Synthesis of Modified Starch/Polyvinyl Alcohol Composite for Treating Textile Wastewater
Abstract
1. Introduction
2. Materials and Methods
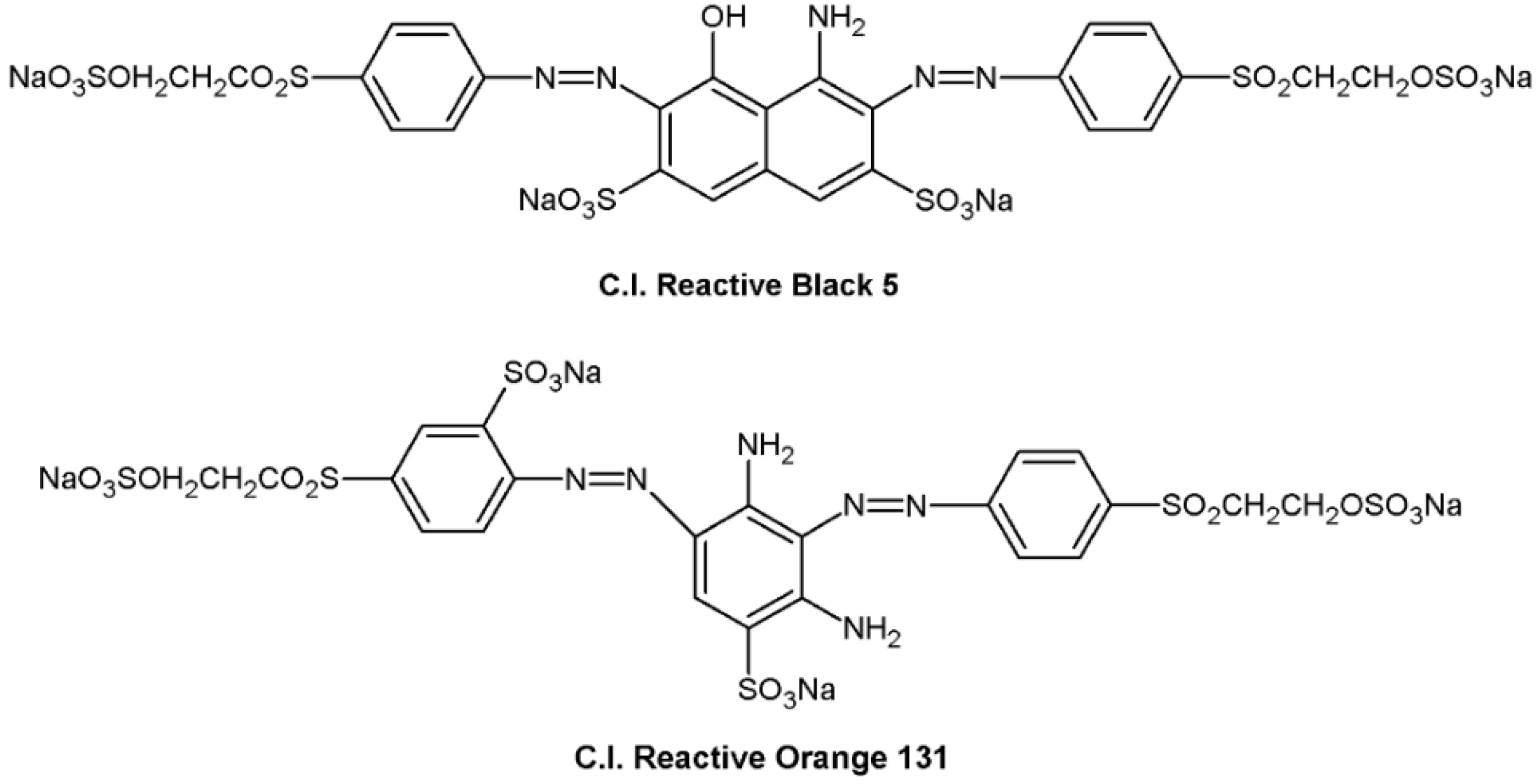
2.1. Materials
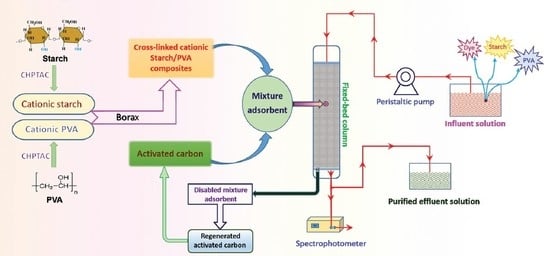
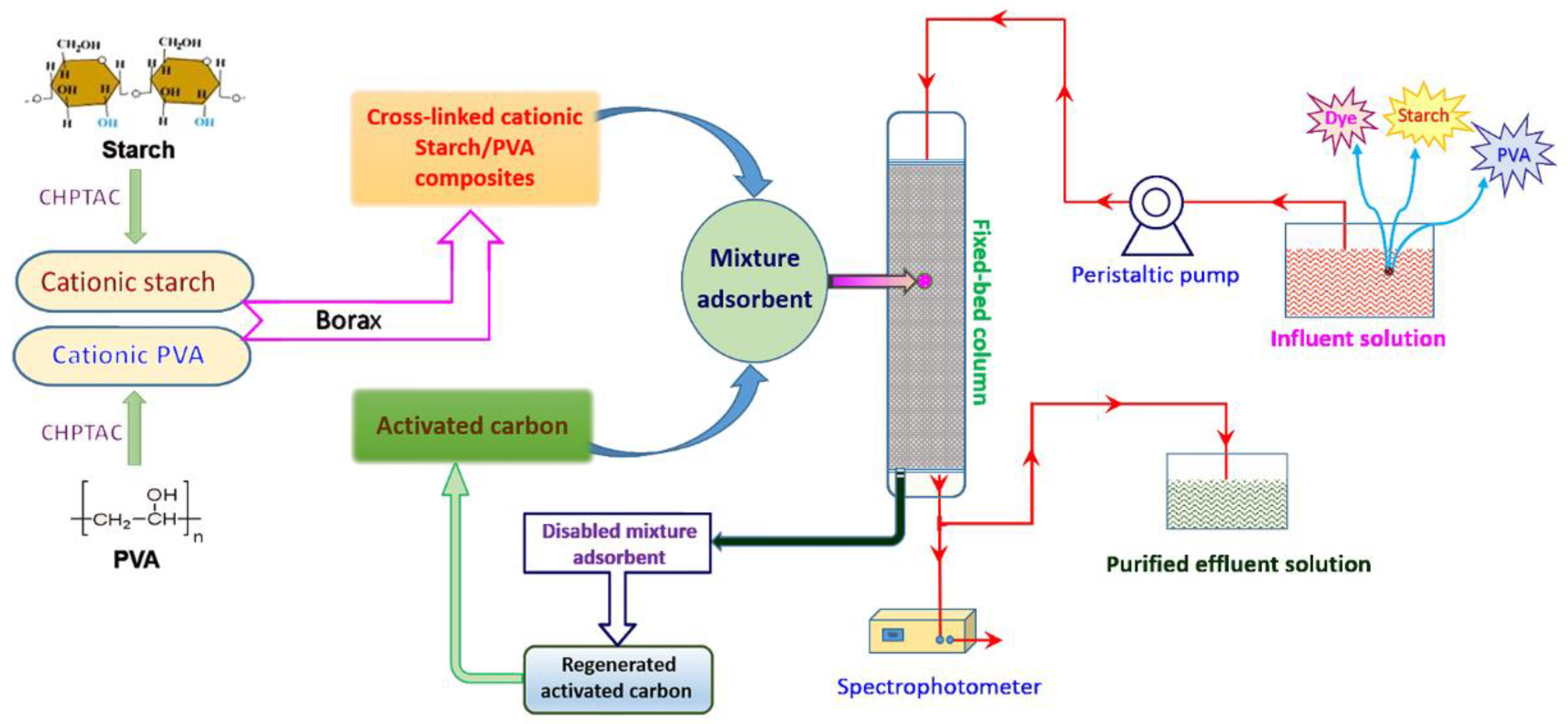
2.2. Preparation of Modified Starch/PVA Composites
2.3. Characterization
2.4. Adsorption Studies
2.5. The Recycle of Disabled Adsorbent
3. Results
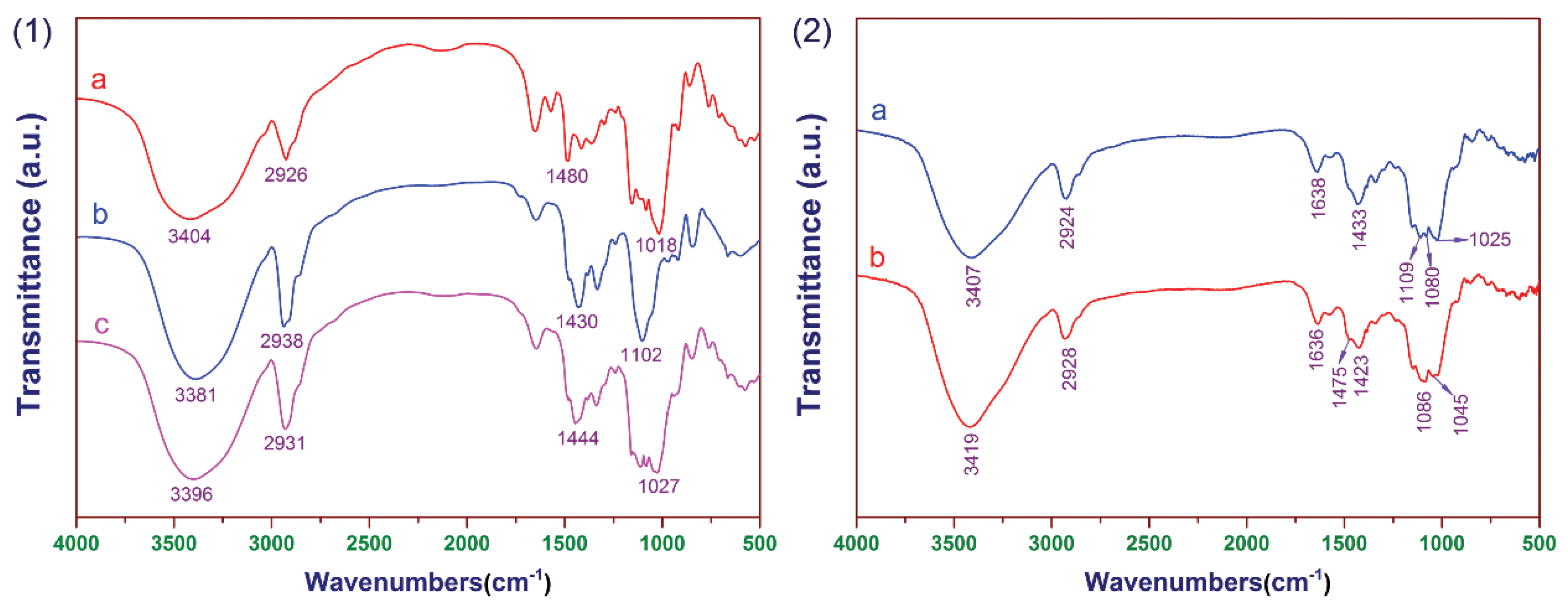
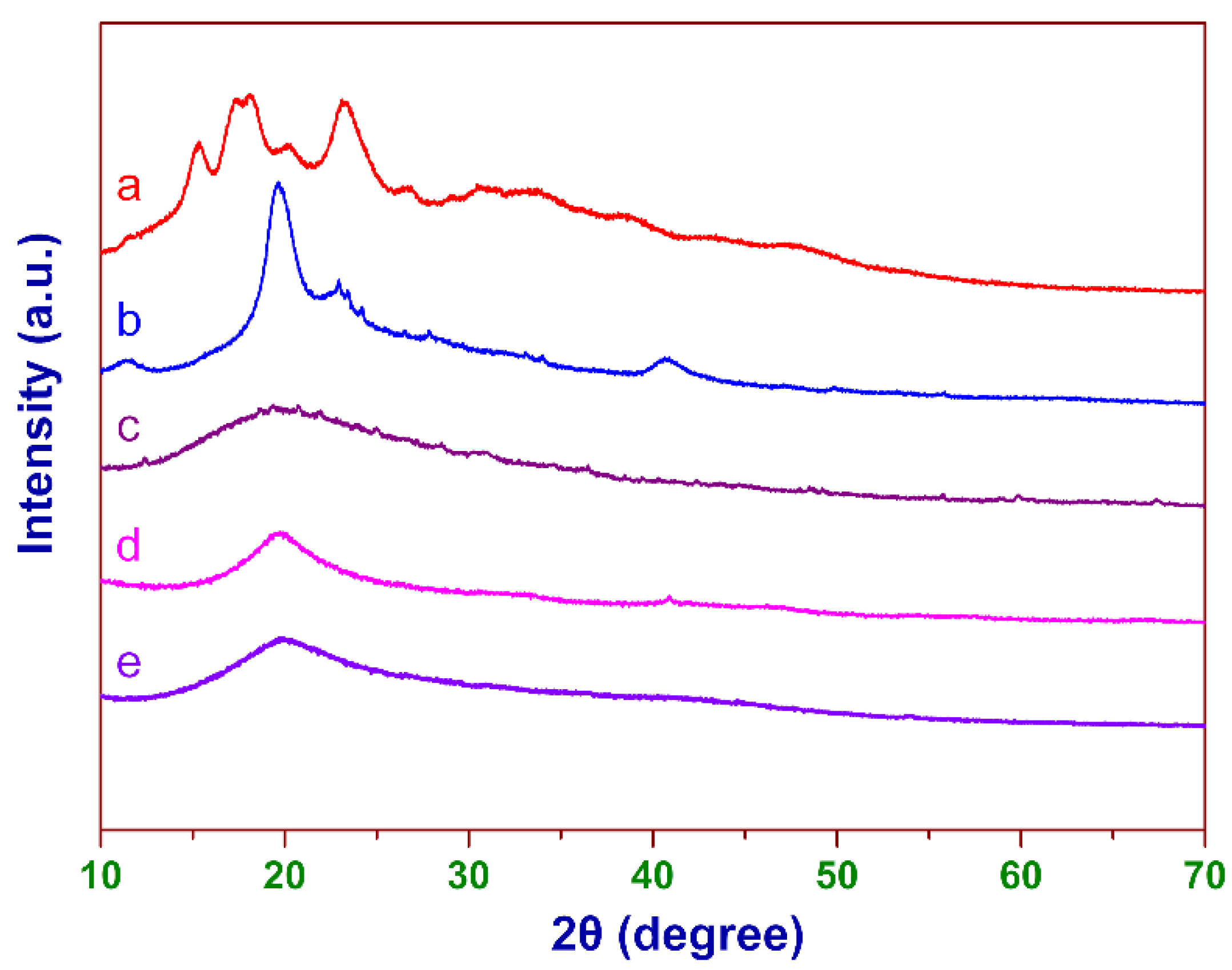
3.1. FT-IR Analysis
3.2. XRD Analysis
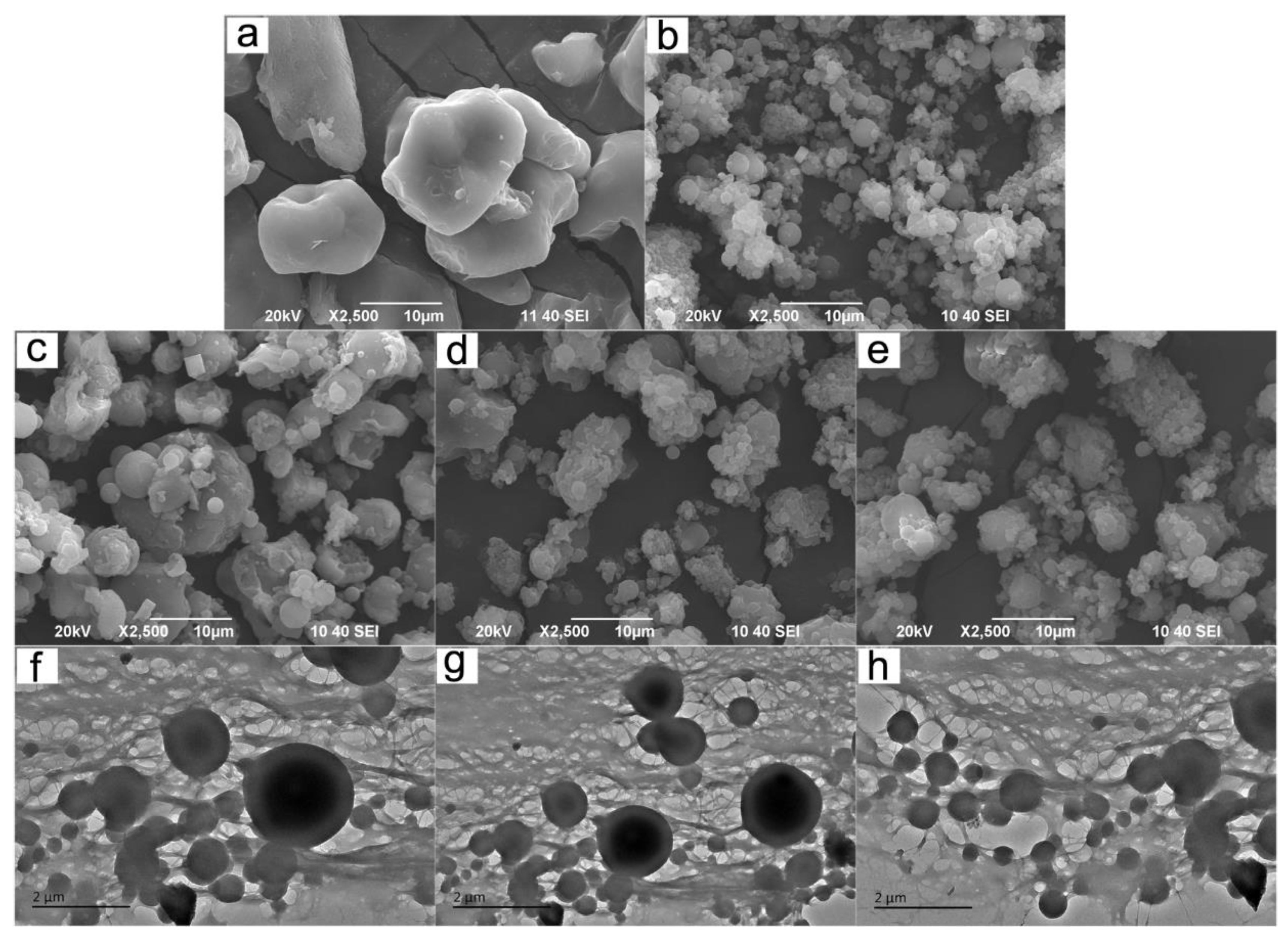
3.3. SEM and TEM Analysis
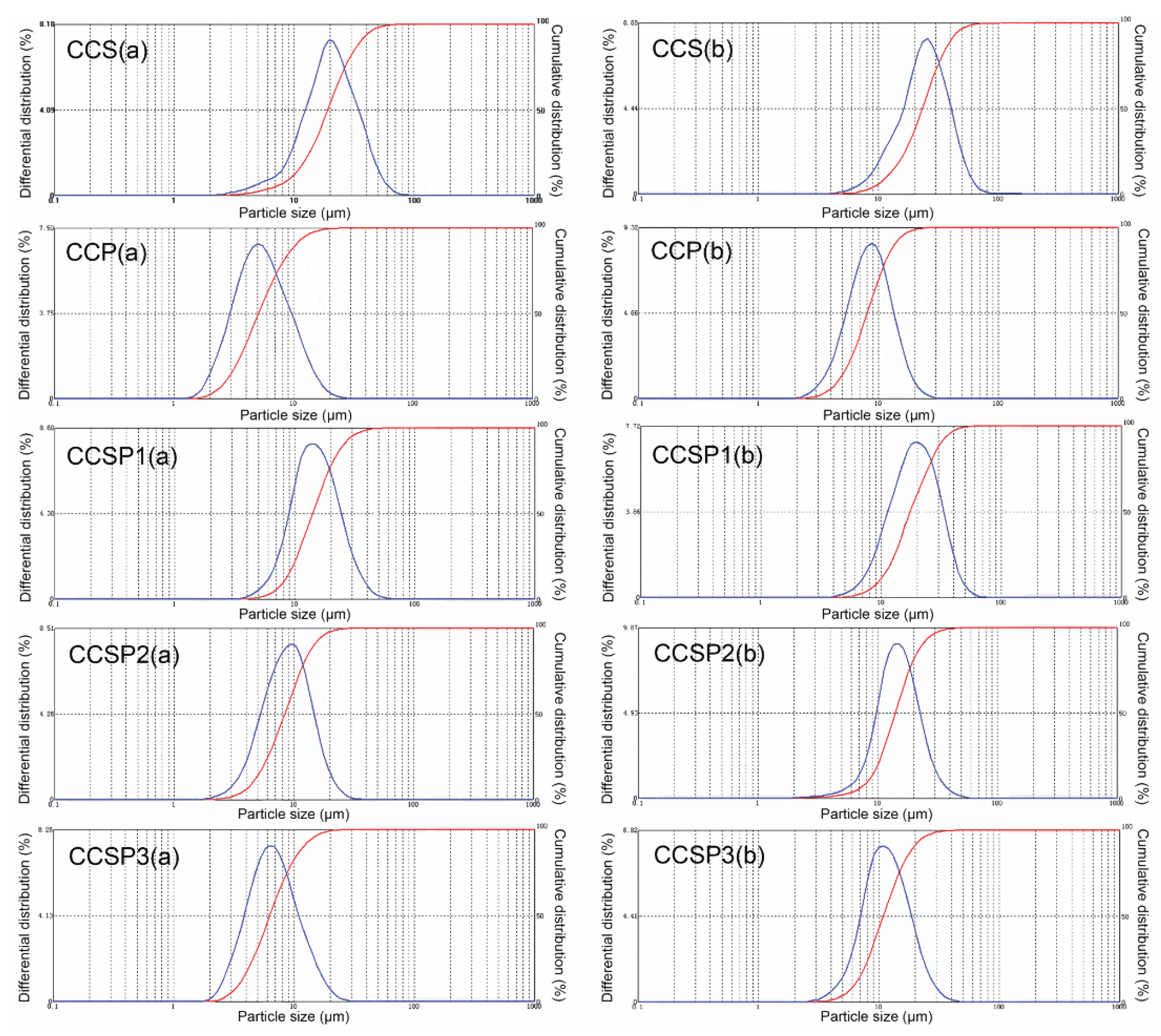
3.4. Particle Size Analysis
3.5. Application in Wastewater Treatment
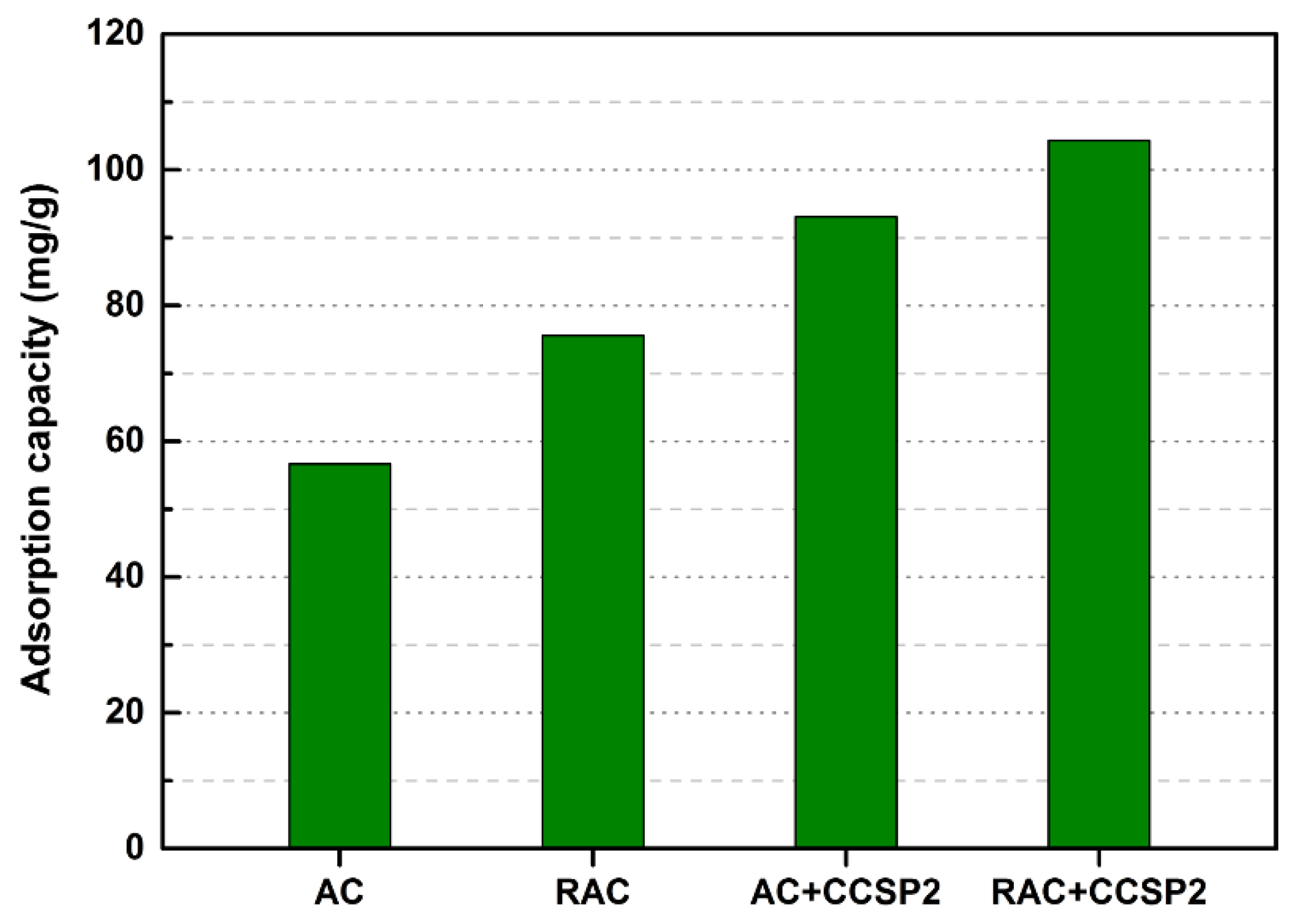
3.6. The Recycle of Disabled Adsorbent
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mirzaie, M.; Rashidi, A.; Tayebi, H.A.; Yazdanshenas, M.E. Optimized removal of acid blue 62 from textile waste water by SBA-15/PAMAM dendrimer hybrid using response surface methodology. J. Polym. Environ. 2018, 26, 1831–1843. [Google Scholar] [CrossRef]
- Pekakis, P.A.; Xekoukoulotakis, N.P.; Mantzavinos, D. Treatment of textile dyehouse wastewater by TiO2 photocatalysis. Water Res. 2006, 40, 1276–1286. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Liu, R.R.; Lu, X.J.; Tian, Q.; Yang, B.; Chen, J.H. The performance evaluation of hybrid anaerobic baffled reactor for treatment of PVA-containing desizing wastewater. Desalination 2011, 271, 287–294. [Google Scholar]
