Effect of Nano-Clay and Surfactant on the Biodegradation of Poly(Lactic Acid) Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Nanocomposite Films
2.3. Characterization of PLA Nanocomposite Films
2.4. Biodegradation Test
2.5. Molecular Weight Determination
2.6. Hydrolysis Test
2.7. Thermal Properties
2.8. Statistical Analysis
3. Results
3.1. Characterization of All Films
3.2. Biodegradation: CO2 Evolution and Mineralization in Non-Inoculated and Inoculated Vermiculite
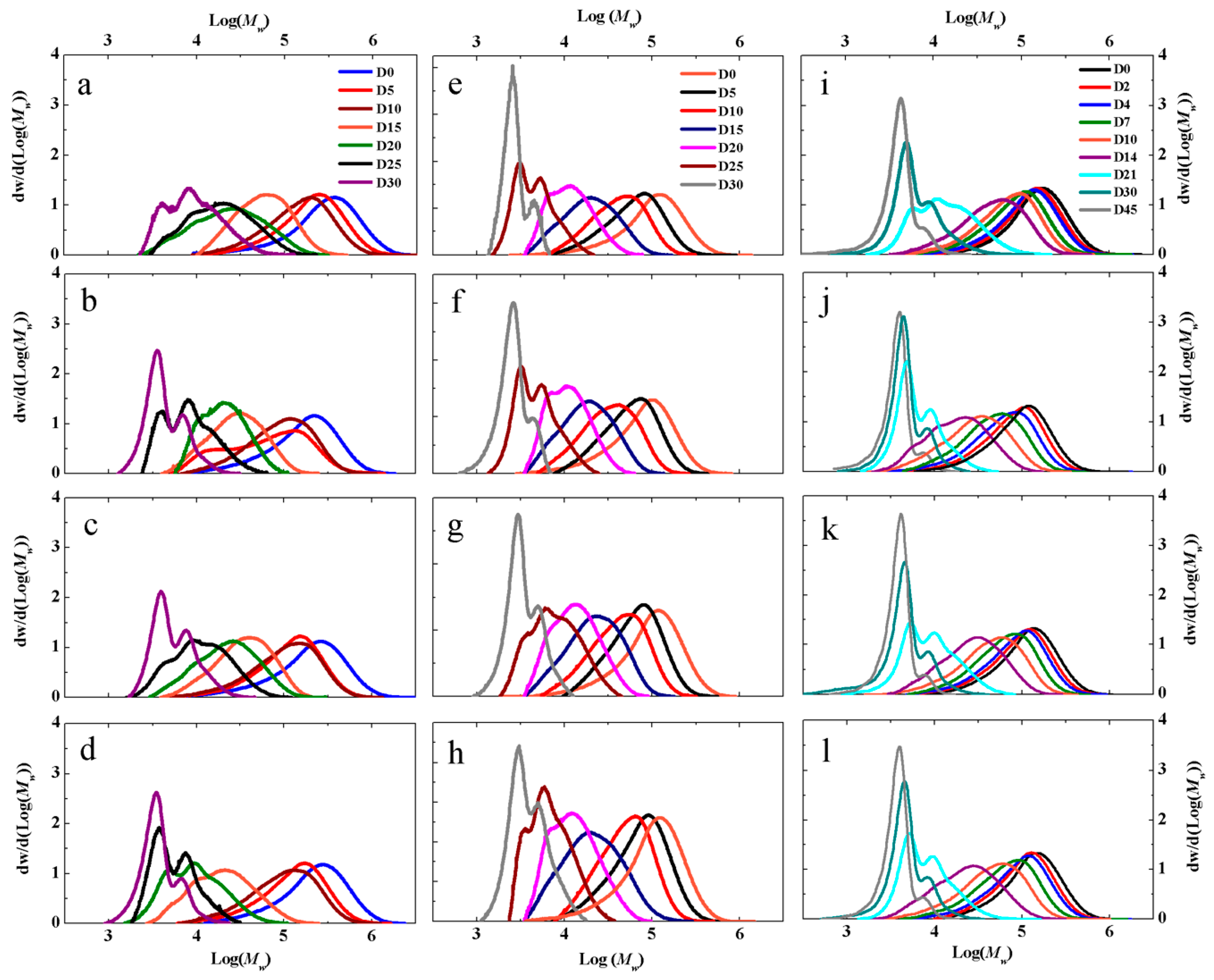
3.3. MWD and Mn Reduction of PLA, PLA QAC, PLA MMT and PLA OMMT in Different Medias
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gironi, F.; Piemonte, V. Bioplastics and petroleum-based plastics: Strengths and weaknesses. Energy Sources Part A Recover. Util. Environ. Eff. 2011, 33, 1949–1959. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Li, S.; Garreau, H.; Vert, M. Selective enzymatic degradations of poly(L-lactide) and poly(E-caprolactone) blend films. Biomacromolecules 2000, 1, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Biodegradable Poly(Lactic Acid) Nanocomposites: Synthesis and Characterization. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2011. [Google Scholar]
- Karkhanis, S.S.; Stark, N.M.; Sabo, R.C.; Matuana, L.M. Blown film extrusion of poly(lactic acid) without melt strength enhancers. J. Appl. Polym. Sci. 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2018, 19, 634–643. [Google Scholar] [CrossRef]
- Lunt, J.; Shafer, A.L. Polylactic acid polymers from corn. Applications in the textiles industry. J. Coat. Fabr. 2000, 29, 191–205. [Google Scholar] [CrossRef]
- Notta-Cuvier, D.; Odent, J.; Delille, R.; Murariu, M.; Lauro, F.; Raquez, J.M.; Bennani, B.; Dubois, P. Tailoring polylactide (PLA) properties for automotive applications: Effect of addition of designed additives on main mechanical properties. Polym. Test. 2014, 36, 1–9. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers-polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Karkhanis, S.S.; Matuana, L.M.; Stark, N.M.; Sabo, R.C. Effect of compounding approaches on fiber dispersion and performance of poly(lactic acid)/cellulose nanocrystal composite blown films; Society of Plastic Engineers: Anaheim, CA, USA, 2017; 6p, ISBN 978-0-692-88309-9. [Google Scholar]
