Degradable Controlled Release Fertilizer Composite Prepared via Extrusion: Fabrication, Characterization, and Release Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.4. Phosphate Release Characteristics
3. Results
3.1. Thermal Decomposition of Urea Phosphate
3.2. Loading Efficiency
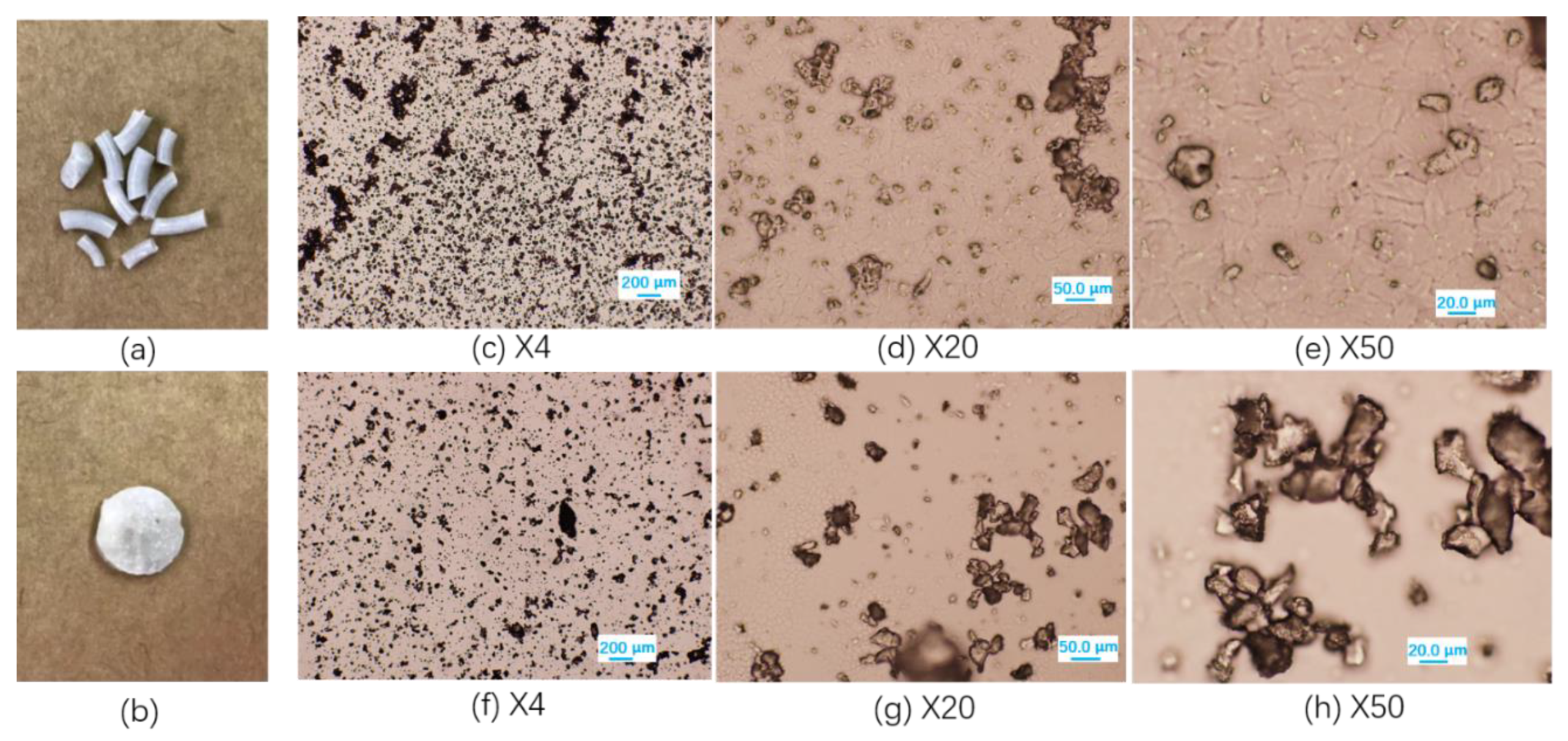
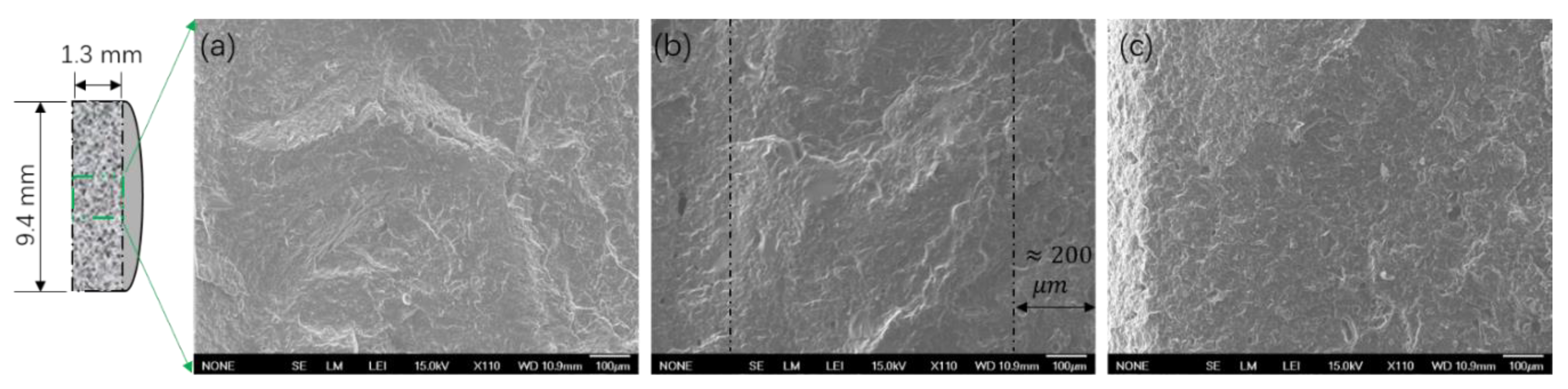
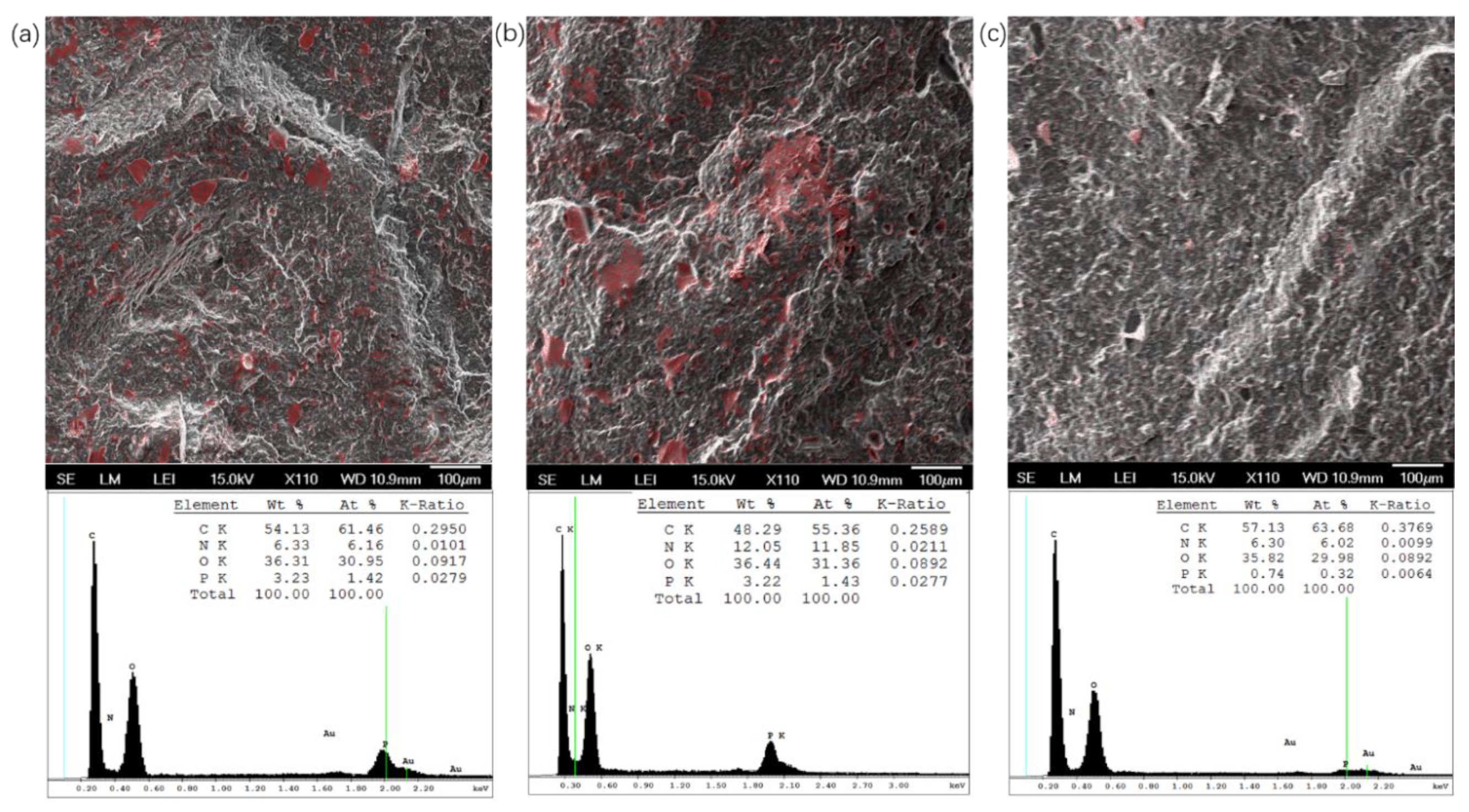
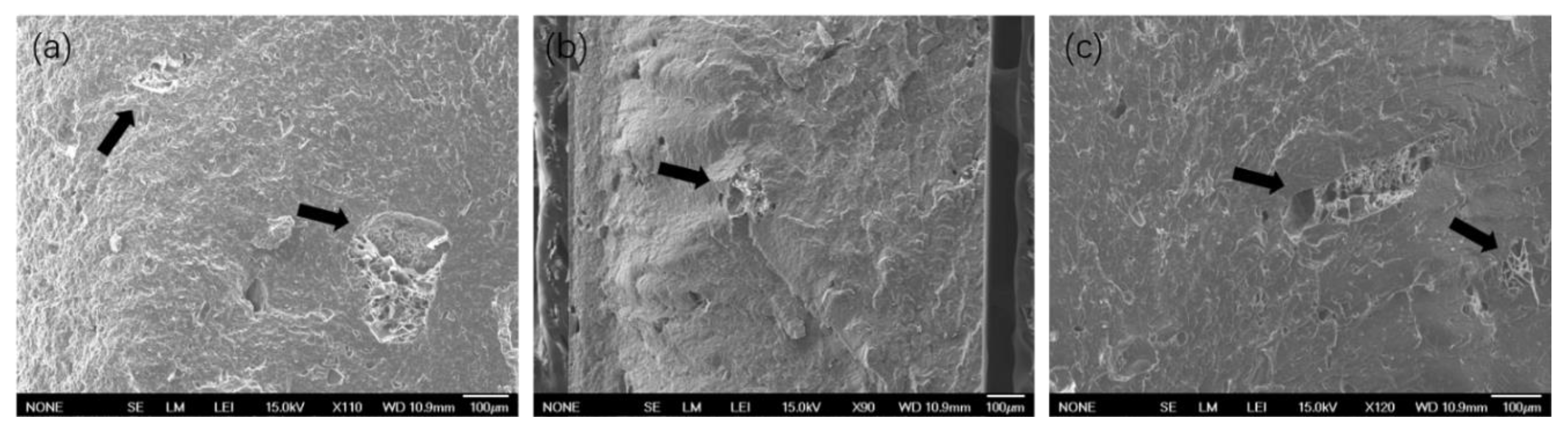
3.3. Characterization of Blends Morphology
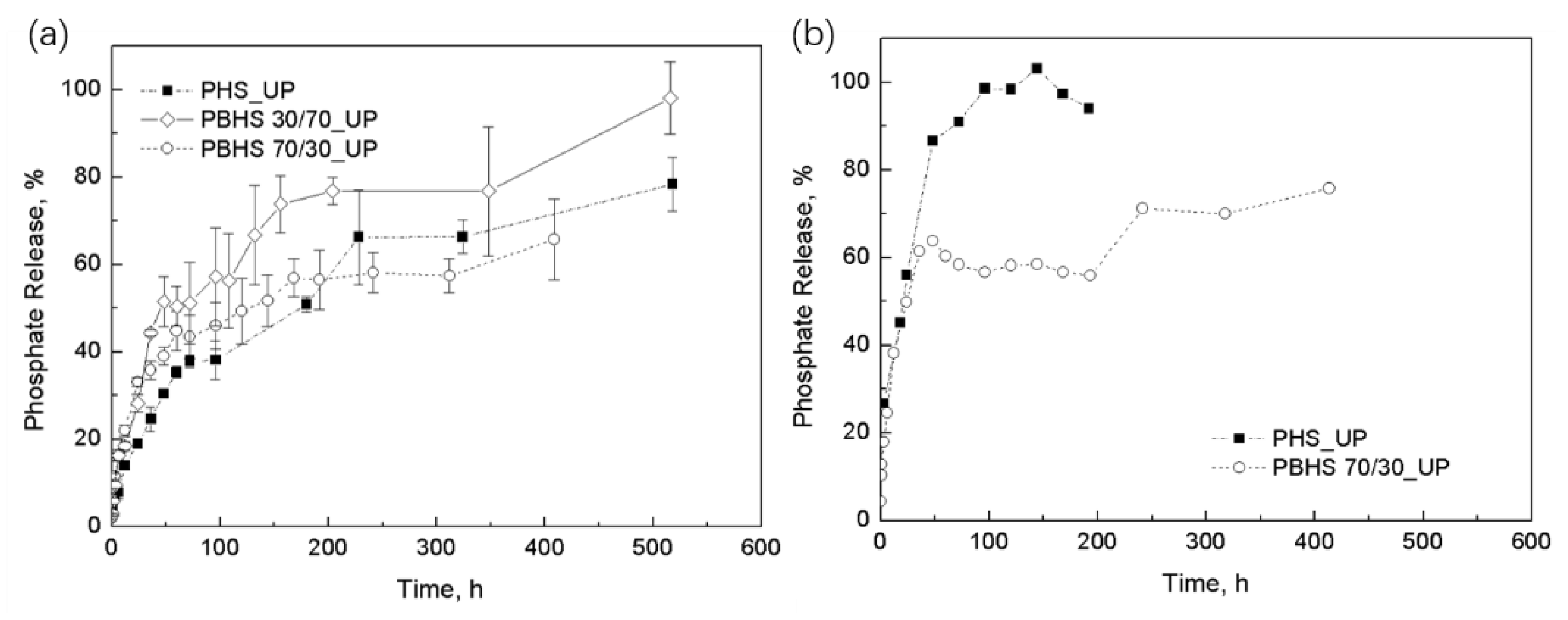
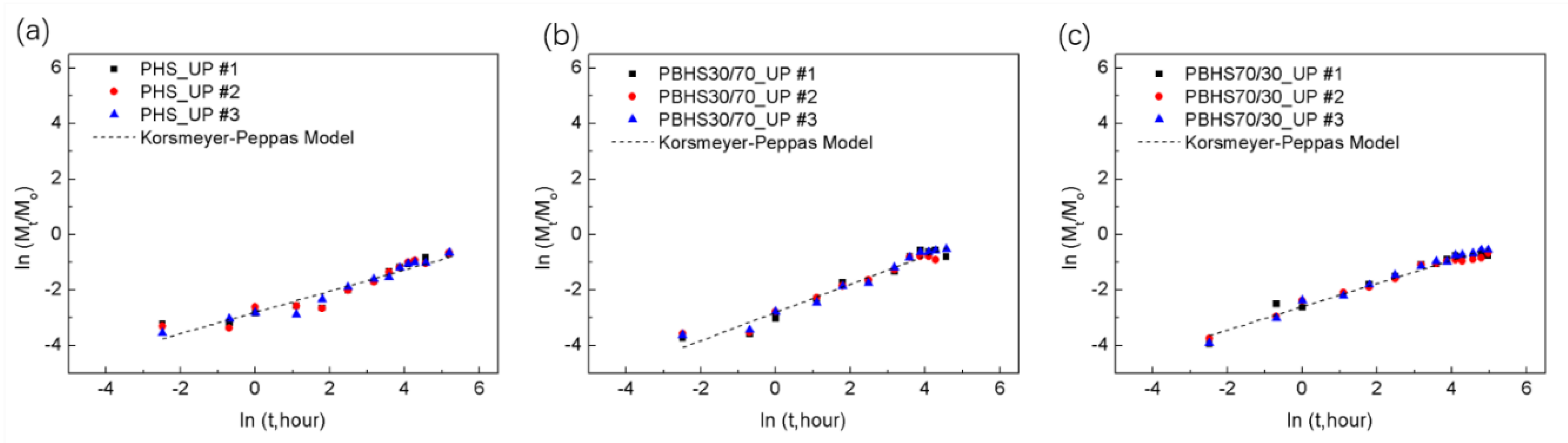
3.4. Phosphate Release and Modeling of Initial Kinetic Behavior
3.5. Discussion and Modeling of Diffusion-Degradation Release Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heffer, P.; Prud, M. Fertilizer Outlook 2019–2023. In Proceedings of the 87th IFA Annual Conference, Montreal, Canada, 11–13 June 2019. [Google Scholar]
- Lubkowski, K.; Smorowska, A.; Grzmil, B.; Kozłowska, A. Controlled-release fertilizer prepared using a biodegradable aliphatic copolyester of poly (butylene succinate) and dimerized fatty acid. J. Agric. Food. Chem. 2015, 63, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture, 2nd ed.; International Fertilizer Industry Association: Paris, France, 2010; pp. 17–54. [Google Scholar]
- Naz, M.Y.; Sulaiman, S.A. Slow release coating remedy for nitrogen loss from conventional urea: A review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.Y.; Sulaiman, S.A. Attributes of natural and synthetic materials pertaining to slow-release urea coating industry. Rev. Chem. Eng. 2017, 33, 293–308. [Google Scholar] [CrossRef]
- Gu, Y.-J.; Han, C.-L.; Fan, J.-W.; Shi, X.-P.; Kong, M.; Shi, X.-Y.; Siddique, K.H.; Zhao, Y.-Y.; Li, F.-M. Alfalfa forage yield, soil water and P availability in response to plastic film mulch and P fertilization in a semiarid environment. Field Crop. Res. 2018, 215, 94–103. [Google Scholar] [CrossRef]