- Sun, W.H.; Chen, J.; Chen, L.J.; Wang, J.L.; Zhang, Y.M. Coupled electron beam radiation and MBR treatment of textile wastewater containing polyvinyl alcohol. Chemosphere 2016, 155, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palácio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Jegatheesan, V.; Pramanik, B.K.; Chen, J.; Navaratna, D.; Chang, C.Y.; Shu, L. Treatment of textile wastewater with membrane bioreactor: A critical review. Bioresour. Technol. 2016, 204, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Bessegato, G.G.; Zanoni, M.V.B. Efficiency comparison of ozonation, photolysis, photocatalysis and photoelectrocatalysis methods in real textile wastewater decolorization. Water Res. 2016, 98, 39–46. [Google Scholar] [CrossRef] [PubMed]
- He, P.Y.; Zhang, Y.J.; Chen, H.; Liu, L.C. Development of an eco-efficient CaMoO4/electroconductive geopolymer composite for recycling silicomanganese slag and degradation of dye wastewater. J. Clean. Prod. 2019, 208, 1476–1487. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Hsu, L.J.; Lee, L.T.; Lin, C.C. Adsorption and photocatalytic degradation of polyvinyl alcohol in aqueous solutions using P-25 TiO2. Chem. Eng. J. 2011, 173, 698–705. [Google Scholar] [CrossRef]
- Ye, B.; Li, Y.; Chen, Z.; Wu, Q.Y.; Wang, W.L.; Wang, T.; Hu, H.Y. Degradation of polyvinyl alcohol (PVA) by UV/chlorine oxidation: Radical roles, influencing factors, and degradation pathway. Water Res. 2017, 124, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Marušincová, H.; Husárová, L.; Růžička, J.; Ingr, M.; Navrátil, V.; Buňková, L.; Koutny, M. Polyvinyl alcohol biodegradation under denitrifying conditions. Int. Biodeterior. Biodegrad. 2013, 84, 21–28. [Google Scholar] [CrossRef]
