Calcined Co(II)-Triethylenetetramine, Co(II)- Polyaniline-Thiourea as the Cathode Catalyst of Proton Exchanged Membrane Fuel Cell
Abstract
:1. Introduction
2. Experimental
2.1. Materials
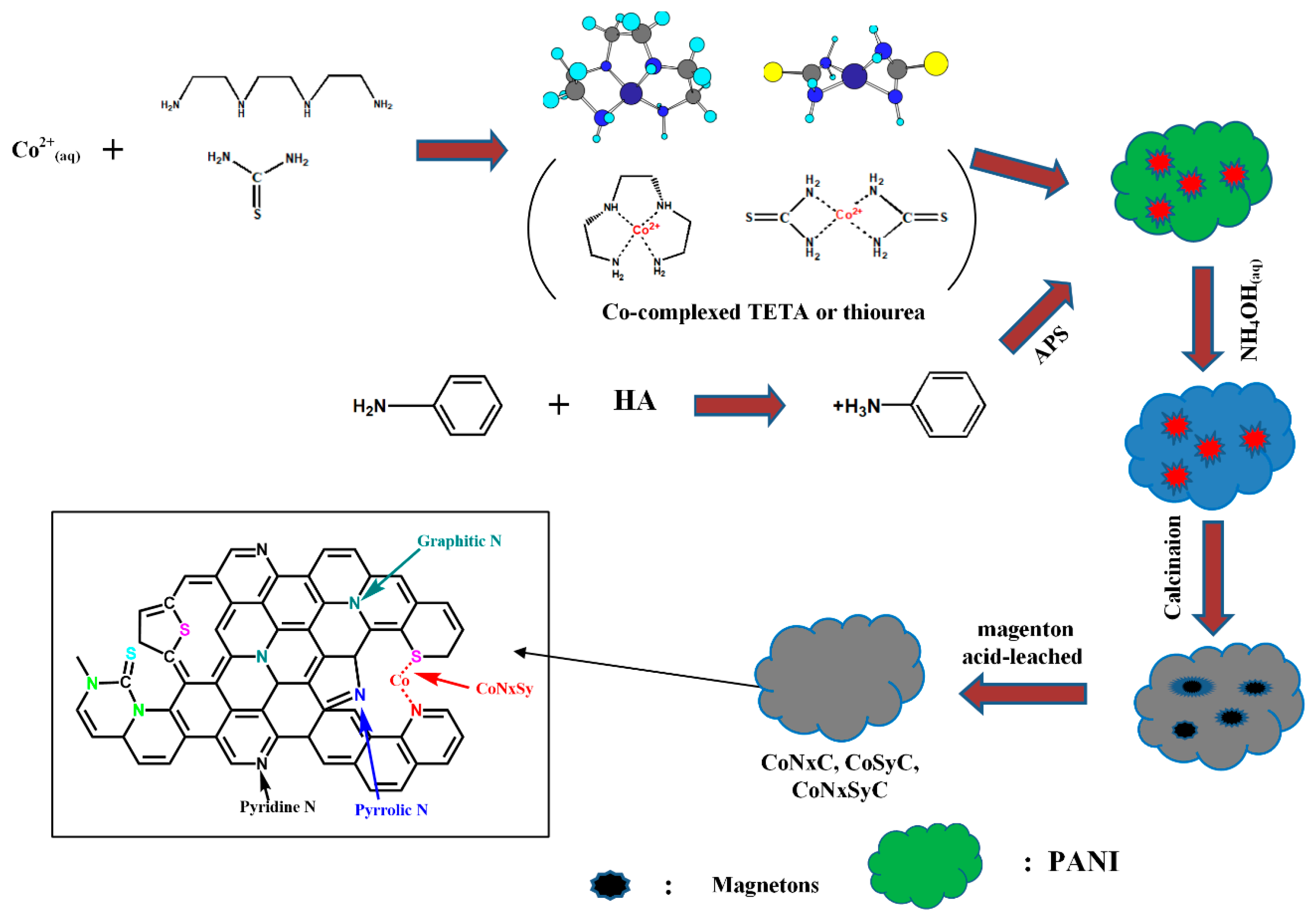
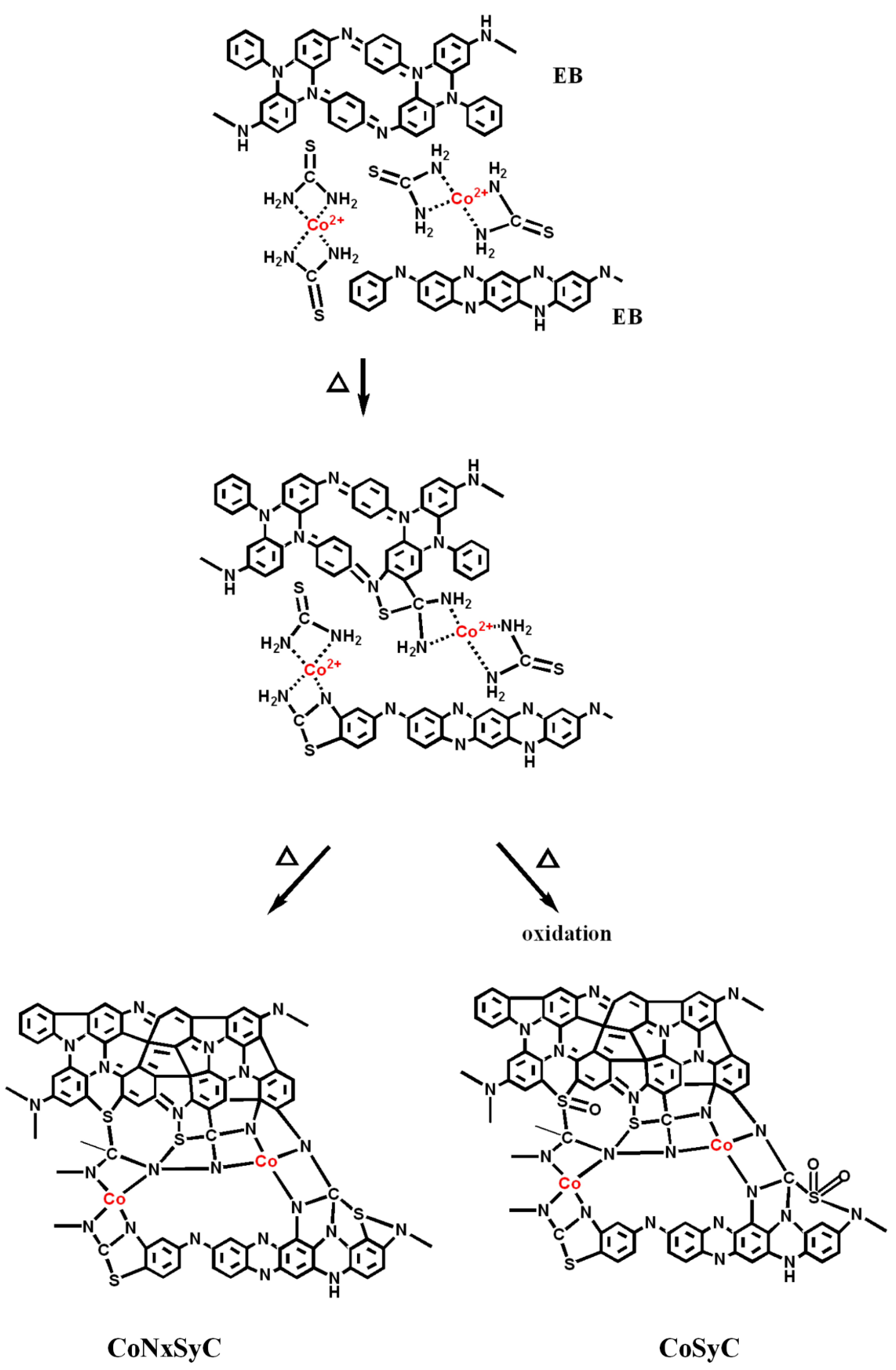
2.2. Preparation of CoNxC Catalyst
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. WXRD (Wide Angle X-ray Diffraction: Powder X-ray Diffraction)
2.6. Raman Spectroscopy
2.7. Scanning Electronic Microscopy (SEM)
2.8. Electrochemical Characterization
2.8.1. Linear Sweep Voltammetry (LSV)
2.8.2. Membrane Electrode Assemblies (MEA) Preparation
2.9. Single-Cell Performance Testing
3. Results and Discussion
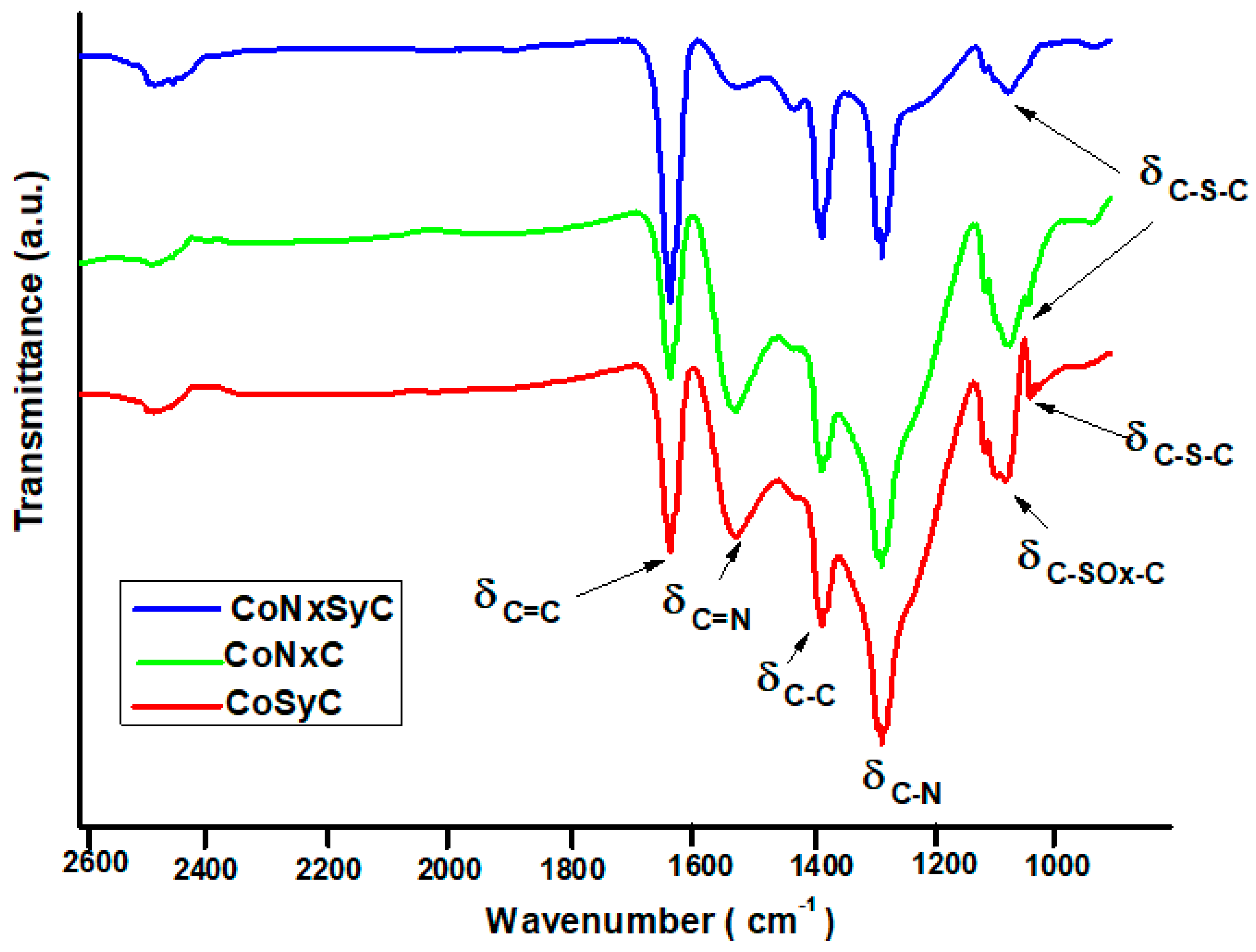
3.1. Fourier-Transform Infrared Spectroscopy (FTIR) Spectra
3.2. X-ray Photoelectron Spectroscopy (XPS)
3.3. Wide Angle X-ray Diffraction: Powder X-ray Diffraction (WXRD)
3.4. Raman Spectroscopy
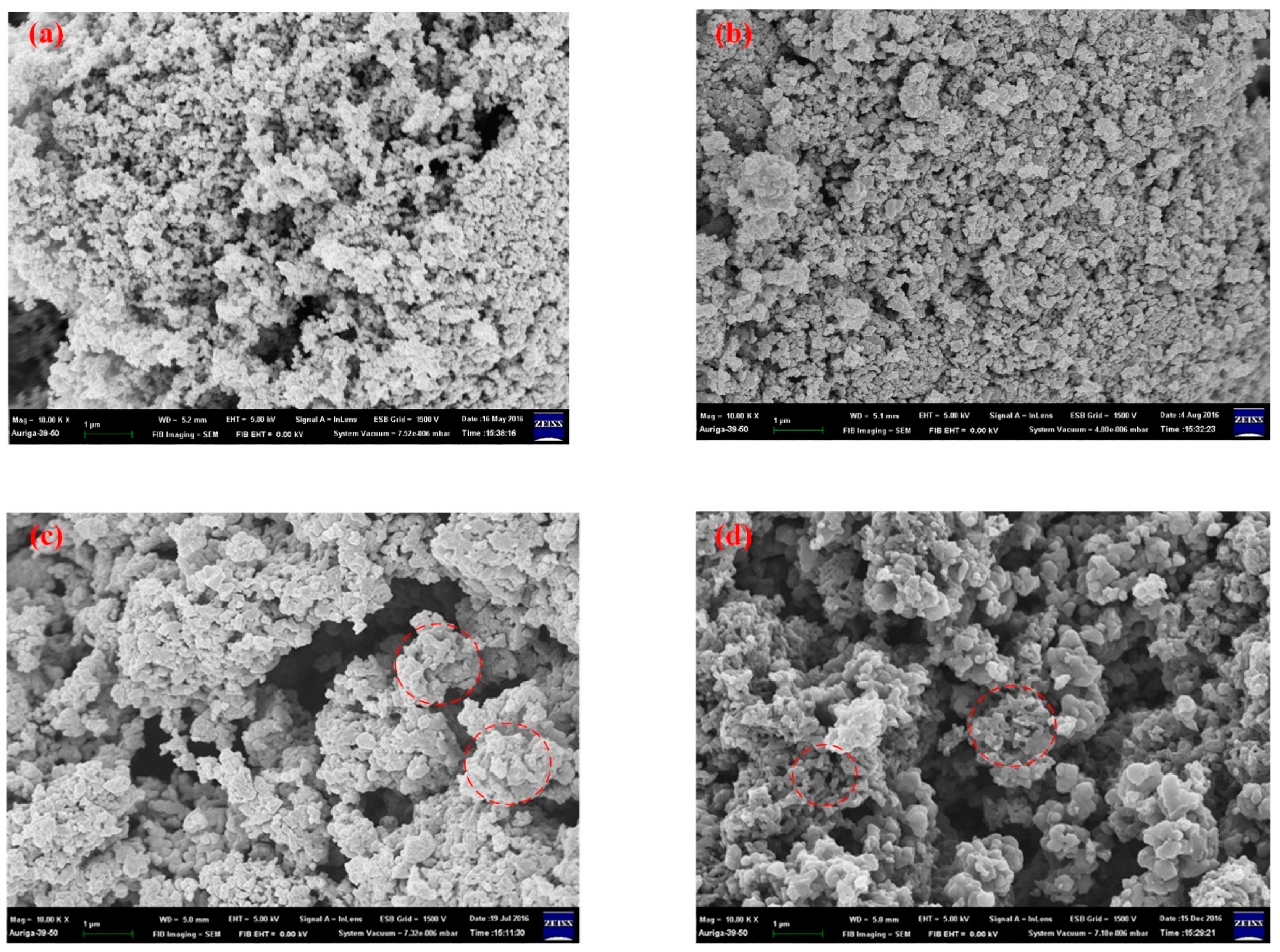
3.5. Scanning Electronic Microscopy (SEM)
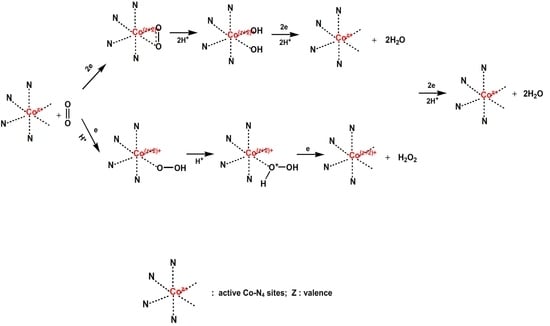
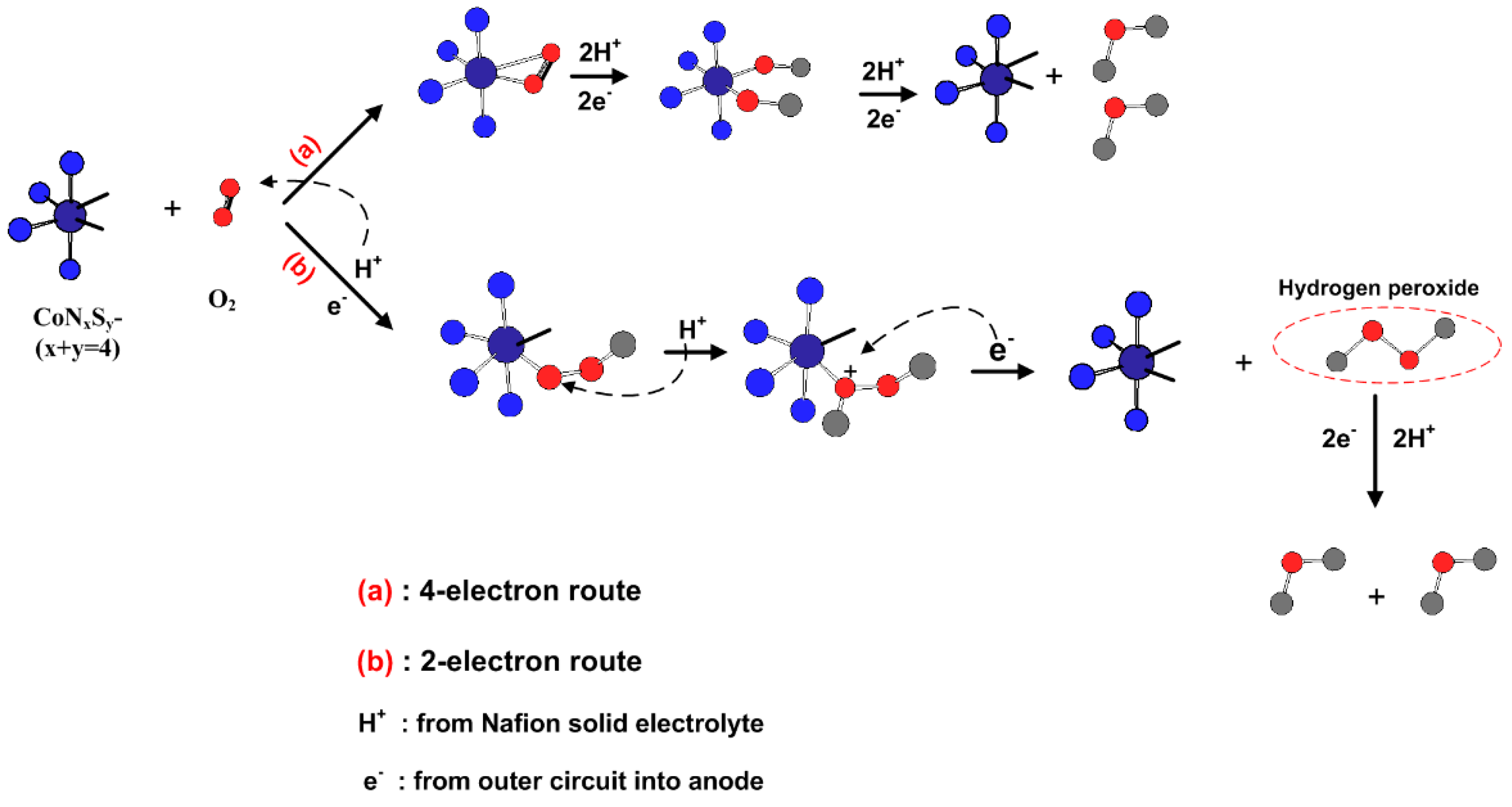
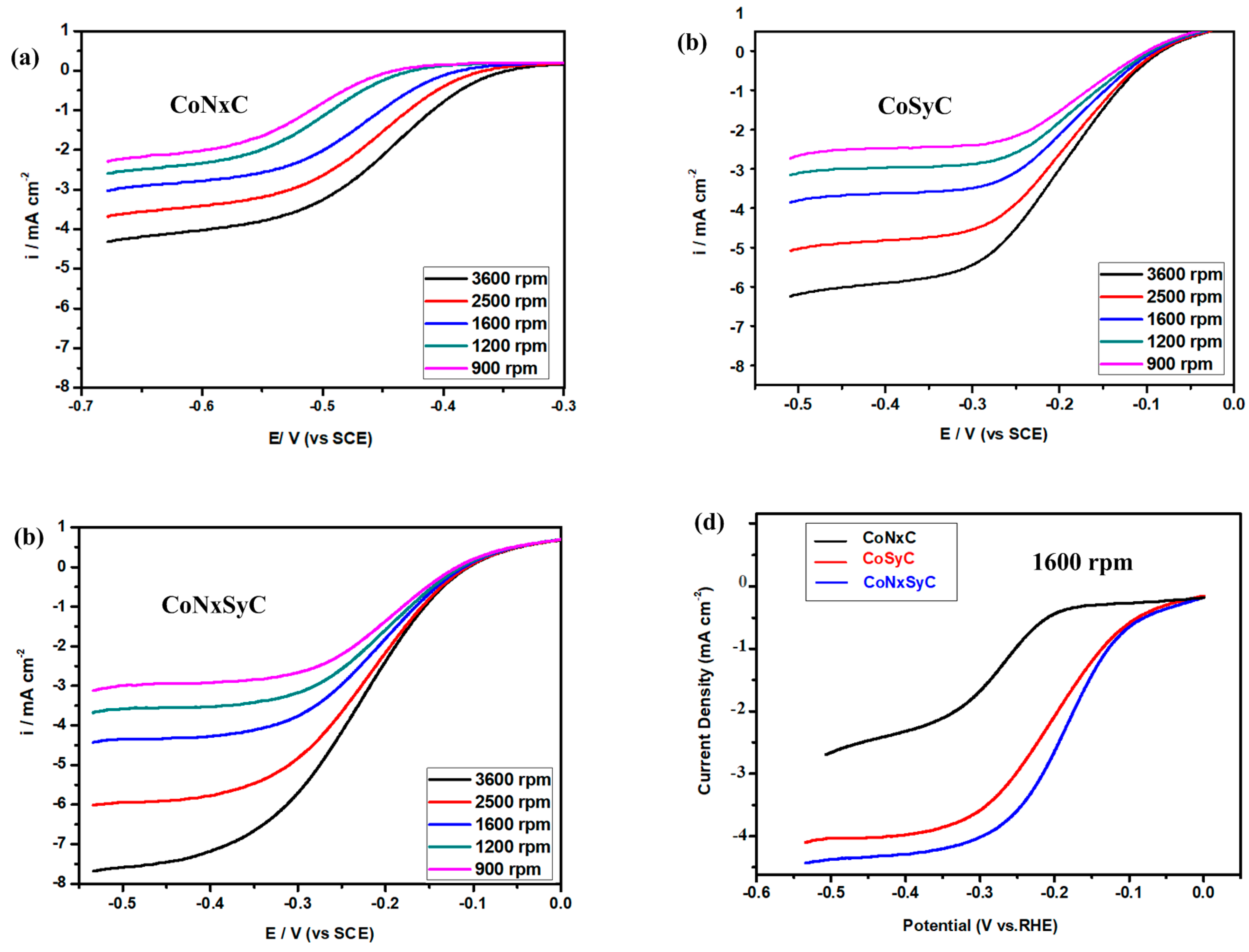
