The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation
Abstract
:1. PEGylation in Bioconjugation and Drug Delivery
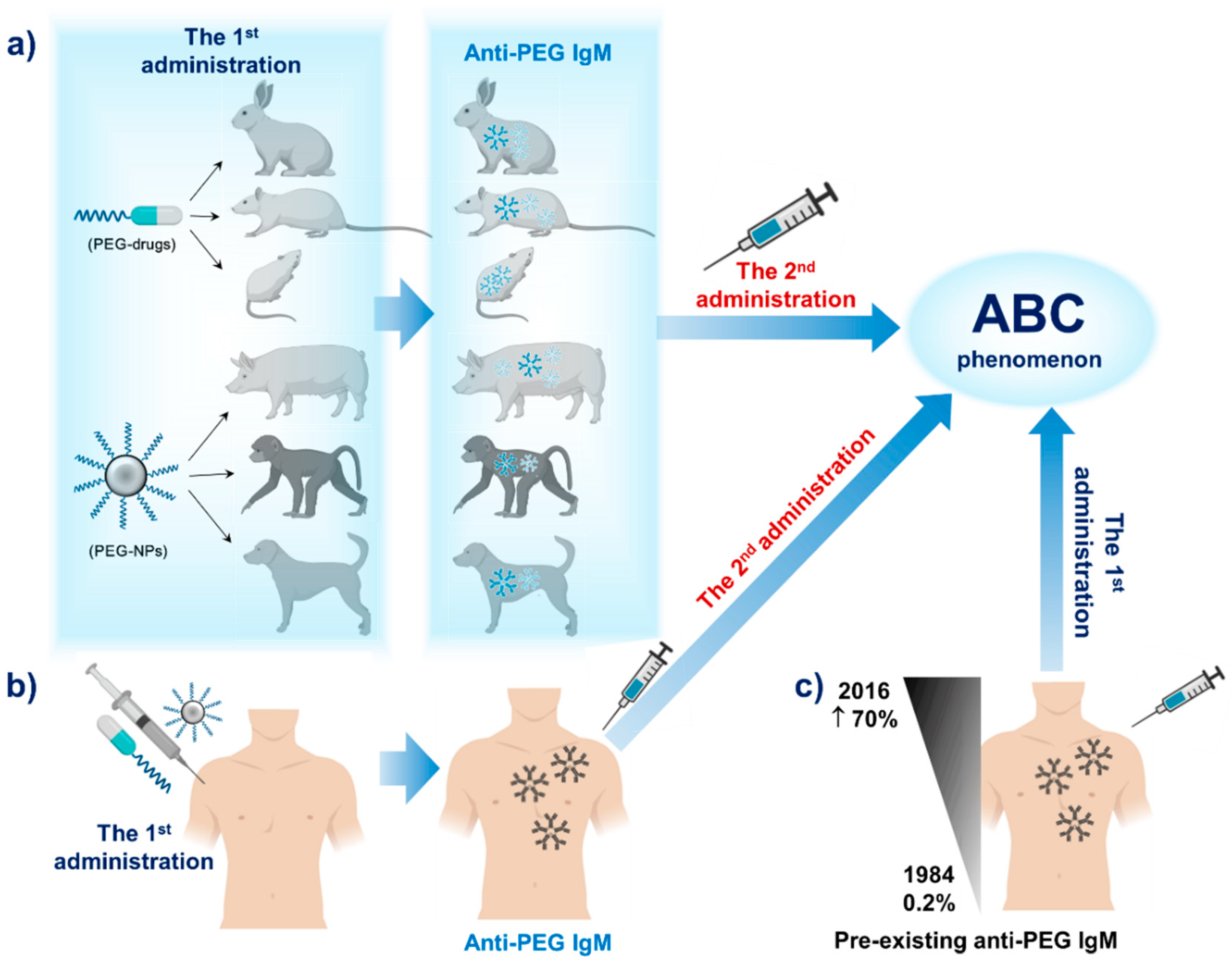
2. PEG Immunogenicity in Animals and Humans
2.1. PEG Immunogenicity in Animals
2.2. PEG Immunogenicity in Humans
3. Factors Affecting PEG Immunogenicity
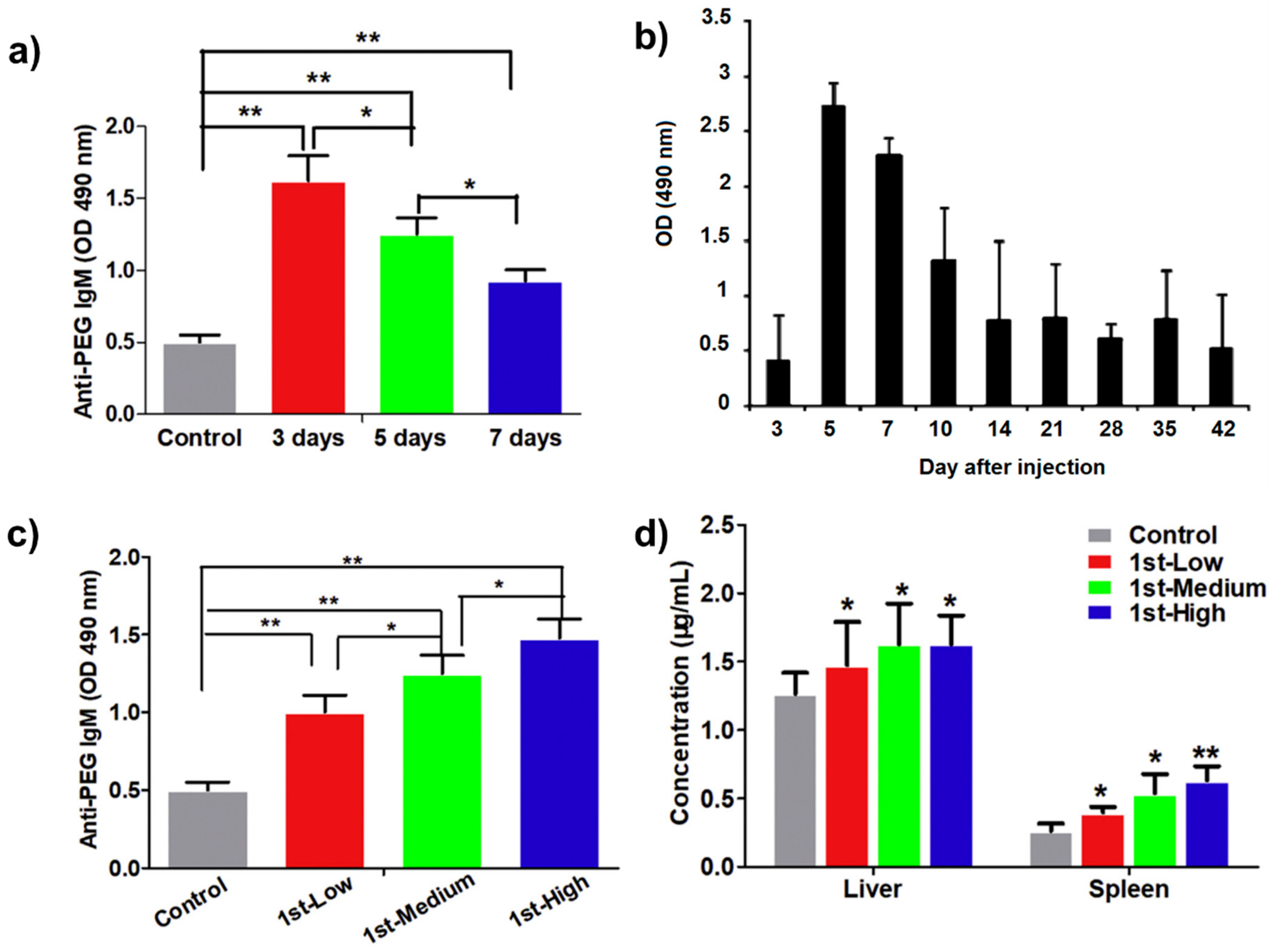
3.1. Effect of Time Intervals between First and Second Doses of PEGylated Drugs
3.2. Effect of Different Doses at the Initial Injection
3.3. Effect of PEG-Surface Density and Content, Molecular Weight and Functional Groups of PEG
3.4. Effect of Nanoparticle Properties
3.5. Effect of Administration Route
3.6. Effect of an Encapsulated Drug
4. Potential PEG Alternatives
4.1. Hydrophilic Polymers
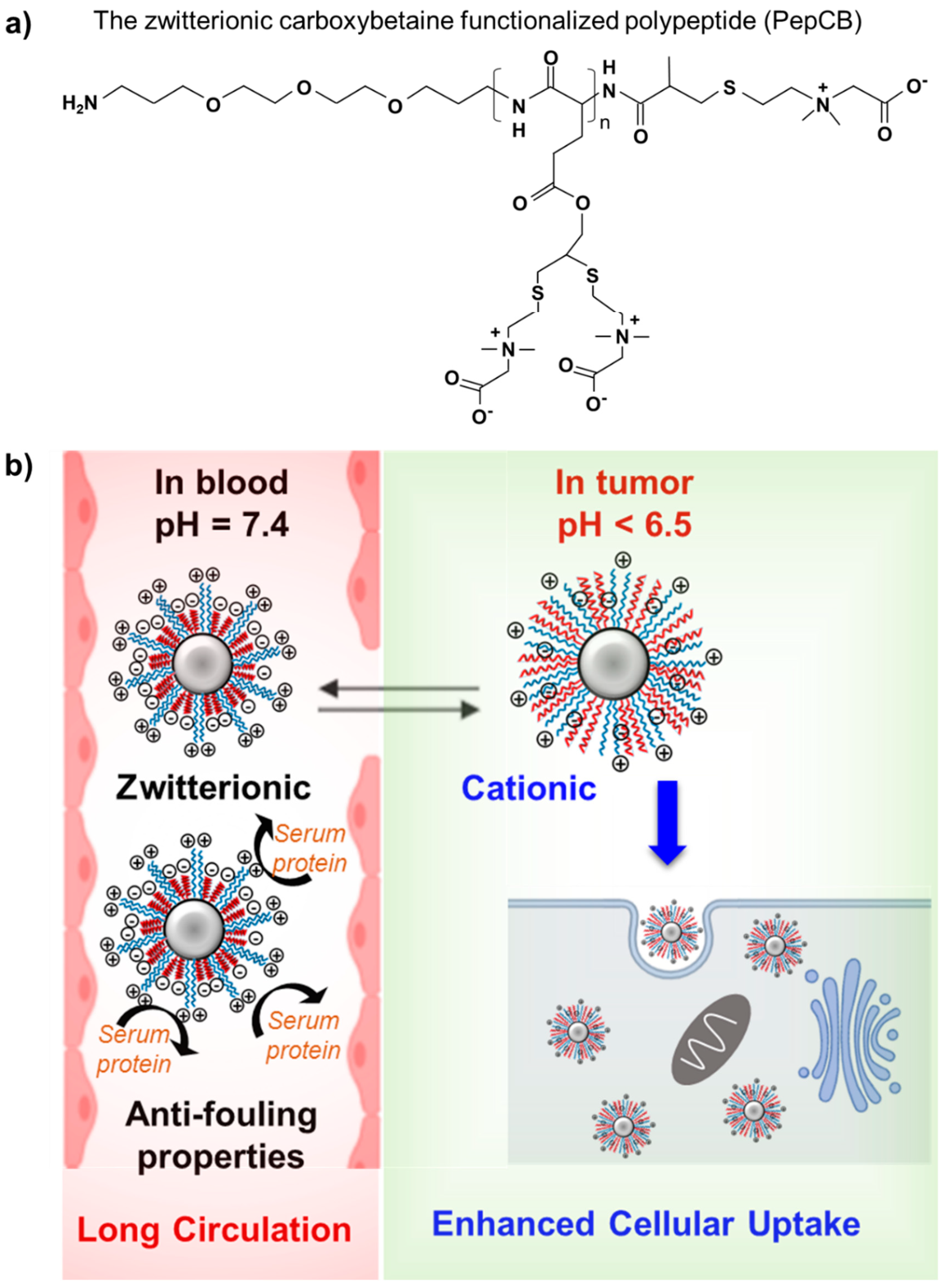
4.2. Zwitterionic Polymers
5. Conclusions and Outlook
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| PEG | poly(ethylene glycol) |
| DNA | deoxyribonucleic acid |
| siRNA | small interfering RNA |
| miRNA | micro RNA |
| FDA | the U.S. Food and Drug Administration |
| MW | molecular weight |
| NPs | nanoparticles |
| RES | reticuloendothelial system |
| ABC | accelerated blood clearance |
| nGO-PEG | PEGylated graphene oxide |
| APC | antigen-presenting cell |
| IgM | anti-PEG immunoglobulin M |
| OVA | ovalbumin |
| SOD | bovine superoxide dismutase |
| mPEG | monomethoxy PEG |
| ALL | acute lymphoblastic leukemia |
| pLT | PEGylated liposomal topotecan |
| PEG-GEA-L | PEGylated liposomal gambogenic acid |
| PL | PEGylated liposomes |
| POEGMA | poly(oligo(ethylene glycol) methyl ether methacrylate) |
| DE | PEGylated emulsions |
| TLR9 | toll-like receptor 9 |
| AG | arabinogalactan |
| SAK | staphylokinase |
| GEA-L | liposomal gambogenic acid |
| POEGMA | poly(oligo(ethylene glycol) methyl ether methacrylate) |
| EG | oligoethylene glycol |
| PGs | poly(glycerols) |
| POXs | poly(oxazolines) |
| PHPMA | poly(hydroxypropyl methacrylate) |
| PHEMA | poly(2-hydroxyethyl methacrylate) |
| HPMA | poly(N-(2-hydroxypropyl) methacrylamide) |
| PVP | poly(vinylpyrrolidone) |
| PDMA | poly(N,N-dimethyl acrylamide) |
| PAcM | poly(N-acryloyl morpholine) |
| HA | hyaluronic acid |
| PSA | polysialic acid |
| pCB | poly(carboxybetaine) |
| pSB | poly(sulfobetaine) |
| pCBAA | zwitterionic poly(carboxybetaine acrylamide) |
| GNP | gold NP |
| PepCB | polypeptides with high zwitterion density |
| PMPC | poly(2-methacryloyloxyethyl phosphorylcholine) |
| PAE | poly(β-amino ester) |
| PCL | poly(ε-caprolactone) |
| MSMs | mixed-shell micelles |