- Paz, A.; Carballo, J.; Pérez, M.J.; Domínguez, J.M. Biological treatment of model dyes and textile wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, K.; Liu, X.; Chen, Z.; Du, H.; Zhang, X. Synthesis of cationic-modified silica gel and its adsorption properties for anionic dyes. J. Taiwan Inst. Chem. Eng. 2019, 102, 1–8. [Google Scholar] [CrossRef]
- Xia, L.; Li, C.; Zhou, S.; Fu, Z.; Wang, Y.; Lyu, P.; Zhang, J.; Liu, X.; Zhang, C.; Xu, W. Utilization of waste leather powders for highly effective removal of dyes from water. Polymers 2019, 11, 1786. [Google Scholar] [CrossRef] [PubMed]
- Afshari, M.; Dinari, M. Synthesis of new imine-linked covalent organic framework as high efficient absorbent and monitoring the removal of direct fast scarlet 4BS textile dye based on mobile phone colorimetric platform. J. Hazard. Mater. 2020, 385, 121514. [Google Scholar] [CrossRef]
- Saeed, A.; Sharif, M.; Iqbal, M. Application potential of grapefruit peel as dye sorbent: Kinetics, equilibrium and mechanism of crystal violet adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar] [CrossRef]
- Jain, M.; Garg, V.K.; Kadirvelu, K.; Sillanpää, M. Combined effect of sunflower stem carbon-calcium alginate beads for the removal and recovery of chromium from contaminated water in column mode. Ind. Eng. Chem. Res. 2015, 54, 1419–1425. [Google Scholar] [CrossRef]
- Zhao, L.; Basly, J.P.; Baudu, M. Macroporous alginate/ferrihydrite hybrid beads used to remove anionic dye in batch and fixed-bed reactors. J. Taiwan Inst. Chem. Eng. 2017, 74, 129–135. [Google Scholar] [CrossRef]
