.6. Influence of Coating on the Sensory Properties of Tilapia Fillets
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vieira, B.B.; Mafra, J.F.; da Rocha Bispo, A.S.; Ferreira, M.A.; de Lima Silva, F.; Rodrigues, A.V.N.; Evangelista-Barreto, N.S. Combination of chitosan coating and clove essential oil reduces lipid oxidation and microbial growth in frozen stored tambaqui (Colossoma macropomum) fillets. LWT 2019, 116, 108546. [Google Scholar] [CrossRef]
- Shankar, S.; Danneels, F.; Lacroix, M. Coating with alginate containing a mixture of essential oils and citrus extract in combination with ozonation or gamma irradiation increased the shelf life of Merluccius sp. fillets. Food Packag. Shelf Life 2019, 22, 100434. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of Nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Chaparro-Hernández, S.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Ocaño-Higuera, V.M.; Valenzuela-López, C.C.; Ornelas-Paz, J.J.; Del-Toro-Sánchez, C.L. Effect of chitosan-carvacrol edible coatings on the quality and shelf life of tilapia (Oreoschromis niloticus) fillets stored in ice. Food Sci. Tech. 2015, 35, 734–741. [Google Scholar] [CrossRef] [Green Version]
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguee, N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019, 139, 94–102. [Google Scholar] [CrossRef]
- Kilincceker, O.; Dogan, I.S.; Kucukoner, E. Effect of edible coatings on the quality of frozen fish fillets. LWT 2009, 42, 868–873. [Google Scholar] [CrossRef]
- Shokri, S.; Parastouei, K.; Taghdir, M.; Abbaszadeh, S. Application an edible active coating based on chitosan- Ferulago angulate essential oil nanoemulsion to shelf life extension of Rainbow trout fillets stored at 4 °C. Int. J. Biol. Macromol. 2020, 153, 846–854. [Google Scholar] [CrossRef]
- Sáez, M.I.; Suárez, M.D.; Martínez, T.F. Effects of alginate coating enriched with tannins on shelf life of cultured rainbow trout (Oncorhynchus mykiss) fillets. LWT 2020, 118, 108767. [Google Scholar] [CrossRef]
- Hassan, B.; Shahid Chatha, S.A.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Perez-Gago, M.B.; Serra, M.; del Río, M.A. Color change of fresh-cut apples coated with whey protein concentrate-based edible coatings. Postharvest Biol. Technol. 2006, 39, 84–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, S.; Zhang, J.; Chi, Y.; Tian, B.; Li, L.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation of whey protein isolate nanofibrils by microwave heating and its application as carriers of lipophilic bioactive substances. LWT 2020, 125, 109213. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Li, D.; Liu, C.; Jiang, B.; Feng, Z. Preparation and characterization of coating based on protein nanofibers and polyphenol and application for salted dick egg yolks. Foods 2020, 9, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruelas-Chacon, X.; Contreras-Esquivel, J.C.; Montañez, J.; Aguilera-Carbo, A.F.; Reyes-Vega, M.L.; Peralta-Rodriguez, R.D.; Sanchéz-Brambila, G. Guar gum as an edible coating for enhancing shelf-life and improving postharvest quality of roma tomato (Solanum lycopersicum L.). J. Food Qual. 2017, 8608304. [Google Scholar] [CrossRef] [Green Version]
- Pires, C.; Ramos, C.; Teixeira, G.; Batista, I.; Mendes, R.; Nunes, L.; Marques, A. Characterization of biodegradable films prepared with hake proteins and thyme oil. J. Food Eng. 2011, 105, 422–428. [Google Scholar] [CrossRef]
