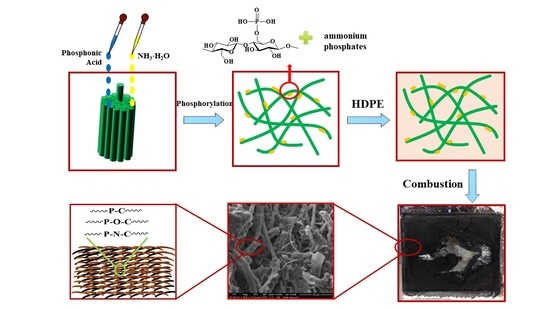
Flame Retardancy of High-Density Polyethylene Composites with P,N-Doped Cellulose Fibrils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
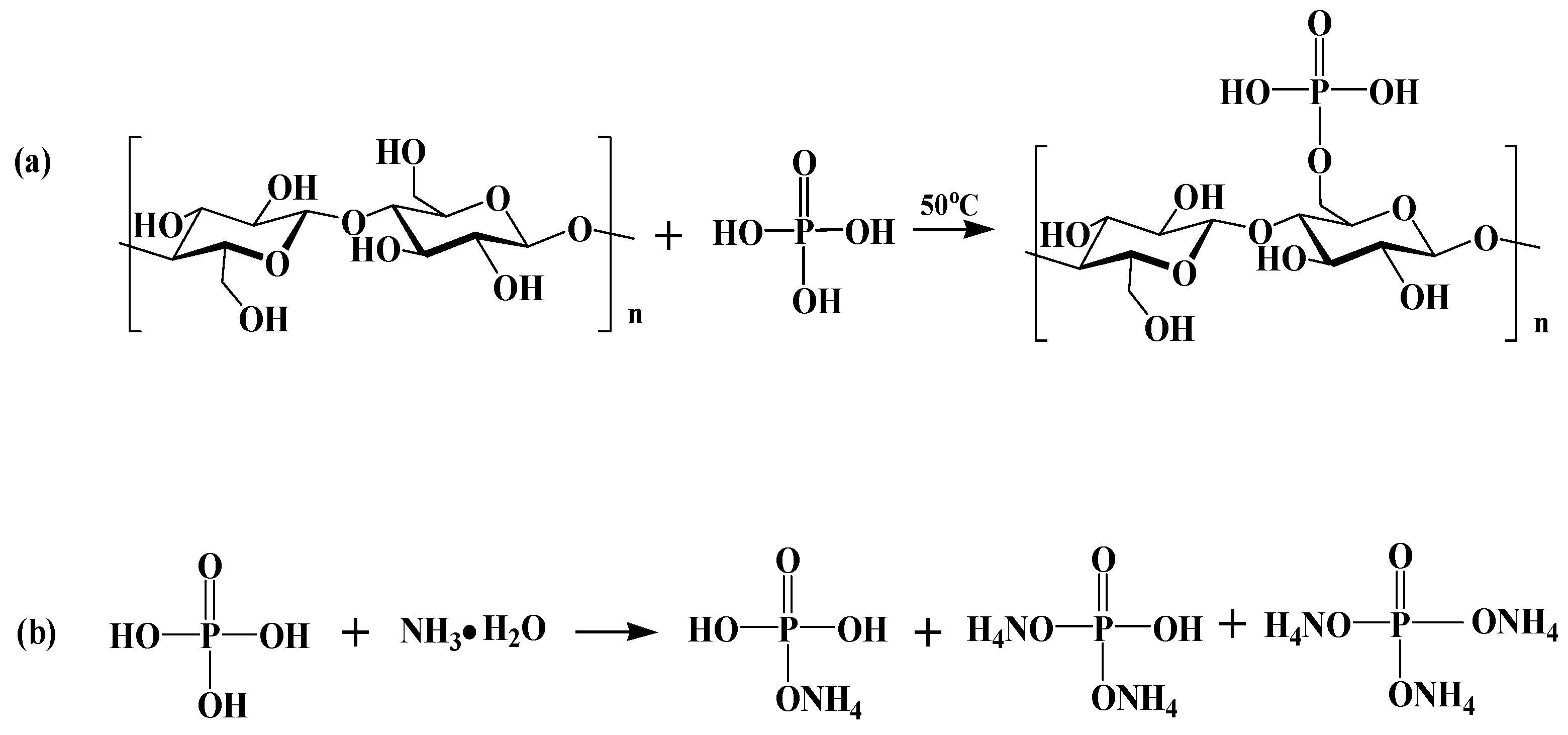