- Jain, M.; Garg, V.K.; Kadirvelu, K. Cadmium (II) sorption and desorption in a fixed bed column using sunflower waste carbon calcium-alginate beads. Bioresour. Technol. 2013, 129, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kurade, M.B.; Waghmode, T.R.; Xiong, J.Q.; Govindwar, S.P.; Jeon, B.H. Decolorization of textile industry effluent using immobilized consortium cells in upflow fixed bed reactor. J. Clean. Prod. 2019, 213, 884–891. [Google Scholar] [CrossRef]
- Demarchi, C.A.; Campos, M.; Rodrigues, C.A. Adsorption of textile dye Reactive Red 120 by the chitosan-Fe (III)-crosslinked: Batch and fixed-bed studies. J. Environ. Chem. Eng. 2013, 1, 1350–1358. [Google Scholar] [CrossRef]
- Faki, A.; Turan, M.; Ozdemir, O.; Turan, A.Z. Analysis of fixed-bed column adsorption of reactive yellow 176 onto surfactant-modified zeolite. Ind. Eng. Chem. Res. 2008, 47, 6999–7004. [Google Scholar] [CrossRef]
- Gong, J.L.; Zhang, Y.L.; Jiang, Y.; Zeng, G.M.; Cui, Z.H.; Liu, K.; Deng, C.H.; Niu, Q.Y.; Deng, J.H.; Huan, S.Y. Continuous adsorption of Pb (II) and methylene blue by engineered graphite oxide coated sand in fixed-bed column. Appl. Surf. Sci. 2015, 330, 148–157. [Google Scholar] [CrossRef]
- Gomez, V.; Larrechi, M.S.; Callao, M.P. Kinetic and adsorption study of acid dye removal using activated carbon. Chemosphere 2007, 69, 1151–1158. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Salehi, R.; Arami, M. Binary system dye removal from colored textile wastewater using activated carbon: Kinetic and isotherm studies. Desalination 2011, 272, 187–195. [Google Scholar] [CrossRef]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.T.