- Balakrishnan, H.; Hassan, A.; Imran, M.; Wahit, M.U. Toughening of polylactic acid nanocomposites: A short review. Polym. Plast. Technol. Eng. 2012, 51, 175–192. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, K.A. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ueda, K. New polylactide-layered silicate nanocomposites. Concurrent improvements of material properties, biodegradability and melt rheology. Polymer 2003, 44, 857–866. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/Layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Sanz, Y.E.; Lagaron, J.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Pathakoti, K.; Manubolu, M.; Hwang, H.-M. Nanostructures: Current uses and future applications in food science. J. Food Drug Anal. 2017, 25, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef] [Green Version]
- Bee, S.-L.; Abdullah, M.A.A.; Bee, S.-T.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Kumar, A.P.; Depan, D.; Tomer, N.S.; Singh, R.P. Nanoscale particles for polymer degradation and stabilization-trends and future perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- De Paiva, L.B.; Morales, A.R.; Díaz, F.R.V. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Souza, P.M.S.; Morales, A.R.; Marin-Morales, M.A.; Mei, L.H.I. PLA and montmorilonite nanocomposites: Properties, biodegradation and potential toxicity. J. Polym. Environ. 2013, 21, 738–759. [Google Scholar] [CrossRef]
- Shah, K.J.; Mishra, M.K.; Shukla, A.D.; Imae, T.; Shah, D.O. Controlling wettability and hydrophobicity of organoclays modified with quaternary ammonium surfactants. J. Colloid Interface Sci. 2013, 407, 493–499. [Google Scholar] [CrossRef]
- Vergnes, B.; Lertwimolnum, W. Impact of processing conditions on the morphology, structure and properties of polymer-organoclay nanocomposites. In Polymer Nanocomposite Research Advanced; Nova Science Publishers, Inc.: New York, NY, USA, 2008; pp. 51–75. [Google Scholar]
- Adame, D.; Beall, G.W. Direct measurement of the constrained polymer region in polyamide/clay nanocomposites and the implications for gas diffusion. Appl. Clay Sci. 2009, 42, 545–552. [Google Scholar] [CrossRef]
- Picard, E.; Espuche, E.; Fulchiron, R. Effect of an organo-modified montmorillonite on PLA crystallization and gas barrier properties. Appl. Clay Sci. 2011, 53, 58–65. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, X.; Zhao, C.; Li, J. The crystalline and mechanical properties of PLA/layered silicate degradable composites. Polym. Sci. Ser. A 2012, 54, 393–400. [Google Scholar] [CrossRef]
- Lai, S.-M.; Wu, S.-H.; Lin, G.-G.; Don, T.-M. Unusual mechanical properties of melt-blended poly(lactic acid) (PLA)/clay nanocomposites. Eur. Polym. J. 2014, 52, 193–206. [Google Scholar] [CrossRef]