| PEGSMs | single PEG-PCL micelles |
| PMPCSMs | single PMPC-PCL micelles |
| MPC | 2-methacryloyloxyethyl phosphorylcholine |
References
- Bré, L.P.; Zheng, Y.; Pêgo, A.P.; Wang, W. Taking tissue adhesives to the future: From traditional synthetic to new biomimetic approaches. Biomater. Sci. 2013, 1, 239–253. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Alconcel, S.N.S.; Baas, A.S.; Maynard, H.D. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym. Chem. 2011, 2, 1442–1448. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B. 2019, 9, 675–689. [Google Scholar] [CrossRef]
- D’Souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Torchilin, V.P. Polymer-coated long-circulating microparticulate pharmaceuticals. J. Microencapsul. 1998, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, K. PEGylation of therapeutic oligonucletides: From linear to highly branched PEG architectures. Nano Res. 2018, 11, 5519–5534. [Google Scholar] [CrossRef] [PubMed]
- Huckaby, J.T.; Lai, S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018, 124, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Nayak, B.; Dey, R.K. PEGylation in anti-cancer therapy: An overview. Asian J. Pharm. Sci. 2016, 11, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, M.E.; Agusti, R.; de Lederkremer, R.M. Carbohydrate PEGylation, an approach to improve pharmacological potency. Beilstein J. Org. Chem. 2014, 10, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.J.; Bentley, M.D.; Harris, J.M. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2012, 64, 116–127. [Google Scholar] [CrossRef]
- Pasut, G.; Sergi, M.; Veronese, F.M. Anti-cancer PEG-enzymes: 30 years old, but still a current approach. Adv. Drug Deliv. Rev. 2008, 60, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Garcia, P.D.; Retamal, J.S.; Shenoy, P.; Imlach, W.; Sykes, M.; Truong, N.P.; Constandil, L.; Pelissier, T.; Nowell, C.J.; Khor, S.Y.; et al. A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. Nat. Nanotechnol. 2019, 14, 1150–1159. [Google Scholar] [CrossRef]
- Truong, N.P.; Gu, W.; Prasadam, I.; Jia, Z.; Crawford, R.; Xiao, Y.; Monteiro, M.J. An influenza virus-inspired polymer system for the timed release of siRNA. Nat. Commun. 2013, 4, 1902. [Google Scholar] [CrossRef] [Green Version]
- Truong, N.P.; Whittaker, M.R.; Anastasaki, A.; Haddleton, D.M.; Quinn, J.F.; Davis, T.P. Facile production of nanoaggregates with tuneable morphologies from thermoresponsive P(DEGMA-co-HPMA). Polym. Chem. 2016, 7, 430–440. [Google Scholar] [CrossRef]