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Garg, V.K.; Sillanpää, M. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr (VI), Cu (II) and Cd (II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.E.; Ciocan, L.T. Progress in hydroxyapatite-starch based sustainable biomaterials for biomedical bone substitution applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of starch composition and molecular weight on physicochemical properties of biodegradable films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Mal, D.; Singh, R.P. Cationic starch: An effective flocculating agent. Carbohydr. Polym. 2005, 59, 417–423. [Google Scholar] [CrossRef]
- Prado, H.J.; Matulewicz, M.C. Cationization of polysaccharides: A path to greener derivatives with many industrial applications. Eur. Polym. J. 2014, 52, 53–75. [Google Scholar] [CrossRef]
- Simanaviciute, D.; Klimaviciute, R.; Rutkaite, R. Equilibrium adsorption of caffeic, chlorogenic and rosmarinic acids on cationic cross-linked starch with quaternary ammonium groups. Int. J. Biol. Macromol. 2017, 95, 788–795. [Google Scholar] [CrossRef]
- Koriche, Y.; Darder, M.; Aranda, P.; Semsari, S.; Ruiz-Hitzky, E. Bionanocomposites based on layered silicates and cationic starch as eco-friendly adsorbents for hexavalent chromium removal. Dalton Trans. 2014, 43, 10512–10520. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Li, A.; Yang, H. Evaluation of starch-based flocculants for the flocculation of dissolved organic matter from textile dyeing secondary wastewater. Chemosphere 2017, 174, 200–207. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, B.; He, M.; Yin, G.; Cui, Y.; Savina, I. Keratin/Polyvinyl alcohol blend films cross-linked by dialdehyde starch and their potential application for drug release. Polymers 2015, 7, 580–591. [Google Scholar] [CrossRef]
- Liu, X.; Fatehi, P.; Ni, Y.; Xiao, H. Using cationic polyvinyl alcohol (C-PVA) to improve the strength of wood-free papers containing high-yield pulp (HYP). Holzforschung 2010, 64, 563–569. [Google Scholar] [CrossRef]
- Santos, A.; de Oliveira, F.W.F.; Silva, F.H.A.; Maria, D.A.; Ardisson, J.D.; de Almeida Macêdo, W.A.; Palmieri, H.E.L.; Franco, M.B. Synthesis and characterization of iron-PVA hydrogel microspheres and their use in the arsenic (V) removal from aqueous solution. Chem. Eng. J. 2012, 210, 432–443. [Google Scholar] [CrossRef]
- Fatehi, P.; Xiao, H. Effect of cationic PVA characteristics on fiber and paper properties at saturation level of polymer adsorption. Carbohydr. Polym. 2010, 79, 423–428. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Y.; Chen, Z.; Liu, D.; Yu, E.; Zhang, X.; Fu, H.; Fu, J.; Zhang, J.; Du, H. Amorphous molybdenum sulfide nanocatalysts simultaneously realizing efficient upgrading of residue and synergistic synthesis of 2D MoS2 nanosheets/carbon hierarchical structures. Green Chem. 2020, 22, 44–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Li, W. Effect of particle size on removal of sunset yellow from aqueous solution by chitosan modified diatomite in a fixed bed column. RSC Adv. 2015, 5, 85673–85681. [Google Scholar] [CrossRef]