- Dawson, C.; Hilton, J. Fertiliser availability in a resource-limited world: Production and recycling of nitrogen and phosphorus. Food Policy 2011, 36, S14–S22. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Global Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Scholz, R.W.; Ulrich, A.E.; Eilittä, M.; Roy, A. Sustainable use of phosphorus: A finite resource. Sci. Total. Environ. 2013, 461, 799–803. [Google Scholar] [CrossRef]
- Elser, J.; Bennett, E. Phosphorus cycle: A broken biogeochemical cycle. Nature 2011, 478, 29–31. [Google Scholar] [CrossRef]
- Calabi-Floody, M.; Medina, J.; Rumpel, C.; Condron, L.M.; Hernandez, M.; Dumont, M.; de la Luz Mora, M. Smart fertilizers as a strategy for sustainable agriculture, in Advances in Agronomy. Adv. Agron. 2018, 147, 119–157. [Google Scholar]
- Campos, E.V.R.; De Oliveira, J.L.; Fraceto, L.F. Applications of Controlled Release Systems for Fungicides, Herbicides, Acaricides, Nutrients, and Plant Growth Hormones: A Review. Adv. Sci. Eng. Med. 2014, 6, 373–387. [Google Scholar] [CrossRef]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Total. Environ. 2018, 613, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered Biochar Reclaiming Phosphate from Aqueous Solutions: Mechanisms and Potential Application as a Slow-Release Fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef] [PubMed]
- Broschat, T.K.; Moore, K.K. Release Rates of Ammonium-Nitrogen, Nitrate-Nitrogen, Phosphorus, Potassium, Magnesium, Iron, and Manganese from Seven Controlled-Release Fertilizers. Commun. Soil Sci. Plant Anal. 2007, 38, 843–850. [Google Scholar] [CrossRef]
- McCauley, A.; Jones, C.; Jacobsen, J. Commercial fertilizers and soil amendments. In Nutrient Management—A Self-Study Course from MSU Extension Continuing Education Series; Montana State University Extension: Bozeman, MT, USA, 2009; pp. 449–510. [Google Scholar]