- Saurabh, C.K.; Gupta, S.; Variyar, P.S.; Sharma, A. Effect of addition of nano clay, beeswax, tween-80 and glycerol on physicochemical properties of guar gum films. Ind. Crop. Prod. 2016, 89, 109–118. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Wang, L.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Fabrication and characterization of microemulsion stabilized by integrated phosvitin and gallic acid. J. Agric. Food Chem. 2020, 68, 5437–5447. [Google Scholar] [CrossRef]
- Yang, W.; Xu, C.; Liu, F.; Yuan, F.; Gao, Y. Native and thermally modified protein-polyphenol coassemblies: Lactoferrin-based nanoparticles and submicrometer particles as protective vehicles for (−)-epigallocatechin-3-gallate. J. Agric. Food Chem. 2014, 62, 10816–10827. [Google Scholar] [CrossRef]
- Radha Krishnan, K.; Babuskin, S.; Rakhavan, K.R.; Tharavin, R.; Azhagu Saravana Babu, P.; Sivarajan, M.; Sukumar, M. Potential application of corn starch edible films with spice essential oils for the shelf life extension of red meat. J. App. Microbiol. 2015, 119, 1613–1623. [Google Scholar] [CrossRef]
- Giteru, S.G.; Oey, I.; Ali, M.A.; Johnson, S.K.; Fang, Z. Effect of kafirin-based films incorporating citral and quercetin on the storage of fresh chicken fillets. Food Control 2017, 80, 37–44. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Carrión-Granda, X.; Maté, J.I. Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control 2014, 36, 69–75. [Google Scholar] [CrossRef]
- Emiroǧlu, Z.K.; Yemiş, G.P.; Coşkun, B.K.; Candoǧan, K. Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010, 86, 283–288. [Google Scholar] [CrossRef]
- Zimoch-Korzycka, A.; Jarmoluk, A. Polysaccharide-based edible coatings containing cellulase for improved preservation of meat quality during storage. Molecules 2017, 22, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpe, M.G.; Siano, F.; Paolucci, M.; Sacco, A.; Sorrentino, A.; Malinconico, M.; Varrichio, E. Active edible coating effectiveness in the shelf-life enhancement of trout (Oncorhynchusmykiss) fillets. Food Sci. Technol. 2015, 60, 615–622. [Google Scholar] [CrossRef]
- Alparslan, Y.; Yapici, H.H.; Metin, C.; Baygar, T.; Günlü, A.; Baygar, T. Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT 2016, 72, 457–466. [Google Scholar] [CrossRef]
- Aşik, E.; Candoǧan, K. Effects of chitosan coatings incorporated with garlic oil on quality characteristics of shrimp. J. Food Qual. 2014, 37, 237–246. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Barber, X.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. Effect of chitosan edible films added with Thymus moroderi and Thymus piperella essential oil on shelf-life of cooked cured ham. J. Food Sci. Technol. 2015, 52, 6493–6501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, W.S.; Ock, S.Y.; Park, G.D.; Lee, I.W.; Lee, M.H.; Park, H.J. Heat-sealing property of cassava starch film plasticized with glycerol and sorbitol. Food Packag. Shelf Life 2020, 26, 100556. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; de Oliveira Rios, A.; Hickmann Flôres, S. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Marrón, M.; Mani-López, E.; Palou, E.; López-Malo, A. Effects of alginate-glycerol-citric acid concentrations on selected physical, mechanical, and barrier properties of papaya puree-based edible films and coatings, as evaluated by response surface methodology. LWT 2019, 101, 83–91. [Google Scholar] [CrossRef]
- Ballesteros-Mártinez, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Effect of glycerol and sorbitol concentrations on mechanical, optical, and barrier properties of sweet potato starch film. NFS J. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- CIE 15: Technical Report: Colorimetry. Available online: https://www.cdvplus.cz/file/3-publikace-cie15-2004/ (accessed on 16 December 2020).