- Ogata, N.; Jimenez, G.; Kawai, H.; Ogihara, T. Structure and thermal/mechanical properties of poly(l-lactide)-clay blend. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 389–396. [Google Scholar] [CrossRef]
- Lewitus, D.; Mccarthy, A.S.; Ophir, A.A.; Kenig, A.S. The effect of nanoclays on the properties of plla-modified polymers part: Mechanical and thermal properties. J. Polym. Environ. 2006, 14, 171–177. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Biodegradable polylactide and its nanocomposites: Opening a new dimension for plastics and composites. Macromol. Rapid Commun. 2003, 24, 815–840. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Fujimoto, Y.; Ogami, A.; Ueda, K. New polylactide/layered silicate nanocomposites. 5. Designing of materials with desired properties. Polymer 2003, 44, 6633–6646. [Google Scholar] [CrossRef] [Green Version]
- Stloukal, P.; Pekařová, S.; Kalendova, A.; Mattausch, H.; Laske, S.; Holzer, C.; Chitu, L.; Bodner, S.; Maier, G.; Slouf, M.; et al. Kinetics and mechanism of the biodegradation of PLA/clay nanocomposites during thermophilic phase of composting process. Waste Manag. 2015, 42, 31–40. [Google Scholar] [CrossRef]
- Paul, M.A.; Delcourt, C.; Alexandre, M.; Degée, P.; Monteverde, F.; Dubois, P. Polylactide/montmorillonite nanocomposites: Study of the hydrolytic degradation. Polym. Degrad. Stab. 2005, 87, 535–542. [Google Scholar] [CrossRef]
- Machado, A.V.; Araújo, A.; Oliveira, M. Assessment of polymer-based nanocomposites biodegradability. Biodegrad. Polym. 2015, 1, 166–196. [Google Scholar]
- Balaguer, M.P.; Aliaga, C.; Fito, C.; Hortal, M. Compostability assessment of nano-reinforced poly(lactic acid) films. Waste Manag. 2016, 48, 143–155. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ogami, A.; Ueda, K. New polylactide/layered silicate nanocomposites, Structure, properties and biodegradability. Compos. Interfaces 2003, 10, 435–450. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ueda, K. Control of biodegradability of polylactide via nanocomposite technology. Macromol. Mater. Eng. 2003, 288, 203–208. [Google Scholar] [CrossRef]
- Pluta, M.; Galeski, A.; Alexandre, M.; Paul, M.-A.; Dubois, P. Polylactide/montmorillonite nanocomposites and microcomposites prepared by melt blending: Structure and some physical properties. J. Appl. Polym. Sci. 2002, 86, 1497–1506. [Google Scholar] [CrossRef]