- Khor, S.Y.; Vu, M.N.; Pilkington, E.H.; Johnston, A.P.R.; Whittaker, M.R.; Quinn, J.F.; Truong, N.P.; Davis, T.P. Elucidating the Influences of Size, Surface Chemistry, and Dynamic Flow on Cellular Association of Nanoparticles Made by Polymerization-Induced Self-Assembly. Small 2018, 14, e1801702. [Google Scholar] [CrossRef]
- Ta, H.T.; Truong, N.P.; Whittaker, A.K.; Davis, T.P.; Peter, K. The effects of particle size, shape, density and flow characteristics on particle margination to vascular walls in cardiovascular diseases. Expert Opin. Drug Deliv. 2017, 15, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Khor, S.Y.; Quinn, J.F.; Whittaker, M.R.; Truong, N.P.; Davis, T.P. Controlling Nanomaterial Size and Shape for Biomedical Applications via Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2019, 40, e1800438. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Zhang, C.; Nguyen, T.A.H.; Anastasaki, A.; Schulze, M.W.; Quinn, J.F.; Whittaker, A.K.; Hawker, C.J.; Whittaker, M.R.; Davis, T.P. Overcoming Surfactant-Induced Morphology Instability of Noncrosslinked Diblock Copolymer Nano-Objects Obtained by RAFT Emulsion Polymerization. ACS Macro Lett. 2018, 7, 159–165. [Google Scholar] [CrossRef]
- Mukhopadhyay, S. Nano drugs: A critical review of their patents and market. In Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 523–551. [Google Scholar]
- Zamboni, W.C.; Torchilin, V.; Patri, A.K.; Hrkach, J.; Stern, S.; Lee, R.; Nel, A.; Panaro, N.J.; Grodzinski, P. Best practices in cancer nanotechnology: Perspective from NCI nanotechnology alliance. Clin. Cancer Res. 2012, 18, 3229–3241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, D.P.; Lin, E.Y.; Lin, K.; Pellegrini, M.; Petter, R.C.; Chen, L.L.; Arduini, R.M.; Brickelmaier, M.; Wen, D.; Hess, D.M.; et al. N-terminally PEGylated human interferon-beta-1a with improved pharmacokinetic properties and in vivo efficacy in a melanoma angiogenesis model. Bioconjug. Chem. 2006, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control Release 2016, 244, 184–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaga, S.; Truong, N.P.; Esser, L.; Senyschyn, D.; Sanyal, A.; Sanyal, R.; Quinn, J.F.; Davis, T.P.; Kaminskas, L.M.; Whittaker, M.R. Influence of Size and Shape on the Biodistribution of Nanoparticles Prepared by Polymerization-Induced Self-Assembly (PISA). Biomacromolecules 2017, 18, 3963–3970. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Beyond PEGylation: Alternative surface-modification of nanoparticles with mucus-inert biomaterials. Adv. Drug Deliv. Rev. 2018, 124, 140–149. [Google Scholar] [CrossRef]