3.6. Oxygen Reduction Reaction (ORR) Performance
Linear Sweep Voltammetry (LSV) Curves
3.7. Single Cell Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du Nguyen, H.; Nguyen, T.T.L.; Nguyen, K.M.; Ha, T.H.; Nguyen, Q.H. Preparation of the vulcan XC-72R-supported Pt nanoparticles for the hydrogen evolution reaction in PEM water electrolysers. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 025012. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; He, L.; Zhang, Y.; Zhang, S.; Wang, L. Facile synthesis of Pt nanoparticles supported on graphene/Vulcan XC-72 carbon and their application for methanol oxidation. Ionics 2016, 23, 435–442. [Google Scholar] [CrossRef]
- Janošević, A.; Pašti, I.A.; Gavrilov, N.; Mentus, S.; Krstić, J.; Mitrić, M.; Travas-Sejdic, J.; Ćirić-Marjanović, G. Microporous conducting carbonized polyaniline nanorods: Synthesis, characterization and electrocatalytic properties. Microporous Mesoporous Mater. 2012, 152, 50–57. [Google Scholar] [CrossRef]
- Michel, M.; Ettingshausen, F.; Scheiba, F.; Wolz, A.; Roth, C. Using layer-by-layer assembly of polyaniline fibers in the fast preparation of high performance fuel cell nanostructured membrane electrodes. Phys. Chem. Chem. Phys. 2008, 10, 3796–3801. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Chang, M.-Y.; Wang, Y.-Z.; Huang, Y.-C.; Ho, K.-S.; Hsieh, T.-H.; Kuo, Y.-C. Polyaniline Based Pt-Electrocatalyst for a Proton Exchanged Membrane Fuel Cell. Polymers 2020, 12, 617. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Z.; Ko, T.-H.; Huang, W.-Y.; Hsieh, T.-H.; Ho, K.-S.; Chen, Y.-Y.; Hsieh, S.-J. Preparation of Pt-Catalyst by Poly(p-phenylenediamine) Nanocomposites Assisted by Microwave Radiation for Proton Exchange Membrane Fuel Cell. Polymers 2018, 10, 1388. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-J.; Hsieh, T.-H.; Wang, Y.-Z.; Ho, K.-S.; Chang, C.-Y. Microwave Assisted Reduction of Pt-Catalyst by N-Phenyl-p-Phenylenediamine for Proton Exchange Membrane Fuel Cells. Polymers 2017, 9, 104. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline, Iron, and Cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Zitolo, A.; Ranjbar-Sahraie, N.; Mineva, T.; Li, J.; Jia, Q.; Stamatin, S.; Harrington, G.F.; Lyth, S.M.; Krtil, P.; Mukerjee, S.; et al. Identification of catalytic sites in cobalt-nitrogen-carbon materials for the oxygen reduction reaction. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Liu, X.; Pu, Z.; Li, W.; Li, Q.; Zhang, J.; Tang, H.; Zhang, H.; Mu, S. From 3D ZIF Nanocrystals to Co-Nx/C Nanorod Array Electrocatalysts for ORR, OER and Zn-air Batteries. Adv. Funct. Mater. 2018, 28, 1704638. [Google Scholar]
- Kattel, S.; Atanassov, P.; Kiefer, B. Catalytic activity of Co-Nx/C electrocatalysts for oxygen reduction reaction: A density functional theory study. Phys. Chem. Chem. Phys. 2013, 15, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ye, D.; Huang, Q.-A.; Zhao, H.; Shao, Q.; Zhang, J. Pyrolyzed Co-Nx/C Electrocatalysts Supported on Different Carbon Materials for Oxygen Reduction Reaction in Neutral Solution. J. Electrochem. Soc. 2020, 167, 024509. [Google Scholar] [CrossRef]
- Xiao, C.; Luo, J.; Tan, M.; Xiao, Y.; Gao, B.; Zheng, Y.; Lin, B. Co/CoNx decorated nitrogen-doped porous carbon derived from melamine sponge as highly active oxygen electrocatalysts for zinc-air batteries. J. Power Sources 2020, 453, 227900. [Google Scholar]