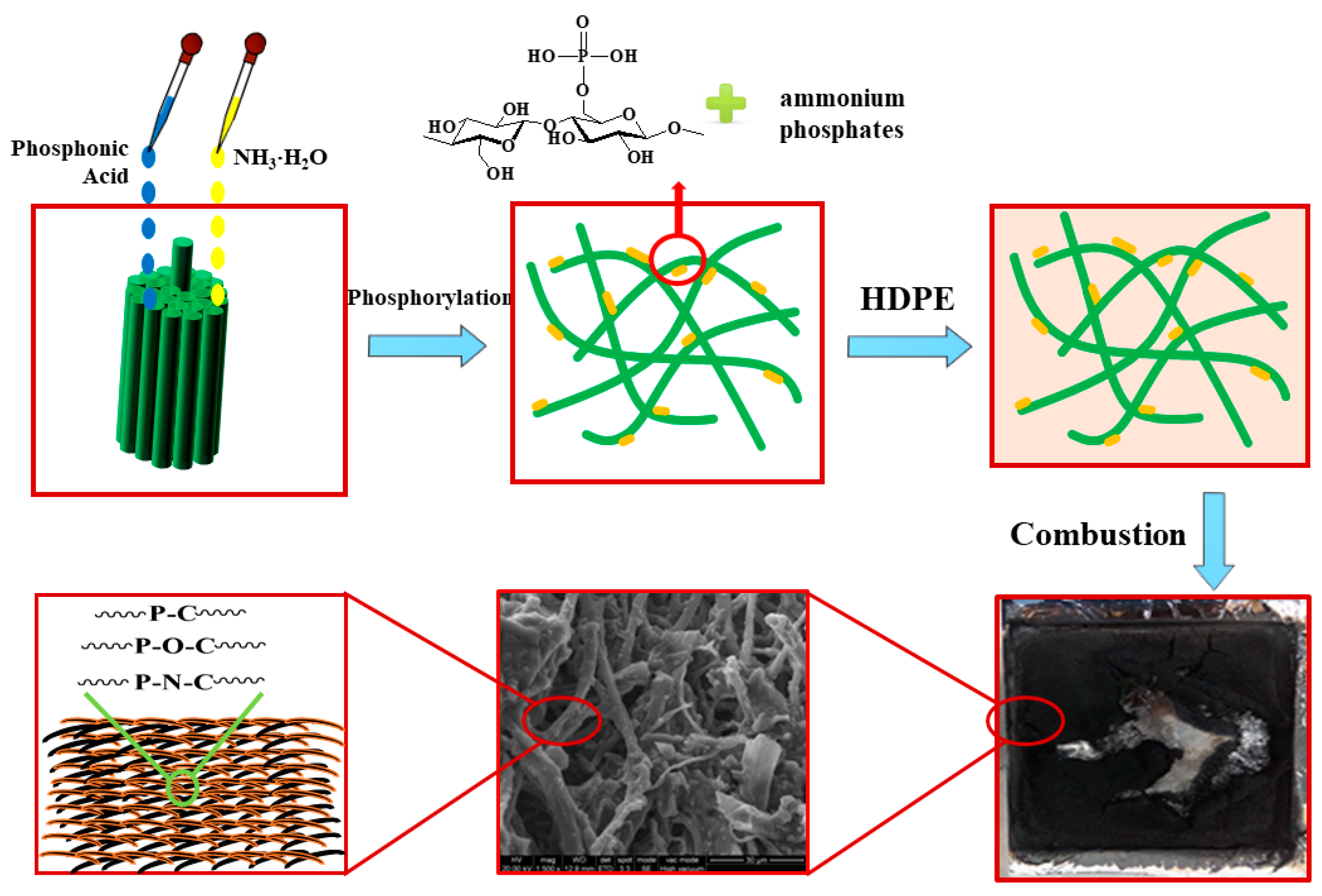
2.2. Fabrication of P,N-Doped Cellulose Fibrils
2.3. HDPE Composite Preparation