- Totten, F.W.; Liu, H.; Mccarty, L.B.; Baldwin, C.M.; Bielenberg, D.G.; Toler, J.E. Efficiency of Foliar Versus Granular Fertilization: A Field Study of Creeping Bentgrass Performance. J. Plant Nutr. 2008, 31, 972–982. [Google Scholar] [CrossRef]
- Liu, L.; Shen, T.; Yang, Y.; Gao, B.; Li, Y.C.; Xie, J.; Tang, Y.; Zhang, S.; Wang, Z.; Chen, J. Bio-based Large Tablet Controlled-Release Urea: Synthesis, Characterization, and Controlled-Released Mechanisms. J. Agric. Food Chem. 2018, 66, 11265–11272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levett, I.; Pratt, S.; Donose, B.C.; Brackin, R.; Pratt, C.; Redding, M.R.; Laycock, B. Understanding the Mobilization of a Nitrification Inhibitor from Novel Slow Release Pellets, Fabricated through Extrusion Processing with PHBV Biopolymer. J. Agric. Food Chem. 2019, 67, 2449–2458. [Google Scholar] [CrossRef]
- Hongsriphan, N.; Pinpueng, A. Properties of Agricultural Films Prepared from Biodegradable Poly (Butylene Succinate) Adding Natural Sorbent and Fertilizer. J. Polym. Environ. 2019, 27, 434–443. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, B.-H. Preparation and characterisation of poly(butylene succinate) microcarriers containing pesticide. Micro Nano Lett. 2019, 14, 81–85. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, L.; Xie, F.; Bao, X.; Liu, H.; Ji, Z.; Chen, L.; Xie, D.F. One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem. Eng. J. 2017, 309, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.G.; Wang, Y.T.; Guo, J.; Liu, T.B.; Wang, X.; Li, B. One-step synthesis of corn starch urea based acrylate superabsorbents. J. Appl. Polym. Sci. 2017, 134, 45175. [Google Scholar] [CrossRef]
- Burg, A.J.L.; Orr, D.E. An overview of bioresorbable materials. In Degradation Rate of Bioresorbable Materials: Prediction and Evaluation. Synthetic Bioresorbable Polymers; Buchanan, F.J., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 3–18. [Google Scholar]