- Rattan, R.; Bhattacharjee, S.; Zong, H.; Swain, C.; Siddiqui, M.A.; Visovatti, S.H.; Kanthi, Y.; Desai, S.; Pinsky, D.J.; Goonewardena, S.N. Nanoparticle-macrophage interactions: A balance between clearance and cell-specific targeting. Bioorg. Med. Chem. 2017, 25, 4487–4496. [Google Scholar] [CrossRef] [Green Version]
- Sebak, A.A. Limitations of Pegylated Nanocarriers: Unfavourable Physicochemical Properties, Biodistribution Patterns and Cellular and Subcellular Fates. Int. J. Pharm. 2018, 10, 6–12. [Google Scholar] [CrossRef]
- Fishburn, C.S. The pharmacology of PEGylation: Balancing PD with PK to generate novel therapeutics. J. Pharm. Sci. 2008, 97, 4167–4183. [Google Scholar] [CrossRef]
- Gorovits, B.; Clements-Egan, A.; Birchler, M.; Liang, M.; Myler, H.; Peng, K.; Purushothama, S.; Rajadhyaksha, M.; Salazar-Fontana, L.; Sung, C.; et al. Pre-existing Antibody: Biotherapeutic Modality-Based Review. AAPS J. 2016, 18, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Lila, A.S.; Shimizu, T.; Ishida, T. PEGylation and anti-PEG antibodies. Eng. Biomater. Drug Deliv. Syst. 2018, 51–68. [Google Scholar] [CrossRef]
- Wenande, E.; Garvey, L.H. Immediate-type hypersensitivity to polyethylene glycols: A review. Clin. Exp. Allergy 2016, 46, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, J.J.; Carpenter, J.F.; Anchordoquy, T.J.; Schellekens, H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov. Today 2014, 19, 1945–1952. [Google Scholar] [CrossRef]
- Schellekens, H.; Hennink, W.E.; Brinks, V. The Immunogenicity of Polyethylene Glycol: Facts and Fiction. Pharm. Res. 2013, 30, 1729–1734. [Google Scholar] [CrossRef]
- Shiraishi, K.; Yokoyama, M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: A review. Sci. Technol. Adv. Mater. 2019, 20, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Lai, S.K. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef] [Green Version]
- Elsadek, N.E.; Abu Lila, A.S.; Ishida, T. 5-Immunological responses to PEGylated proteins: Anti-PEG antibodies. In Polymer-Protein Conjugates; Pasut, G., Zalipsky, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 103–123. [Google Scholar]
- Abu Lila, A.S.; Ishida, T. Anti-PEG IgM Production via a PEGylated Nanocarrier System for Nucleic Acid Delivery. Methods Mol. Biol. 2019, 1943, 333–346. [Google Scholar]