2.4. Characterization
3. Results
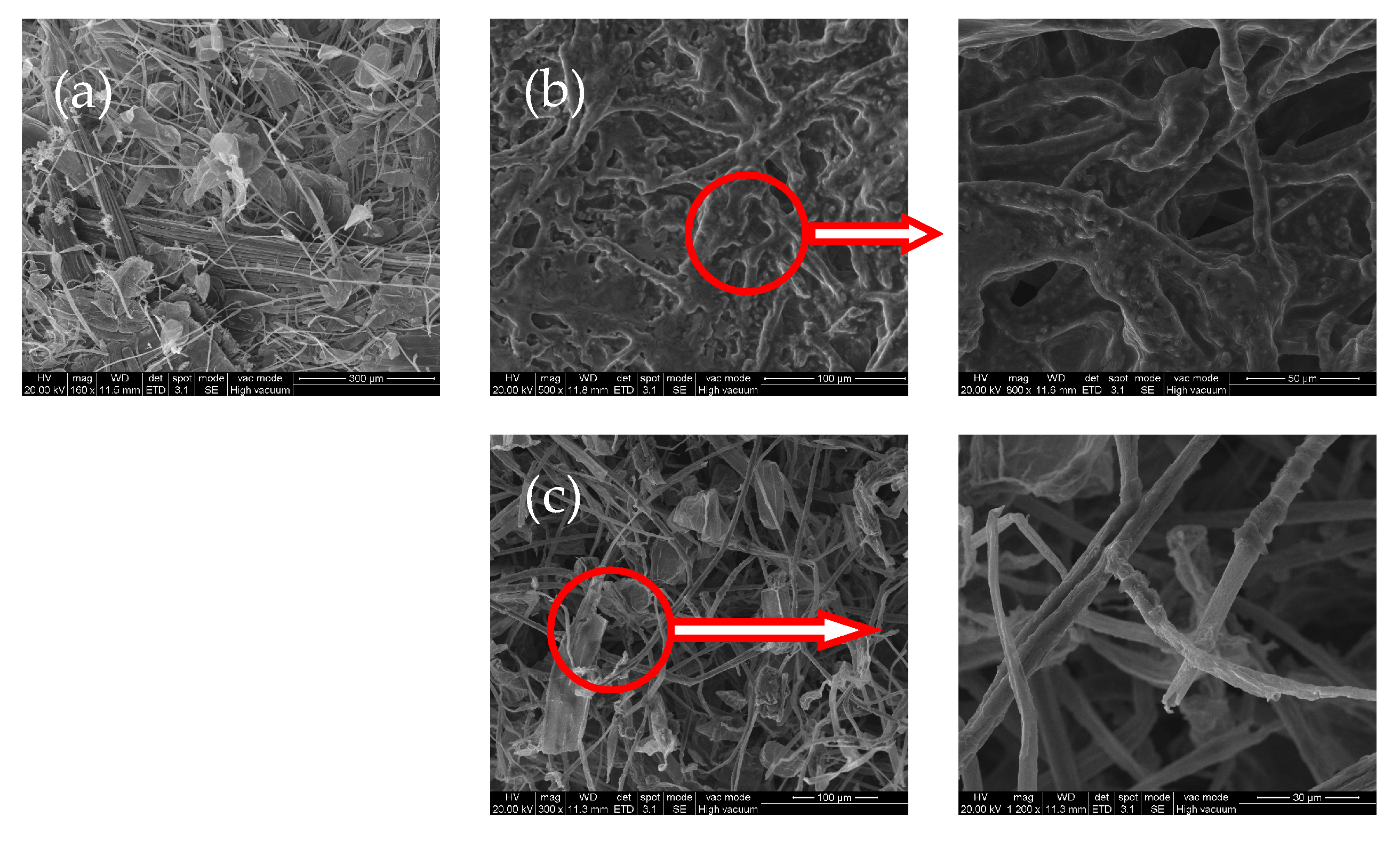
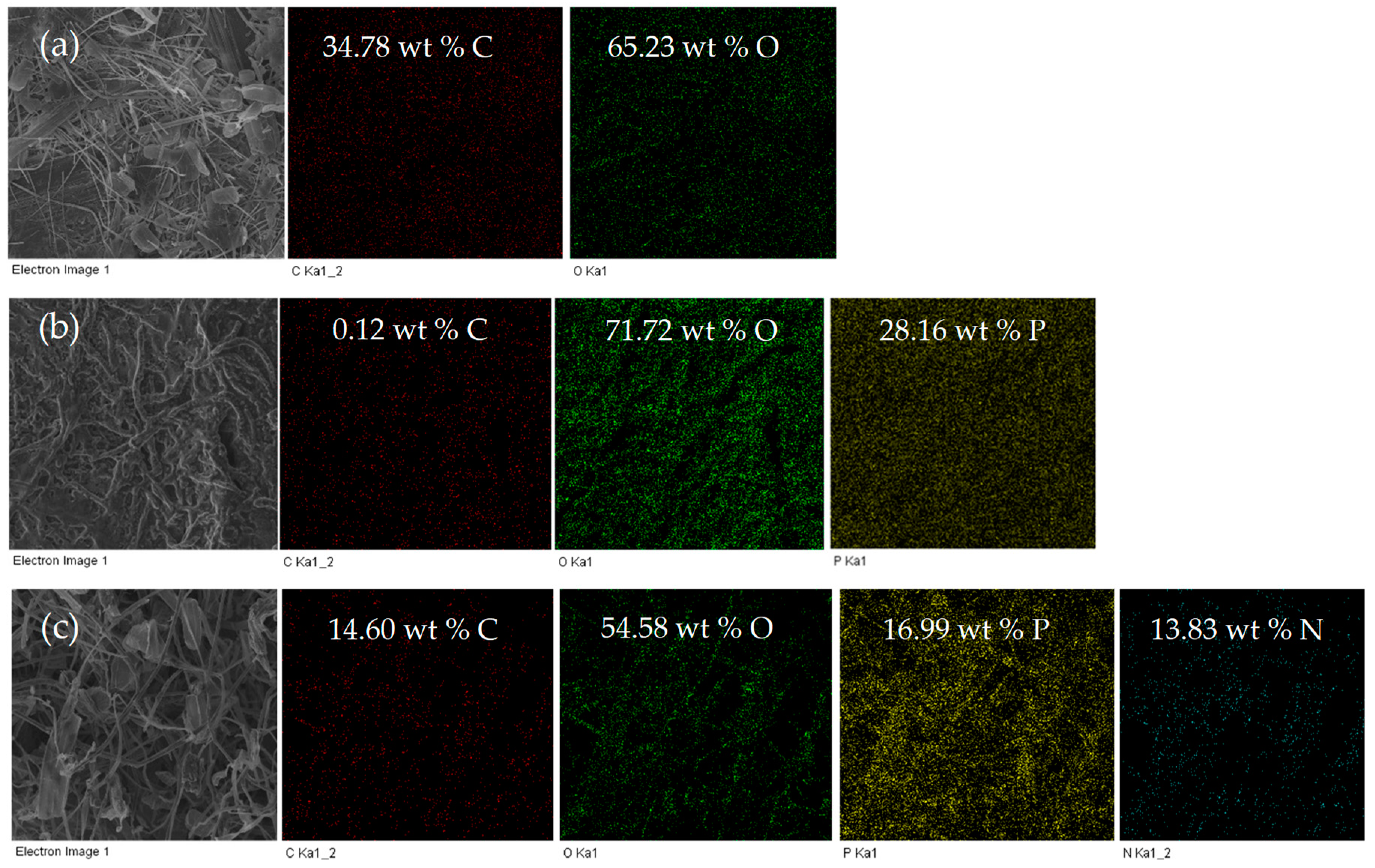