- ASTM-D Standard. Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials under Controlled Composting Conditions; ASTM-D Standard: West Conshohocken, PA, USA, 2015. [Google Scholar]
- International Organization for Standardization. Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 1: General Method; ISO 14855-1:2012; ISO: Geneva, Switzerland, 2012; p. 2. [Google Scholar]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Ngouajio, M.; Fernandez, R.T. Development of an automatic laboratory-scale respirometric system to measure polymer biodegradability. Polym. Test. 2006, 25, 1006–1016. [Google Scholar] [CrossRef]
- Bellia, G.; Tosin, M.; Degli-Innocenti, F. Test method of composting in vermiculite is unaffected by the priming effect. Polym. Degrad. Stab. 2000, 69, 113–120. [Google Scholar] [CrossRef]
- Bellia, G.; Tosin, M.; Floridi, G.; Degli-Innocenti, F. Activated vermiculite, a solid bed for testing biodegradability under composting conditions. Polym. Degrad. Stab. 1999, 66, 65–79. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Insights on the aerobic biodegradation of polymers by analysis of evolved carbon dioxide in simulated composting conditions. Polym. Degrad. Stab. 2017, 137, 251–271. [Google Scholar] [CrossRef] [Green Version]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Perejón, A.; Sánchez-Jiménez, P.E.; Criado, J.M.; Pérez-Maqueda, L.A. Kinetic analysis of complex solid-state reactions. A new deconvolution procedure. J. Phys. Chem. B 2011, 115, 1780–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASTM-D Standard. Standard Test Method for Two-Sided Liquid Extraction of Plastic Materials Using FDA Migration Cell; ASTM-D Standard: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Alex, A.; Ilango, N.K.; Ghosh, P. Comparative Role of Chain Scission and Solvation in the Biodegradation of Polylactic Acid (PLA). J. Phys. Chem. B 2018, 122, 9516–9526. [Google Scholar] [CrossRef]
- Gleadall, A.C. Modelling Degradation of Biodegradable Polymers and Their Mechanical Properties. Ph.D. Dissertation, University of Leicester, Leicester, UK, 2015. [Google Scholar]
- Gorrasi, G.; Pantani, R. Hydrolysis and biodegradation of poly(lactic acid). In Romanian Reports in Physics; Springer: Berlin/Heidelberg, Germany, 2017; pp. 119–151. [Google Scholar] [CrossRef]
- Limsukon, W.; Auras, R.; Selke, S. Hydrolytic degradation and lifetime prediction of poly(lactic acid) modified with a multifunctional epoxy-based chain extender. Polym. Test. 2019, 80, 106108. [Google Scholar] [CrossRef]