- Li, M.; Al-Jamal, K.T.; Kostarelos, K.; Reineke, J. Physiologically Based Pharmacokinetic Modeling of Nanoparticles. ACS Nano 2010, 4, 6303–6317. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Kiwada, H.; Ishida, T. The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. J. Control. Release 2013, 172, 38–47. [Google Scholar] [CrossRef]
- Park, K. Impact of anti-PEG antibodies on PEGylated nanoparticles fate in vivo. J. Control Release 2018, 287, 257. [Google Scholar] [CrossRef] [PubMed]
- Neun, B.W.; Barenholz, Y.; Szebeni, J.; Dobrovolskaia, M.A. Understanding the Role of Anti-PEG Antibodies in the Complement Activation by Doxil in Vitro. Molecules 2018, 23, 1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, N.; Weber, J.K.; Wang, S.; Luan, B.; Yue, H.; Xi, X.; Du, J.; Yang, Z.; Wei, W.; Zhou, R.; et al. PEGylated graphene oxide elicits strong immunological responses despite surface passivation. Nat. Commun. 2017, 8, 14537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.; Li, C.; Liu, M.; Qiu, Q.; Luo, X.; Liu, X.; Hu, L.; Deng, Y.; Song, Y. Effect of Kupffer cells depletion on ABC phenomenon induced by Kupffer cells-targeted liposomes. Asian J. Pharm. Sci. 2018, 14, 455–464. [Google Scholar] [CrossRef]
- Seppälä, J.T.S.; Mäkelä, O. Hapten. In Encyclopedia of Immunology, 2nd ed.; Roitt, I., Delves, P., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 1998; pp. 1050–1052. [Google Scholar]
- Caliceti, P.; Schiavon, O.; Veronese, F.M. Immunological properties of uricase conjugated to neutral soluble polymers. Bioconjug. Chem. 2001, 12, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Dams, E.T.; Laverman, P.; Oyen, W.J.; Storm, G.; Scherphof, G.L.; Corstens, F.H.; Boerman, O.C. Accelerated Blood Clearance and Altered Biodistribution of Repeated Injections of Sterically Stabilized Liposomes. J. Pharmacol. Exp. Ther. 2000, 292, 1071–1079. [Google Scholar] [PubMed]
- Mohamed, M.; Lila, A.S.A.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [Green Version]
- Moreno, A.; Pitoc, G.A.; Ganson, N.J.; Layzer, J.M.; Hershfield, M.S.; Tarantal, A.F.; Sullenger, B.A. Anti-PEG Antibodies Inhibit the Anticoagulant Activity of PEGylated Aptamers. Cell Chem. Biol. 2019, 26, 634–644. [Google Scholar] [CrossRef]
- Armstrong, J.K. The occurrence, induction, specificity and potential effect of antibodies against poly(ethylene glycol). In PEGylated Protein Drugs: Basic Science and Clinical Applications; Veronese, F.M., Ed.; Birkhäuser Basel: Basel, Switzerland, 2009; pp. 147–168. [Google Scholar]