- Versypt, A.N.; Pack, D.W.; Braatz, R.D. Mathematical modeling of drug delivery from autocatalytically degradable PLGA microspheres—A review. J. Control. Release 2013, 165, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Arifin, D.Y.; Lee, L.Y.; Wang, C.-H. Mathematical modeling and simulation of drug release from microspheres: Implications to drug delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 1274–1325. [Google Scholar] [CrossRef]
- Sackett, C.K.; Narasimhan, B. Mathematical modeling of polymer erosion: Consequences for drug delivery. Int. J. Pharm. 2011, 418, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Fertahi, S.; Bertrand, I.; Amjoud, M.; Oukarroum, A.; Arji, M.; Barakat, A. Properties of Coated Slow-Release Triple Superphosphate (TSP) Fertilizers Based on Lignin and Carrageenan Formulations. ACS Sustain. Chem. Eng. 2019, 7, 10371–10382. [Google Scholar] [CrossRef]
- Wu, C.-S. Controlled release evaluation of bacterial fertilizer using polymer composites as matrix. J. Control. Release 2008, 132, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Irfan, S.A.; Razali, R.; KuShaari, K.; Mansor, N.; Azeem, B.; Versypt, A.N.F. A review of mathematical modeling and simulation of controlled-release fertilizers. J. Control. Release 2018, 271, 45–54. [Google Scholar] [CrossRef]
- Bi, S.; Tan, B.; Soule, J.L.; Sobkowicz, M.J. Enzymatic degradation of poly (butylene succinate-co-hexamethylene succinate). Polym. Degrad. Stab. 2018, 155, 9–14. [Google Scholar] [CrossRef]
- Tan, B.; Bi, S.; Emery, K.; Sobkowicz, M.J. Bio-based poly(butylene succinate-co-hexamethylene succinate) copolyesters with tunable thermal and mechanical properties. Eur. Polym. J. 2017, 86, 162–172. [Google Scholar] [CrossRef]
- Nagul, E.A.; McKelvie, I.D.; Worsfold, P.; Kolev, S.D. The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box. Anal. Chim. Acta 2015, 890, 60–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcilla, A.; Beltran, M.I.; Gómez-Siurana, A.; Martinez-Castellanos, I.; Berenguer, D.; Pastor, V.; García, A.N. TGA/FTIR study of the pyrolysis of diammonium hydrogen phosphate–tobacco mixtures. J. Anal. Appl. Pyrolysis 2015, 112, 48–55. [Google Scholar] [CrossRef]