- Da Silva Santos, F.M.; Martins da Silva, A.I.; Brandão Vieira, C.; Horácio de Araújo, M.; Coelho da Silva, A.L.; Carneiro-da-Cunha, M.G.; Silva de Souza, B.W.; De Souza Bezerra, R. Use of chitosan coating in increasing the shelf life liquid smoked Nile tilapia (Oreoschromis niloticus) fillet. J. Food Sci. Technol. 2017, 54, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Cherian, G.; Zhao, Y. Quality enhancement in fresh and frozen lingcod (Ophiodon elongates) fillets by employment of fish oil incorporated chitosan coatings. Food Chem. 2010, 119, 524–532. [Google Scholar] [CrossRef]
- Cao, X.; Islam, N.; Chitrakar, B.; Duan, Z.; Xu, W.; Zhong, S. Effect of combined chlorogenic acid and chitosan coating on antioxidant, antimicrobial, and sensory properties of snakehead fish in cold storage. Food Sci. Nutr. 2020, 8, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Arannilewa, S.T.; Salawu, S.O.; Sorungbe, A.A.; Ola-Salawu, B.B. Effect of frozen period on the chemical, microbiological and sensory quality of frozen tilapia fish (Sarotherodun galiaenus). Afr. J. Biotechnol. 2005, 4, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Joukar, F.; Hashem Hosseini, S.M.; Moosavi-Nasab, M.; Mesbahi, G.R.; Behzadnia, A. Effect of Farsi gum-based antimicrobial adhesive coatings on the refrigeration shelf life of rainbow trout fillets. LWT 2017, 80, 1–9. [Google Scholar] [CrossRef]
- Vital, A.C.P.; Guerrero, A.; Ornaghi, M.G.; Kempinski, E.M.B.C.; Sary, C.; Monteschio, J.O.; Pintro, P.T.M.; Ribeiro, R.R.; Prado, I.N. Quality and sensory acceptability of fish fillet (Oreoschromis niloticus) with alginate-based coating containing essential oils. J. Food Sci. Tech. 2018, 55, 4945–4955. [Google Scholar] [CrossRef]
- Pinto, L.; Nascimento, K.; Monteschio, J.; Vital, A.; Scapim, M.; Madrona, G.; Prado, I. Effect of Alginate-based Edible Coating with Oatmeal on the Quality of Nile tilapia Fillets. Chem. Eng. Trans. 2019, 75, 589–594. [Google Scholar]
- Gharibzahedia, S.M.T.; Mohammadnabi, S. Effect of novel bioactive edible coatings based on jujube gum and nettle oil-loaded nanoemulsions on the shelf-life of Beluga sturgeosn fillets. Inter. J. Biol. Macromol. 2016, 95, 769–777. [Google Scholar] [CrossRef]
- Uçak, I. Physicochemical and antimicrobial effects pf gelatin-based edible films incorporated with garlic peel extract on the rainbow trout fillets. Prog. Nutr. 2019, 21, 232–240. [Google Scholar]
- Jouki, M.; Tabatabaei Yazdi, F.; Mortazavi, S.A.; Koocheki, A.; Khazaei, N. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Inter. J. Food Microbiol. 2014, 174, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Lee, J.H.; Yang, H.J.; Song, K.B. Production and characterization of skate skin gelatin films incorporated with thyme essential oil and their application in chicken tenderloin packaging. Int. J. Food Sci. Tech. 2016, 51, 1465–1472. [Google Scholar] [CrossRef]