- Ganson, N.J.; Povsic, T.J.; Sullenger, B.A.; Alexander, J.H.; Zelenkofske, S.L.; Sailstad, J.M.; Rusconi, C.P.; Hershfield, M.S. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J. Allergy Clin. Immunol. 2016, 137, 1610–1613.e7. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.C.; Wang, H.E.; Lin, W.W.; Roffler, S.R.; Cheng, T.C.; Su, Y.C.; Li, J.J.; Chen, C.C.; Huang, C.H.; Chen, B.M.; et al. Pre-existing anti-polyethylene glycol antibody reduces the therapeutic efficacy and pharmacokinetics of PEGylated liposomes. Theranostics 2018, 8, 3164–3175. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, Y.; Yang, H.; Li, H.; Li, H.; Tian, W.; Yang, J.; Cui, J. Prolongation of time interval between doses could eliminate accelerated blood clearance phenomenon induced by pegylated liposomal topotecan. Int. J. Pharm. 2013, 443, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mima, Y.; Hashimoto, Y.; Shimizu, T.; Kiwada, H.; Ishida, T. Anti-PEG IgM Is a Major Contributor to the Accelerated Blood Clearance of Polyethylene Glycol-Conjugated Protein. Mol. Pharm. 2015, 12, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ye, X.; Wu, Y.; Wang, H.; Sheng, C.; Peng, D.; Chen, W. Time Interval of Two Injections and First-Dose Dependent of Accelerated Blood Clearance Phenomenon Induced by PEGylated Liposomal Gambogenic Acid: The Contribution of PEG-Specific IgM. J. Pharm. Sci. 2019, 108, 641–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichihara, M.; Shimizu, T.; Imoto, A.; Hashiguchi, Y.; Uehara, Y.; Ishida, T.; Kiwada, H. Anti-PEG IgM Response against PEGylated Liposomes in Mice and Rats. Pharmaceutics 2010, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Yan, M.; Ma, Y.; Zang, G.; She, Z.; Deng, Y. Repeated injection of PEGylated solid lipid nanoparticles induces accelerated blood clearance in mice and beagles. Int. J. Nanomed. 2012, 7, 2891–2900. [Google Scholar]
- Ishihara, T.; Takeda, M.; Sakamoto, H.; Kimoto, A.; Kobayashi, C.; Takasaki, N.; Yuki, K.; Tanaka, K.; Takenaga, M.; Igarashi, R.; et al. Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharm. Res. 2009, 26, 2270–2279. [Google Scholar] [CrossRef]
- Quach, Q.H.; Kong, R.L.X.; Kah, J.C.Y. Complement Activation by PEGylated Gold Nanoparticles. Bioconjug. Chem. 2018, 29, 976–981. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, Z.; Zhang, P.; Jiang, S. Zwitterlation mitigates protein bioactivity loss in vitro over PEGylation. Chem. Sci. 2018, 9, 8561–8566. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: A review of challenges to clinical transition and promising resolution. Drug Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef]