- Fan, M.X.; MacKenzie, A.F. Urea and Phosphate Interactions in Fertilizer Microsites: Ammonia Volatilization and pH Changes. Soil Sci. Soc. Am. J. 1993, 57, 839–845. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, P.; Hu, Y.; Hu, K. Thermal degradation study of intumescent flame retardants by TG and FTIR: Melamine phosphate and its mixture with pentaerythritol. J. Anal. Appl. Pyrolysis 2009, 86, 207–214. [Google Scholar] [CrossRef]
- Xia, Z.; Singh, A.; Kiratitanavit, W.; Mosurkal, R.; Kumar, J.; Nagarajan, R. Unraveling the mechanism of thermal and thermo-oxidative degradation of tannic acid. Thermochim. Acta 2015, 605, 77–85. [Google Scholar] [CrossRef]
- Saihi, D.; Vroman, I.; Giraud, S.; Bourbigot, S. Microencapsulation of ammonium phosphate with a polyurethane shell part I: Coacervation technique. React. Funct. Polym. 2005, 64, 127–138. [Google Scholar] [CrossRef]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy, 2nd ed.; Acadenuc Press: New York, NY, USA, 2012; pp. 278–310, 321–364. [Google Scholar]
- Balabanovich, A. Thermal decomposition study of intumescent additives: Pentaerythritol phosphate and its blend with melamine phosphate. Thermochim. Acta 2005, 435, 188–196. [Google Scholar] [CrossRef]
- Abdel-Kader, A.; Ammar, A.; Saleh, S. Thermal behaviour of ammonium dihydrogen phosphate crystals in the temperature range 25–600° C. Thermochim. Acta 1991, 176, 293–304. [Google Scholar] [CrossRef]
- Pretsch, E.; Buehlmann, P.; Affolter, C.; Pretsch, E.; Bhuhlmann, P.; Affolter, C. Structure Determination of Organic Compounds; Springer: Berlin, Germany, 2000; pp. 328–331. [Google Scholar]
- Jiang, W.; Hao, J.; Han, Z. Study on the thermal degradation of mixtures of ammonium polyphosphate and a novel caged bicyclic phosphate and their flame retardant effect in polypropylene. Polym. Degrad. Stab. 2012, 97, 632–637. [Google Scholar] [CrossRef]