- Mexis, S.F.; Chouliara, E.; Kontominas, M.G. Combined effect of an O2 absorber and oregano essential oil on shelf-life extension of Greek cod roe paste (tarama salad) stored at 4 °C. Innov. Food Sci. Emerg. Technol. 2009, 10, 572–579. [Google Scholar] [CrossRef]
- Ozogul, Y.; Yuvka, Y.; Ucar, Y.; Durmus, M.; Kosker, A.R.; Oz, M.; Ozogul, F. Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme, and sage) on the sensory, chemical, and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT 2017, 75, 677–684. [Google Scholar] [CrossRef]
- Öz, M. Effects of garlic (Allium sativum) supplemented fish diet on sensory, chemical and microbiological properties of rainbow trout during storage at −18 °C. LWT 2018, 92, 155–160. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Effect of fish gelatin coating enriched with oregano essential oil on the quality of refrigerated rainbow trout fillet. J. Aquat. Food Prod. Technol. 2016, 25, 835–842. [Google Scholar] [CrossRef]
- Heydari, R.; Bavandi, S.; Javadian, S.R. Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4 °C. Food Sci. Nutr. 2015, 3, 188–194. [Google Scholar] [CrossRef]
- Ariaii, P.; Tavakolipour, H.; Rezaei, M.; Elhami Rad, A.H.; Bahram, S. Effect of methylcellulose coating enriched with Pimpinella affinis oil on the quality of silver carp fillet during refrigerator storage condition. J. Food Process. Pres. 2015, 39, 1647–1655. [Google Scholar] [CrossRef]
- Raeisi, M.; Tajik, H.; Aliakbarlu, J.; Mirhosseini, S.H.; Hosseini, S.M.H. Effect of carboxymethyl cellulose-based coatings incorporated with Zataria multiflora Boiss. essential oil and grape seed extract on the shelf life of rainbow trout fillets. LWT 2015, 64, 898–904. [Google Scholar] [CrossRef]




| Physicochemical Variables | Storage Period (Days) | Treatments | ||
|---|---|---|---|---|
| UC | GGC | GGCTH | ||
| Moisture, % | 0 | 89.95 ± 0.33 aB | 90.02 ± 0.25 aAB | 90.01 ± 0.18 aA |
| 5 | 82.65 ± 0.75 bB | 83.41 ± 0.42 bAB | 84.17 ± 0.28 bA | |
| 10 | 74.16 ± 0.53 cB | 75.31 ± 0.03 cAB | 76.46 ± 0.53 cA | |
| 15 | 62.19 ± 0.93 dB | 62.97 ± 0.33 dAB | 63.76 ± 0.04 dA | |
| Ash content, % | 0 | 1.69 ± 0.03 aA | 1.70 ± 0.04 aA | 1.68 ± 0.05 aA |
| 5 | 1.75 ± 0.01 aA | 1.85 ± 0.01 aA | 1.96 ± 0.02 aA | |
| 10 | 2.04 ± 0.01 aA | 1.99 ± 0.01 aA | 1.95 ± 0.03 aA | |
| 15 | 2.11 ± 0.21 aA | 1.99 ± 0.01 aA | 1.88 ± 0.05 aA | |
| Crude fat, % | 0 | 1.47 ± 0.57 aA | 1.47 ± 0.25 aA | 1.48 ± 0.42 aA |
| 5 | 1.87 ± 0.58 aA | 1.60 ± 0.17 aA | 1.51 ± 0.20 aA | |
| 10 | 1.72 ± 0.03 aA | 1.64 ± 0.01 aA | 1.70 ± 0.01 aA | |
| 15 | 1.66 ± 0.03 aA | 1.83 ± 0.02 aA | 1.87 ± 0.04 aA | |
| TBARS, mg of malonaldehyde/kg fillet | 0 | 0.17 ± 0.22 dA | 0.19 ± 0.60 dA | 0.18 ± 0.11 dA |