- Zhang, H.-J.; Yuan, X.; Wen, W.; Zhang, N.-Y.; Sun, L.; Jiang, Q.-Z.; Ma, Z.-F. Electrochemical performance of a novel CoTETA/C catalyst for the oxygen reduction reaction. Electrochem. Commun. 2009, 11, 206–208. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Yuan, X.; Sun, L.; Zeng, X.; Jiang, Q.-Z.; Shao, Z.; Ma, Z.-F. Pyrolyzed CoN4-chelate as an electrocatalyst for oxygen reduction reaction in acid media. Int. J. Hydrogen Energy 2010, 35, 2900–2903. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Yuan, X.; Ma, Z.-F.; Wen, W.; Yang, J.; Magrasó, A.; Fontaine, M.-L.; Haugsrud, R.; Norby, T. Investigation of Non-Precious Metal CoN4-Based Oxygen Reduction Catalyst by Electrochemical and X-ray Absorption Spectroscopy Techniques. J. Electrochem. Soc. 2014, 161, H155–H160. [Google Scholar] [CrossRef]
- Fu, L.; Chen, Y.; Zhao, S.; Liu, Z.; Zhu, R. Sulfur-mediated synthesis of N-doped carbon supported cobalt catalysts derived from cobalt porphyrin for ethylbenzene oxidation. RSC Adv. 2016, 6, 19482–19491. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S. Sulfur and Nitrogen Dual-Doped Mesoporous Graphene Electrocatalyst for Oxygen Reduction with Synergistically Enhanced Performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Wu, Z.; Wang, S.; Wu, Y.; Liu, X. Heteroatom (Nitrogen/Sulfur)-Doped Graphene as an Efficient Electrocatalyst for Oxygen Reduction and Evolution Reactions. Catalysts 2018, 8, 475. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Li, R.; Ma, G.; Gou, X.; Zhu, Y.; Xia, Y. Cobalt sulfide/N, S Co-doped porous carbon core-shell nanocomposites as superior bifunctional electrocatalysts for oxygen reduction and evolution reactions. Nanoscale 2015, 7, 20674–20684. [Google Scholar]
- Qiu, X.; Yu, Y.; Peng, Z.; Asif, M.; Wang, Z.; Jiang, L.; Wang, W.; Xu, Z.; Wang, H.; Liu, H. Cobalt sulfides nanoparticles encapsulated in N, S co-doped carbon substrate for highly efficient oxygen reduction. J. Alloys Compd. 2020, 815, 152457. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.-P.; Wu, G.; Chung, H.T.; Johnston, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuelcells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Park, S.-J.; Jang, Y.-S. Preparation and characterization of activated carbon fibers supported with silver metal for antibacterial behavior. J. Colloid Interface Sci. 2003, 261, 238–243. [Google Scholar] [CrossRef]
- Bikshapathi, M.; Mandal, S.; Mathur, G.N.; Sharma, A.; Verma, N. Modification of Activated Carbon Fiber by Metal Dispersion and Surface Functionalization for the Removal of 2-Chloroethanol. Ind. Eng. Chem. Res. 2011, 50, 13092–13104. [Google Scholar] [CrossRef]
- Wang, H.; Da, H.; Ji, S.; Liao, S.; Wang, R. Selenium-Functionalized Carbon as a Support for Platinum Nanoparticles with Improved Electrochemical Properties for the Oxygen Reduction Reaction and CO Tolerance. J. Electrochem. Soc. 2013, 160, H266–H270. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-J.; Bera, S.; Kim, C.M.; Koh, E.-K.; Hong, W.-P.; Oh, S.-J.; Cho, E.; Kwon, S.H. Synthesis of highly dispersed Pt nanoparticles into carbon supports by fluidized bed reactor atomic layer deposition to boost PEMFC performance. NPG Asia Mater. 2020, 12, 1–13. [Google Scholar] [CrossRef]