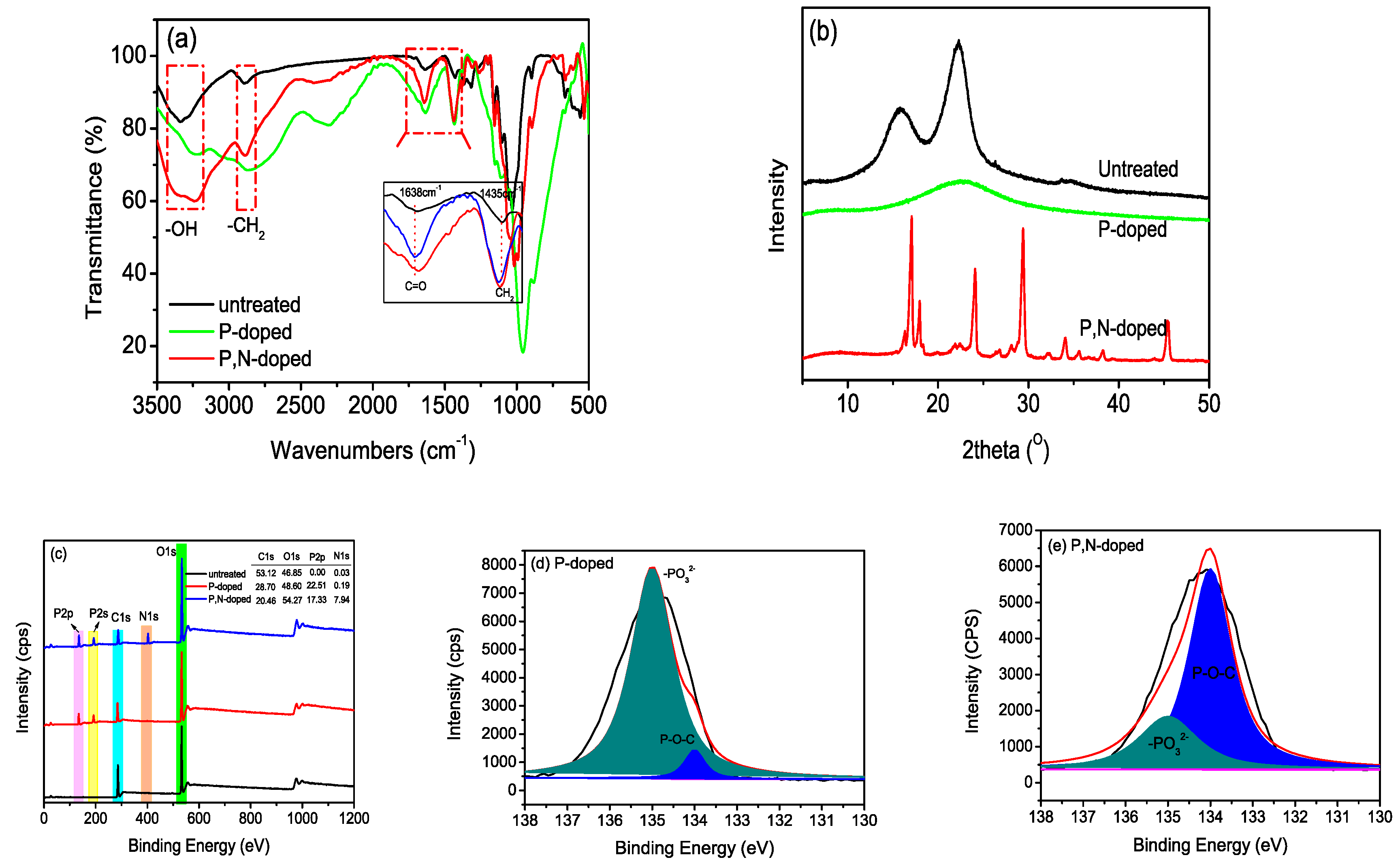
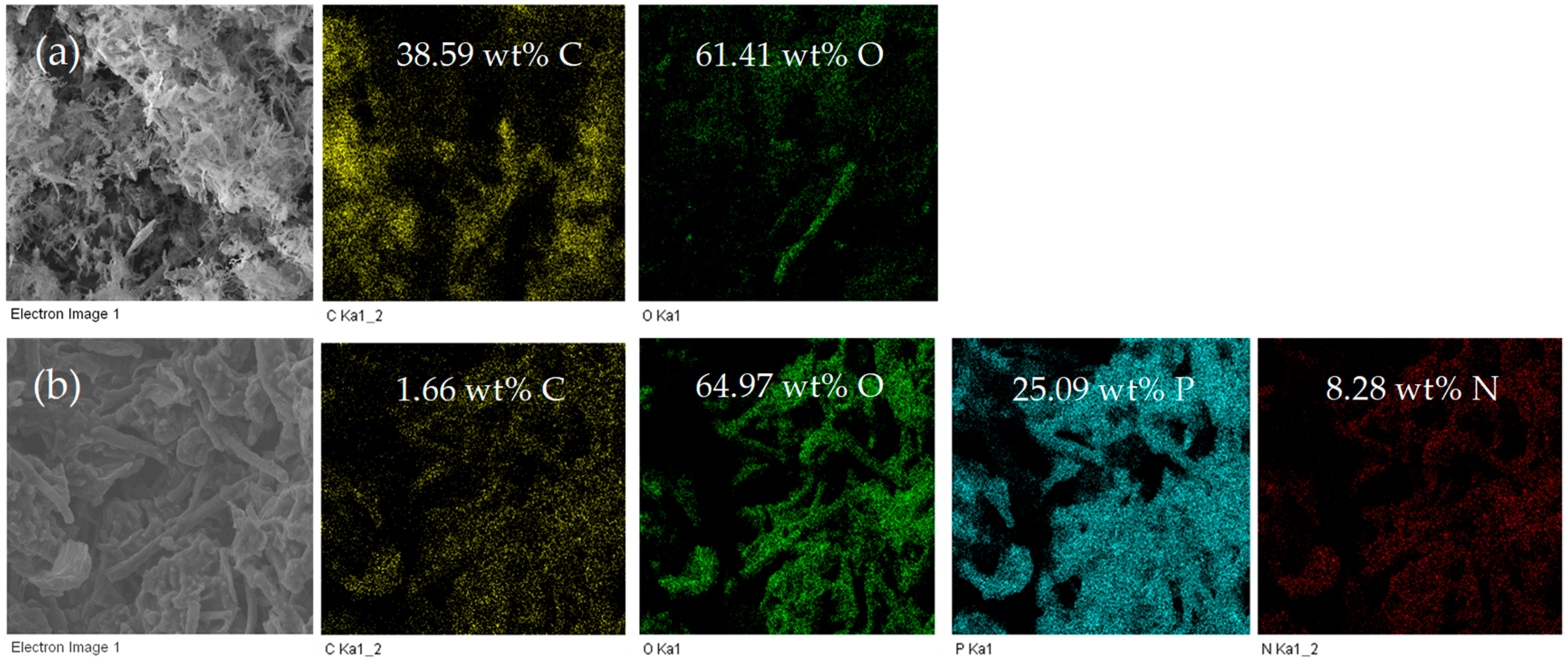
3.1. Morphology of P,N-Doped