- Sudaryanto, Y.; Hartono, S.B.; Irawaty, W.; Hindarso, H.; Ismadji, S. High surface area activated carbon prepared from cassava peel by chemical activation. Bioresour. Technol. 2006, 97, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhang, X.; Ma, J.; Sun, D.; Zhang, B.; Luan, J. Eco-Friendly cationic modification of cotton fabrics for improving utilization of reactive dyes. RSC Adv. 2015, 57, 45654–45661. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, W. Synthesis of cationic starch with a high degree of substitution in an ionic liquid. Carbohydr. Polym. 2010, 80, 1172–1177. [Google Scholar] [CrossRef]
- Klimaviciute, R.; Riauka, A.; Zemaitaitis, A. The binding of anionic dyes by cross-linked cationic starches. J. Polym. Res. 2007, 14, 67–73. [Google Scholar] [CrossRef]
- Shen, F.; Liu, J.; Dong, Y.; Wu, D. Mercury removal by biomass-derived porous carbon: Experimental and theoretical insights into the effect of H2S. Chem. Eng. J. 2018, 348, 409–415. [Google Scholar] [CrossRef]
- Zhang, S.J.; Yu, H.Q.; Feng, H.M. PVA-based activated carbon fibers with lotus root-like axially porous structure. Carbon 2006, 44, 2059–2068. [Google Scholar] [CrossRef]


| Simulated Wastewater Solutions | The Adsorption Capacity (mg/g) | ||||||
|---|---|---|---|---|---|---|---|
| CCS | CCP | CCSP1 | CCSP2 | CCSP3 | AC | Mixture Adsorbent (CCSP2 and AC) | |
| Reactive Black 5 | 572.0 | 693.5 | 605.0 | 629.5 | 661.0 | 54.0 | 378.0 |
| Reactive Orange 131 | 494.0 | 605.5 | 539.0 | 560.0 | 573.5 | 48.0 | 340.5 |