- Shimizu, T.; Abu Lila, A.S.; Fujita, R.; Awata, M.; Kawanishi, M.; Hashimoto, Y.; Okuhira, K.; Ishima, Y.; Ishida, T. A hydroxyl PEG version of PEGylated liposomes and its impact on anti-PEG IgM induction and on the accelerated clearance of PEGylated liposomes. Eur. J. Pharm. Biopharm. 2018, 127, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, X.; Sui, Y.; Luo, X.; Jiang, G.; Wang, Y.; Huang, Z.; She, Z.; Deng, Y. A noticeable phenomenon: Thiol terminal PEG enhances the immunogenicity of PEGylated emulsions injected intravenously or subcutaneously into rats. Eur. J. Pharm. Biopharm. 2013, 85 Pt A, 744–751. [Google Scholar] [CrossRef]
- Joh, D.Y.; Zimmers, Z.; Avlani, M.; Heggestad, J.T.; Aydin, H.B.; Ganson, N.; Kumar, S.; Fontes, C.M.; Achar, R.K.; Hershfield, M.S.; et al. Architectural Modification of Conformal PEG-Bottlebrush Coatings Minimizes Anti-PEG Antigenicity While Preserving Stealth Properties. Adv. Healthc. Mater. 2019, 8, e1801177. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Qi, J.; Hu, C.; Shen, L.; Yu, W.; Hu, T. Conjugation of staphylokinase with the arabinogalactan-PEG conjugate: Study on the immunogenicity, in vitro bioactivity and pharmacokinetics. Int. J. Biol. Macromol. 2019, 131, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Quinn, J.F.; Anastasaki, A.; Rolland, M.; Vu, M.; Haddleton, D.; Whittaker, M.R.; Davis, T.P. Surfactant-free RAFT emulsion polymerization using a novel biocompatible thermoresponsive polymer. Polym. Chem. 2017, 8, 1353–1363. [Google Scholar] [CrossRef]
- Truong, N.P.; Dussert, M.V.; Whittaker, M.R.; Quinn, J.F.; Davis, T.P. Rapid synthesis of ultrahigh molecular weight and low polydispersity polystyrene diblock copolymers by RAFT-mediated emulsion polymerization. Polym. Chem. 2015, 6, 3865–3874. [Google Scholar] [CrossRef]
- Nunvářová, K.; Charvátová, B.; Šlouf, M.; Hermanová, S.; Merna, J. Synthesis of amphiphilic copolymers based on dendritic polyethylene grafted by polyhydroxyethylmethacrylate and polyhydroxypropylmethacrylate and their use for construction of nanoparticles. Eur. Polym. J. 2019, 115, 193–200. [Google Scholar] [CrossRef]
- Abbina, S.; Parambath, A. PEGylation and its alternatives: A summary. In Engineering of Biomaterials for Drug Delivery Systems: Beyond Polyethylene Glycol; Parambath, A., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 363–376. [Google Scholar]
- Hadjesfandiari, N.; Parambath, A. Stealth coatings for nanoparticles. Eng. Biomater. Drug Deliv. Syst. 2018, 345–361. [Google Scholar] [CrossRef]