- Castro-Aguirre, E. Increasing the Biodegradation Rate of Poly(Lactic Acid) In Composting Conditions. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2018. [Google Scholar]
- Lee, S.; Park, H.; Lim, H.; Kang, T.; Li, X.; Cho, W.; Ha, C. Microstructure, tensile properties and biodegradability of aliphatic polyester/clay nanocomposites. Polymer 2002, 43, 2495–2500. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Enhancing the biodegradation rate of poly(lactic acid) films and PLA bio-nanocomposites in simulated composting through bioaugmentation. Polym. Degrad. Stab. 2018, 154, 46–54. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, J.H.; An, I.-G.; Kim, C.; Lee, D.S.; Lee, Y.K.; Nam, J.-D. Electrospun dual-porosity structure and biodegradation morphology of Montmorillonite reinforced PLLA nanocomposite scaffolds. Biomaterials 2005, 26, 3165–3172. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Ikada, Y. Blends of crystalline and amorphous poly(lactide). III. Hydrolysis of solution-cast blend films. J. Appl. Polym. Sci. 1997, 63, 855–863. [Google Scholar] [CrossRef]
- Fukushima, K.; Giménez, E.; Cabedo, L.; Lagarón, J.M.; Feijoo, J.L. Biotic degradation of poly(dl-lactide) based nanocomposites. Polym. Degrad. Stab. 2012, 97, 1278–1284. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Auras, R.; Dolan, K.; Selke, S.; Holmes, D.; Rubino, M.; Soto-Valdez, H. Chemical recycling of poly(lactic acid) by water-ethanol solutions. Polym. Degrad. Stab. 2018, 149, 28–38. [Google Scholar] [CrossRef]
- SebastiaÄo; Canevarolo, V. Chain scission distribution function for polypropylene degradation during multiple extrusions. Polym. Degrad. Stab. 2000, 70, 71–76. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Auras, R.; Burgess, G.; Holmes, D.; Fang, X.; Rubino, M.; Soto-Valdez, H. Concurrent solvent induced crystallization and hydrolytic degradation of PLA by water-ethanol solutions. Polymer 2016, 99, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Chen, X. Modelling degradation of amorphous biodegradable polyesters: Basic model. Model. Degrad. Bioresorbable Polym. Med. Devices. 2015, 15–31. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Impact of Nanoclays on the Biodegradation of Poly(Lactic Acid) Nanocomposites. Polymers 2018, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Xanthos, M. Nanoclay and crystallinity effects on the hydrolytic degradation of polylactides. Polym. Degrad. Stab. 2018, 93, 1450–1459. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Dottori, M.; Armentano, I.; Kenny, J.M.; Camino, G. Effect of temperature and nanoparticle type on hydrolytic degradation of poly(lactic acid) nanocomposites. Polym. Degrad. Stab. 2011, 96, 2120–2129. [Google Scholar] [CrossRef]