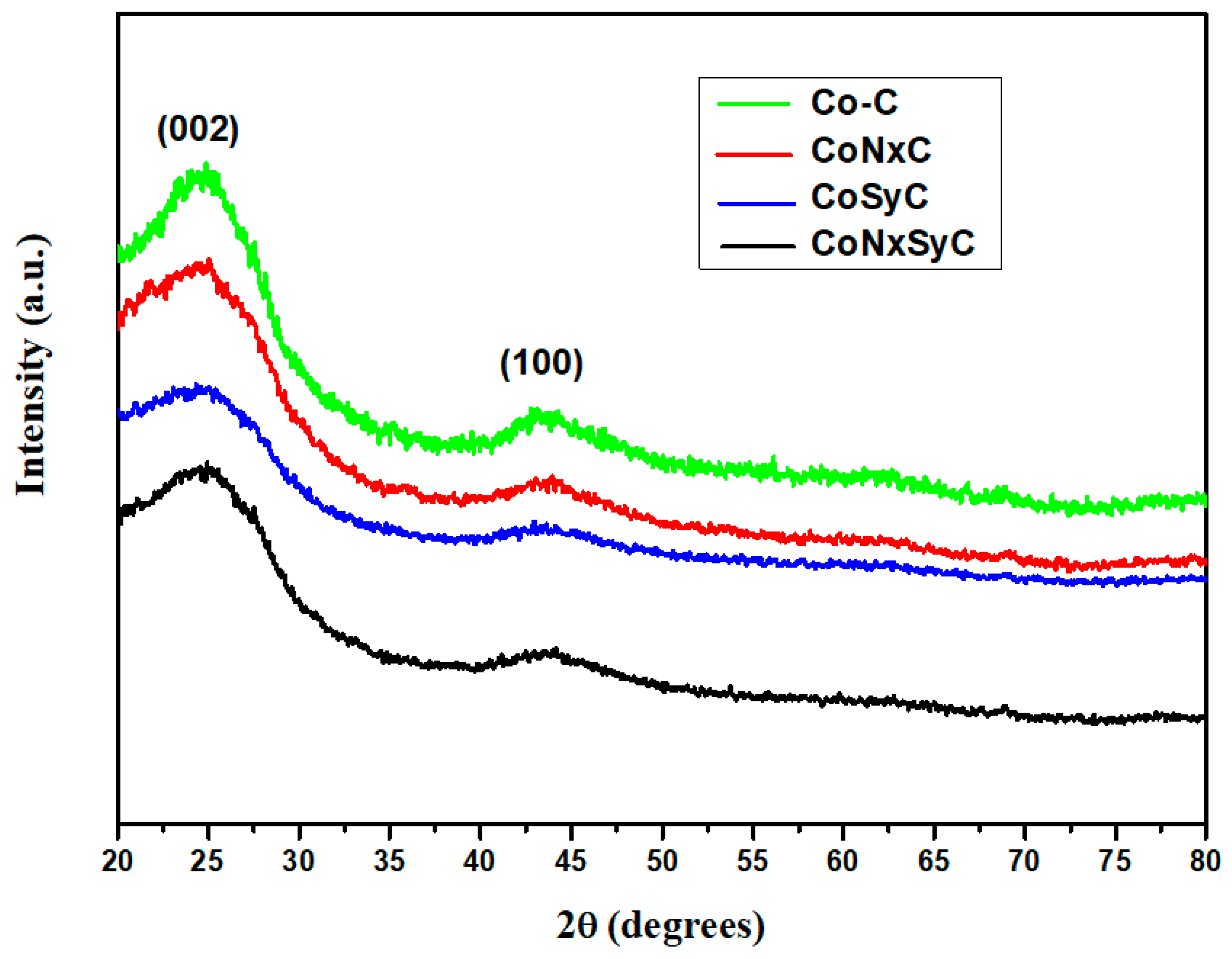


| I100/I002 (a) | ID/IG (b) | |
|---|---|---|
| Co–C | 0.459 | 0.970 |
| CoNxC | 0.432 | 0.973 |
| CoSyC | 0.427 | 0.978 |
| CoNxSyC | 0.412 | 0.987 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-Y.; Jheng, L.-C.; Hsieh, T.-H.; Ho, K.-S.; Wang, Y.-Z.; Gao, Y.-J.; Tseng, P.-H. Calcined Co(II)-Triethylenetetramine, Co(II)- Polyaniline-Thiourea as the Cathode Catalyst of Proton Exchanged Membrane Fuel Cell. Polymers 2020, 12, 3070. https://doi.org/10.3390/polym12123070
Huang W-Y, Jheng L-C, Hsieh T-H, Ho K-S, Wang Y-Z, Gao Y-J, Tseng P-H. Calcined Co(II)-Triethylenetetramine, Co(II)- Polyaniline-Thiourea as the Cathode Catalyst of Proton Exchanged Membrane Fuel Cell. Polymers. 2020; 12(12):3070. https://doi.org/10.3390/polym12123070
Chicago/Turabian StyleHuang, Wen-Yao, Li-Cheng Jheng, Tar-Hwa Hsieh, Ko-Shan Ho, Yen-Zen Wang, Yi-Jhun Gao, and Po-Hao Tseng. 2020. "Calcined Co(II)-Triethylenetetramine, Co(II)- Polyaniline-Thiourea as the Cathode Catalyst of Proton Exchanged Membrane Fuel Cell" Polymers 12, no. 12: 3070. https://doi.org/10.3390/polym12123070