| Starch solution | 12.4 | 9.3 | 11.4 | 10.6 | 9.9 | 16.2 | 14.4 |
| PVA solution | 7.7 | 16.2 | 10.1 | 13.4 | 15.1 | 19.6 | 17.7 |
| Textile wastewater | 40.6 | 52.5 | 68.6 | 73.5 | 60.9 | 56.7 | 93.1 |
| Adsorbents | Zeta Potential (mV) |
|---|---|
| CCS | 20.3 |
| CCP | 30.4 |
| CCSP1 | 22.7 |
| CCSP2 | 26.2 |
| CCSP3 | 28.1 |
| Bed Height (cm) | Flow Rate (mL/min) | The Adsorption Capacity (mg/g) |
|---|---|---|
| 9.5 (10g) | 2 | 93.1 |
| 7.6 (8g) | 2 | 88.9 |
| 5.7 (6g) | 2 | 80.5 |
| 9.5 (10g) | 3 | 86.8 |
| 9.5 (10g) | 4 | 77.0 |
| Characterization | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| AC | 386 | 0.39 | 4.12 |
| RAC | 581 | 0.53 | 3.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, K.; Liu, X.; Wang, W.; Yang, X.; Zhang, X. Synthesis of Modified Starch/Polyvinyl Alcohol Composite for Treating Textile Wastewater. Polymers 2020, 12, 289. https://doi.org/10.3390/polym12020289
Xia K, Liu X, Wang W, Yang X, Zhang X. Synthesis of Modified Starch/Polyvinyl Alcohol Composite for Treating Textile Wastewater. Polymers. 2020; 12(2):289. https://doi.org/10.3390/polym12020289
Chicago/Turabian StyleXia, Kai, Xin Liu, Weiwei Wang, Xizi Yang, and Xiaodong Zhang. 2020. "Synthesis of Modified Starch/Polyvinyl Alcohol Composite for Treating Textile Wastewater" Polymers 12, no. 2: 289. https://doi.org/10.3390/polym12020289
APA StyleXia, K., Liu, X., Wang, W., Yang, X., & Zhang, X. (2020). Synthesis of Modified Starch/Polyvinyl Alcohol Composite for Treating Textile Wastewater. Polymers, 12(2), 289. https://doi.org/10.3390/polym12020289