| 5 | 0.20 ± 0.65 cB | 0.23 ± 0.01 cA | 0.23 ± 0.10 cA | |
| 10 | 0.39 ± 0.18 bA | 0.28 ± 0.24 bB | 0.28 ± 0.17 bB | |
| 15 | 0.54 ± 0.90 aA | 0.31 ± 0.65 aB | 0.30 ± 0.03 aB | |
| TVB-N, mg N/100 g fillet | 0 | 16.03 ± 0.32 dA | 16.04 ± 0.20 dA | 15.99 ± 0.47 dA |
| 5 | 27.70 ± 0.65 cA | 19.56 ± 0.01 cB | 16.44 ± 0.10 cC | |
| 10 | 37.68 ± 0.18 bA | 26.04 ± 0.24 bB | 19.72 ± 0.17 bC | |
| 15 | 51.32 ± 0.90 aA | 29.24 ± 0.65 aB | 22.03 ± 0.03 aC | |
| Color Variables | Storage Period (Days) | Treatments | ||
|---|---|---|---|---|
| UC | GGC | GGCTH | ||
| L* | 0 | 52.80 ± 0.23 cA | 51.38 ± 0.48 bB | 49.62 ± 0.49 bC |
| 5 | 52.95 ± 0.34 cA | 51.54 ± 0.22 bB | 50.12 ± 0.76 aC | |
| 10 | 53.10 ± 0.25 bA | 49.91 ± 0.45 cB | 46.71 ± 0.65 cC | |
| 15 | 55.70 ± 0.68 aA | 52.84 ± 0.28 aB | 49.97 ± 0.39 bC | |
| a* | 0 | −0.78 ± 0.82 dA | −0.59 ± 0.52 cAB | −0.45 ± 0.61 cB |
| 5 | −0.84 ± 0.13 cA | −0.65 ± 0.81 cAB | −0.46 ± 0.26 cB | |
| 10 | 2.36 ± 0.87 aB | 3.66 ± 0.35 aAB | 4.96 ± 0.61 aA | |
| 15 | 1.33 ± 0.41 bA | 1.15 ± 0.76 bAB | 0.96 ± 0.74 bB | |
| b* | 0 | 2.75 ± 0.24 bA | 1.99 ± 0.50 bB | 0.97 ± 0.23 bB |
| 5 | 2.94 ± 0.58 bA | 2.01 ± 0.27 bB | 1.07 ± 0.51 bB | |
| 10 | 5.22 ± 0.33 aB | 6.77 ± 0.60 aA | 6.32 ± 0.43 aA | |
| 15 | 2.61 ± 0.52 bB | 7.79 ± 0.42 aA | 7.97 ± 0.55 aA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruelas-Chacon, X.; Aguilar-González, A.; de la Luz Reyes-Vega, M.; Peralta-Rodríguez, R.D.; Corona-Flores, J.; Rebolloso-Padilla, O.N.; Aguilera-Carbo, A.F. Bioactive Protecting Coating of Guar Gum with Thyme Oil to Extend Shelf Life of Tilapia (Oreoschromis niloticus) Fillets. Polymers 2020, 12, 3019. https://doi.org/10.3390/polym12123019
Ruelas-Chacon X, Aguilar-González A, de la Luz Reyes-Vega M, Peralta-Rodríguez RD, Corona-Flores J, Rebolloso-Padilla ON, Aguilera-Carbo AF. Bioactive Protecting Coating of Guar Gum with Thyme Oil to Extend Shelf Life of Tilapia (Oreoschromis niloticus) Fillets. Polymers. 2020; 12(12):3019. https://doi.org/10.3390/polym12123019
Chicago/Turabian StyleRuelas-Chacon, Xochitl, Alfredo Aguilar-González, María de la Luz Reyes-Vega, René Darío Peralta-Rodríguez, José Corona-Flores, Oscar Noé Rebolloso-Padilla, and Antonio Francisco Aguilera-Carbo. 2020. "Bioactive Protecting Coating of Guar Gum with Thyme Oil to Extend Shelf Life of Tilapia (Oreoschromis niloticus) Fillets" Polymers 12, no. 12: 3019. https://doi.org/10.3390/polym12123019
APA StyleRuelas-Chacon, X., Aguilar-González, A., de la Luz Reyes-Vega, M., Peralta-Rodríguez, R. D., Corona-Flores, J., Rebolloso-Padilla, O. N., & Aguilera-Carbo, A. F. (2020). Bioactive Protecting Coating of Guar Gum with Thyme Oil to Extend Shelf Life of Tilapia (Oreoschromis niloticus) Fillets. Polymers, 12(12), 3019. https://doi.org/10.3390/polym12123019