- Lorson, T.; Lubtow, M.M.; Wegener, E.; Haider, M.S.; Borova, S.; Nahm, D.; Jordan, R.; Sokolski-Papkov, M.; Kabanov, A.V.; Luxenhofer, R. Poly(2-oxazoline)s based biomaterials: A comprehensive and critical update. Biomaterials 2018, 178, 204–280. [Google Scholar] [CrossRef]
- Gulati, N.M.; Stewart, P.L.; Steinmetz, N.F. Bioinspired Shielding Strategies for Nanoparticle Drug Delivery Applications. Mol. Pharm. 2018, 15, 2900–2909. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, L.; Wang, S.; White, A.D.; Jiang, S. Functionalizable and ultra stable nanoparticles coated with zwitterionic poly(carboxybetaine) in undiluted blood serum. Biomaterials 2009, 30, 5617–5621. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jain, P.; Tsao, C.; Yuan, Z.; Li, W.; Li, B.; Wu, K.; Hung, H.C.; Lin, X.; Jiang, S. Polypeptides with High Zwitterion Density for Safe and Effective Therapeutics. Angew. Chem. 2018, 57, 7743–7747. [Google Scholar] [CrossRef]
- Ou, H.; Cheng, T.; Zhang, Y.; Liu, J.; Ding, Y.; Zhen, J.; Shen, W.; Xu, Y.; Yang, W.; Niu, P.; et al. Surface-adaptive zwitterionic nanoparticles for prolonged blood circulation time and enhanced cellular uptake in tumor cells. Acta Biomater. 2018, 65, 339–348. [Google Scholar] [CrossRef]





| PEG alternatives | Advantages | Limitations | |
|---|---|---|---|
| Synthetic polymers | |||
| Polyoxazolines (POX/POZ) |
|
| |
| Poly(N-vinylpyrrolidone) (PVP) | |||
| Poly(glycerols) (PG) |
| ||
| Polyacrylamides |
| ||
| Natural polymers | |||
| Lipids, Carbohydrates (Heparin, GAGs, PSA, HA, …) Proteins (ELPs, serum albumin, CD47, …) | |||
| Polyaminoacids |
| ||
| Zwitterionic polymers: Potential next-generation biomaterials, an excellent alternative to PEG [78] | |||
| Poly(carboxybetaine) (pCB), poly(sulfobetaine) (pSB), phosphobetaine-base polymers. |
| ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. https://doi.org/10.3390/polym12020298
Hoang Thi TT, Pilkington EH, Nguyen DH, Lee JS, Park KD, Truong NP. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers. 2020; 12(2):298. https://doi.org/10.3390/polym12020298
Chicago/Turabian StyleHoang Thi, Thai Thanh, Emily H. Pilkington, Dai Hai Nguyen, Jung Seok Lee, Ki Dong Park, and Nghia P. Truong. 2020. "The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation" Polymers 12, no. 2: 298. https://doi.org/10.3390/polym12020298
APA StyleHoang Thi, T. T., Pilkington, E. H., Nguyen, D. H., Lee, J. S., Park, K. D., & Truong, N. P. (2020). The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers, 12(2), 298. https://doi.org/10.3390/polym12020298