- Hakkarainen, M.; Albertsson, A.C.; Karlsson, S. Weight losses and molecular weight changes correlated with the evolution of hydroxyacids in simulated in vivo degradation of homo- and copolymers of PLA and PGA. Polym. Degrad. Stab. 1996, 52, 283–291. [Google Scholar] [CrossRef]
- Tabasi, R.Y.; Ajji, A. Selective degradation of biodegradable blends in simulated laboratory composting. Polym. Degrad. Stab. 2015, 120, 435–442. [Google Scholar] [CrossRef]
- Degli-Innocenti, F. Biodegradation of plastics and ecotoxicity testing: When should it be done. Front. Microbiol. 2014, 5, 475. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly(lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]

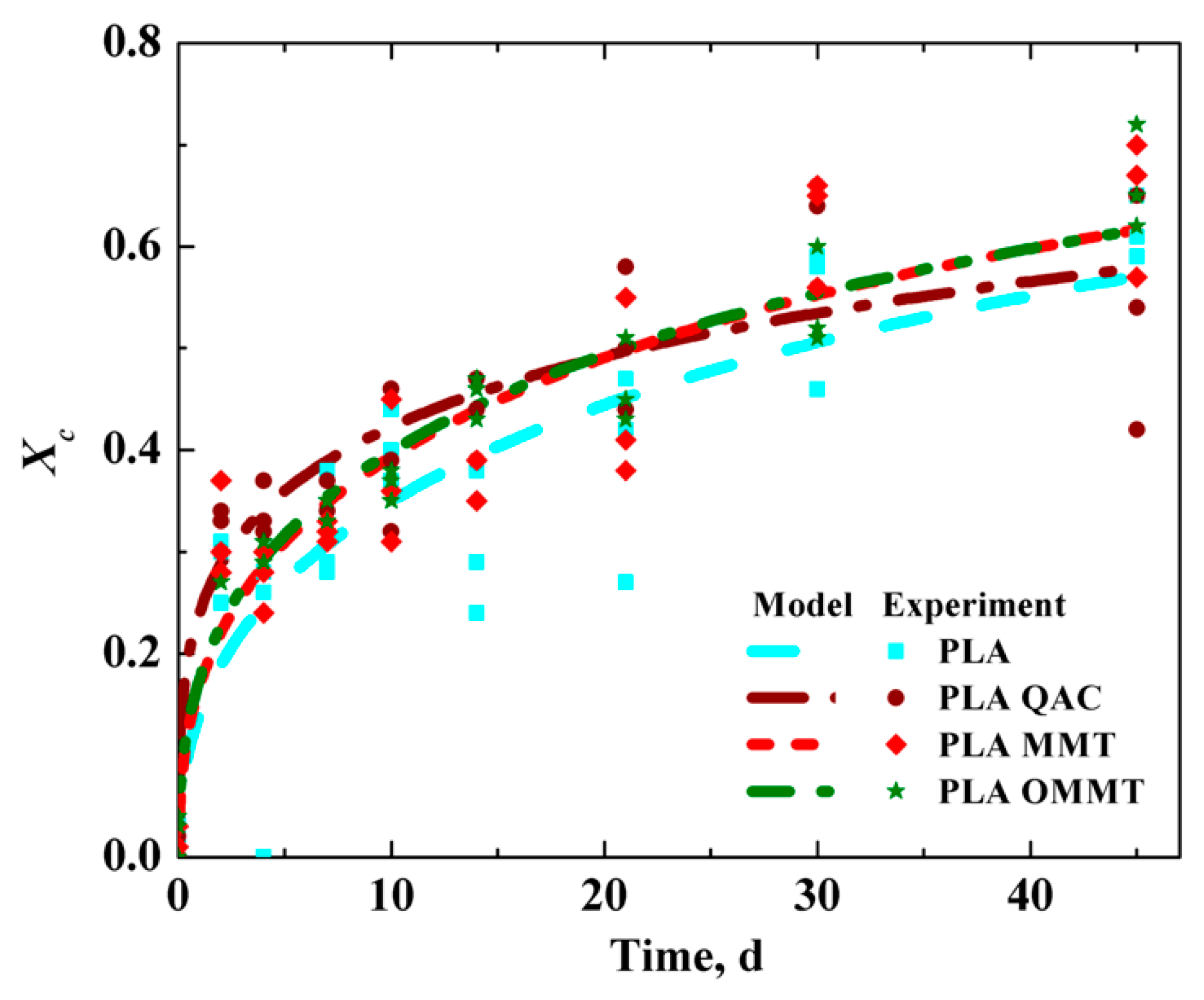

| Films | Mno, kDa | Mwo, kDa | SEM-Thickness (µm) | Crystallinity, Xc (%) |
|---|---|---|---|---|
| PLA | 109.8 ± 3.1 a | 185.5 ± 9.3 a | 19.8 ± 0.4 a | 3.7 ± 4.7 a |
| PLA QAC | 87.6 ± 4.2 b | 128.6 ± 4.8 b | 19.0 ± 3.8 a | 1.0 ± 1.0 a |
| PLA MMT | 111.1 ± 4.0 a | 160.4 ± 5.7 a | 17.4 ± 1.0 a | 2.3 ± 2.1 a |
| PLA OMMT | 114.3 ± 11.8 a | 170.4 ± 5.8 a | 21.5 ± 1.3 a | 1.7 ± 1.2 a |
| k (d−1) for Films in Different Media | |||
|---|---|---|---|
| Films | Inoculated Vermiculite | Non-inoculated Vermiculite | Water |
| PLA | 0.0735 ± 0.0137 a,A | 0.1035 ± 0.0120 a,A | 0.0828 ± 0.0081 a,A |
| PLA QAC | 0.0874 ± 0.0149 a,A | 0.0996 ± 0.0086 a,A | 0.1060 ± 0.0174 a,A |
| PLA MMT | 0.0843 ± 0.0113 a,A | 0.0986 ± 0.0085 a,A | 0.0973 ± 0.0126 a,A |
| PLA OMMT | 0.1087 ± 0.0211 a,A | 0.0959 ± 0.0077 a,A | 0.1002 ± 0.0110 a,A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayekar, P.C.; Castro-Aguirre, E.; Auras, R.; Selke, S.; Narayan, R. Effect of Nano-Clay and Surfactant on the Biodegradation of Poly(Lactic Acid) Films. Polymers 2020, 12, 311. https://doi.org/10.3390/polym12020311
Mayekar PC, Castro-Aguirre E, Auras R, Selke S, Narayan R. Effect of Nano-Clay and Surfactant on the Biodegradation of Poly(Lactic Acid) Films. Polymers. 2020; 12(2):311. https://doi.org/10.3390/polym12020311
Chicago/Turabian StyleMayekar, Pooja C., Edgar Castro-Aguirre, Rafael Auras, Susan Selke, and Ramani Narayan. 2020. "Effect of Nano-Clay and Surfactant on the Biodegradation of Poly(Lactic Acid) Films" Polymers 12, no. 2: 311. https://doi.org/10.3390/polym12020311