- Wang, A.; Liu, N.; Yin, H.; Wu, H.; Wada, Y.; Ren, M.; Jiang, T.; Cheng, X.; Xu, Y. Size-controlled synthesis of hydroxyapatite nanorods by chemical precipitation in the presence of organic modifiers. Mater. Sci. Eng. C 2007, 27, 865–869. [Google Scholar] [CrossRef]
- Tang, J.W.; Mu, R.Z.; Zhang, B.L.; Fan, X.S. Solubility of urea phosphate in water+ phosphoric acid from (277.00 to 354.50) K. J. Chem. Eng. Data 2007, 52, 1179–1181. [Google Scholar] [CrossRef]
- Ruiz-Beviá, F.; Fernández-Sempere, J.; Boluda-Botella, N. Variation of phosphoric acid diffusion coefficient with concentration. AIChE J. 1995, 41, 185–189. [Google Scholar] [CrossRef]
- Winkelmann, J. Diffusion coefficient of urea in water. In Diffusion in Gases, Liquids and Electrolytes; Lechner, M.D., Ed.; Springer: Berlin, Germany, 2017; p. 583. [Google Scholar]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. Drug Res. 2010, 67, 217–223. [Google Scholar]
- Boyandin, A.N.; Zhila, N.O.; Kiselev, E.G.; Volova, T.G. Constructing Slow-Release Formulations of Metribuzin Based on Degradable Poly(3-hydroxybutyrate). J. Agric. Food Chem. 2016, 64, 5625–5632. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Jung, J.H.; Ree, M.; Kim, H. Acid- and base-catalyzed hydrolyses of aliphatic polycarbonates and polyesters. Catal. Today 2006, 115, 283–287. [Google Scholar] [CrossRef]
- Lee, J.M.; Ishak, Z.M.; Taib, R.M.; Law, T.T.; Thirmizir, M.A. Mechanical, thermal and water absorption properties of kenaf-fiber-based polypropylene and poly (butylene succinate) composites. J. Poly. Environ. 2013, 21, 293–302. [Google Scholar] [CrossRef]
- Siepmann, J.; Elkharraz, K.; Siepmann, F.; Klose, D. How Autocatalysis Accelerates Drug Release from PLGA-Based Microparticles: A Quantitative Treatment. Biomacromolecules 2005, 6, 2312–2319. [Google Scholar] [CrossRef]
- Mylonaki, I.; Allémann, E.; Delie, F.; Jordan, O. Imaging the porous structure in the core of degrading PLGA microparticles: The effect of molecular weight. J. Control. Release 2018, 286, 231–239. [Google Scholar] [CrossRef]
- Upadrashta, S.M.; Katikaneni, P.R.; Hileman, G.A.; Keshary, P.R. Direct Compression Controlled Release Tablets Using Ethylcellulose Matrices. Drug Dev. Ind. Pharm. 1993, 19, 449–460. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
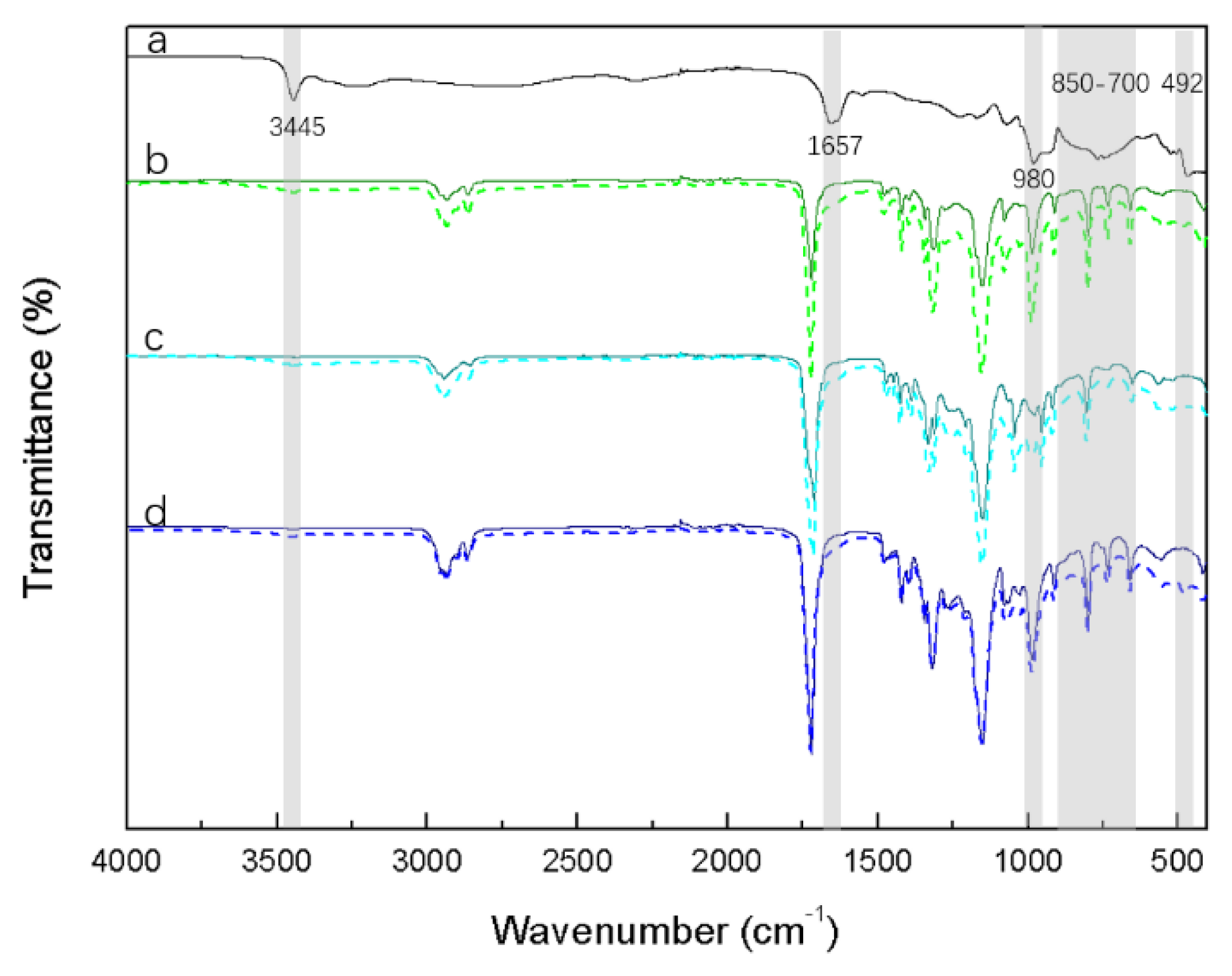



| Gaseous Species | Characteristic IR Bands (cm−1) | Ref. |
|---|---|---|
| CO2 | 2362, 670 | [38,39] |
| NH3 | 3337, 1627, 967, 930 | [36,38] |
| H2O (minor) | 4000–3500, 1850–1640, 1560–1400 | [38] |
| Assignment | Ref. | Assignment | Ref. | ||
|---|---|---|---|---|---|
| Urea Phosphate | - | Urea Phosphate after TGA-FTIR | - | ||
| 3445 | N-H stretching in secondary amide | [40] | 3200–3600 | O-H vibration (broad band) | [40] |
| 3170 | N-H vibration (free/bonded) | [40] | |||
| 3218 (broad) | O-H/N-H valence vibration | [43] | 3028, 2857 | C-H stretching/deformation | [36,41] |
| 1650–1690 | C=O stretching (amide I band) | [40] | 1241, 1295 | P=O stretching vibration | [44] |
| 1160–1260 | P-O stretching | [42] | 1062, 1027 | P-O stretching vibration (P-O-P or P-O-C) | [45] |
| 970–1010 1050–1140 | P=O for ROPO32- | [42,46] | 877 | Typical of bicyclic O=PO3 structure | [42] |
| 700–850 (broad) | N-H bending/wagging | [41,42] | 760, 679 | Typical of cyclic phosphate structure | [42] |
| 492 | O=P-OH | [42] | 492 | O=P-OH | [42] |
| Blends | Feed Ratio, % | UP Loading, % From UV-Vis | UP Loading, % From TGA |
|---|---|---|---|
| PHS_UP | 10 | 9.9 ± 0.1 | 9.9 ± 0.6 |
| PBHS 30/70_UP | 10 | 10.4 ± 0.5 | 9.3 ± 0.1 |
| PBHS 70/30_UP | 10 | 7.8 ± 0. 4 | 8.1 ± 0.7 |
| Blends | Korsmeyer–Peppas Model | |||
|---|---|---|---|---|
| n | k1 | R2 | Type of Diffusion | |
| 25 °C | - | |||
| PHS_UP | 0.38 ± 0.01 | 0.060 ± 0.002 | 0.94 ± 0.02 | Fickian |
| PBHS 30/70_UP | 0.51 ± 0.01 | 0.060 ± 0.002 | 0.963 ± 0.006 | Non-Fickian |
| PBHS 70/30_UP | 0.42 ± 0.02 | 0.074 ± 0.002 | 0.98 ± 0.009 | Fickian |
| 37 °C | - | |||
| PHS_UP | 0.3333 | 0.001838 | 0.9752 | Fickian |
| PBHS 70/30_ UP | 0.4227 | 0.125093 | 0.9914 | Fickian |
| Blends | Diffusion–Relaxation Model | Erosion–Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | R2 | |||||||
| 25 °C | - | |||||||
| PHS_UP | 0.053 ± 0.001 | −0.0005 ± 0.0003 | 0.97 ± 0.01 | 0.038 ± 0.003 | 0.0008 ± 0.0003 | −3 × 10−6 ± 1 × 10−6 | −3 × 10−9 ± 2 × 10−9 | 0.98 ± 0.01 |
| PBHS 30/70_UP | 0.09 ± 0.01 | −0.0021 ± 0.0006 | 0.94 ± 0.04 | 0.059 ± 0.006 | 0.0016 ± 0.0009 | −1.4 × 10−5 ± 6 × 10−6 | −1.9 × 10−8 ± 9 × 10−9 | 0.97 ± 0.01 |
| PBHS 70/30_UP | 0.077 ± 0.007 | −0.0024 ± 0.0003 | 0.973 ± 0.09 | 0.0595 ± 0.005 | 7 × 10−26 ± 1 × 10−25 | −1.1 × 10−5 ± 2 × 10−6 | −1 × 10−9 ± 1 × 10−9 | 0.97 ± 0.02 |
| 37 °C | - | |||||||
| PHS_UP | 0.18144 | −0.00819 | 0.93165 | 0.11544 | 0.00181 | 4.12 × 10−5 | 7.5 × 10−8 | 0.96772 |
| PBHS 70/30_ UP | 0.11332 | −0.00458 | 0.85088 | 0.15763 | −0.012 | 2.4 × 10−6 | −2.3 × 10−8 | 0.96073 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, S.; Barinelli, V.; Sobkowicz, M.J. Degradable Controlled Release Fertilizer Composite Prepared via Extrusion: Fabrication, Characterization, and Release Mechanisms. Polymers 2020, 12, 301. https://doi.org/10.3390/polym12020301
Bi S, Barinelli V, Sobkowicz MJ. Degradable Controlled Release Fertilizer Composite Prepared via Extrusion: Fabrication, Characterization, and Release Mechanisms. Polymers. 2020; 12(2):301. https://doi.org/10.3390/polym12020301
Chicago/Turabian StyleBi, Siwen, Vincenzo Barinelli, and Margaret J. Sobkowicz. 2020. "Degradable Controlled Release Fertilizer Composite Prepared via Extrusion: Fabrication, Characterization, and Release Mechanisms" Polymers 12, no. 2: 301. https://doi.org/10.3390/polym12020301