Cellulose Fibrils
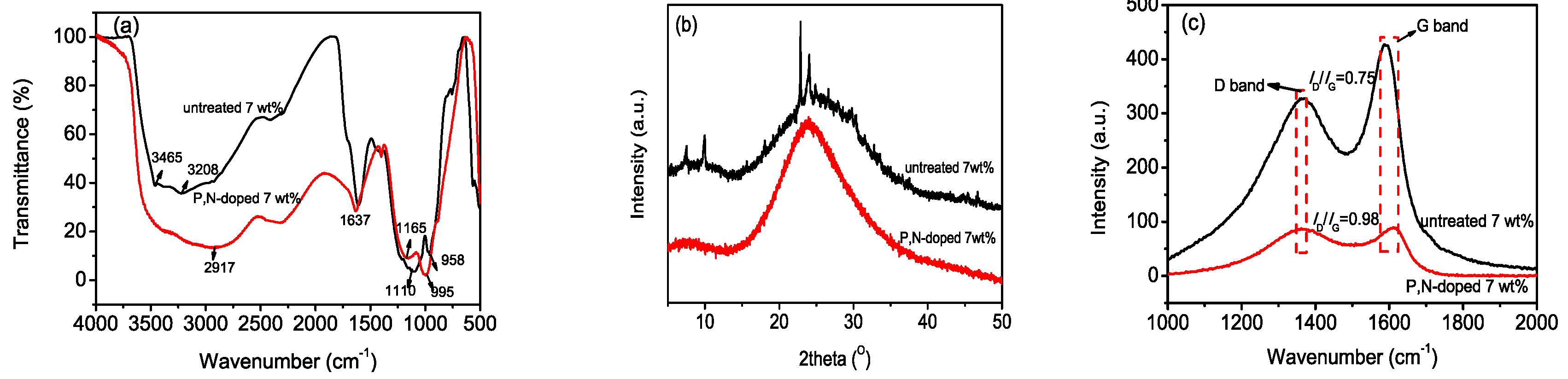
3.2. Chemical Property of P,N-Doped Cellulose Fibrils
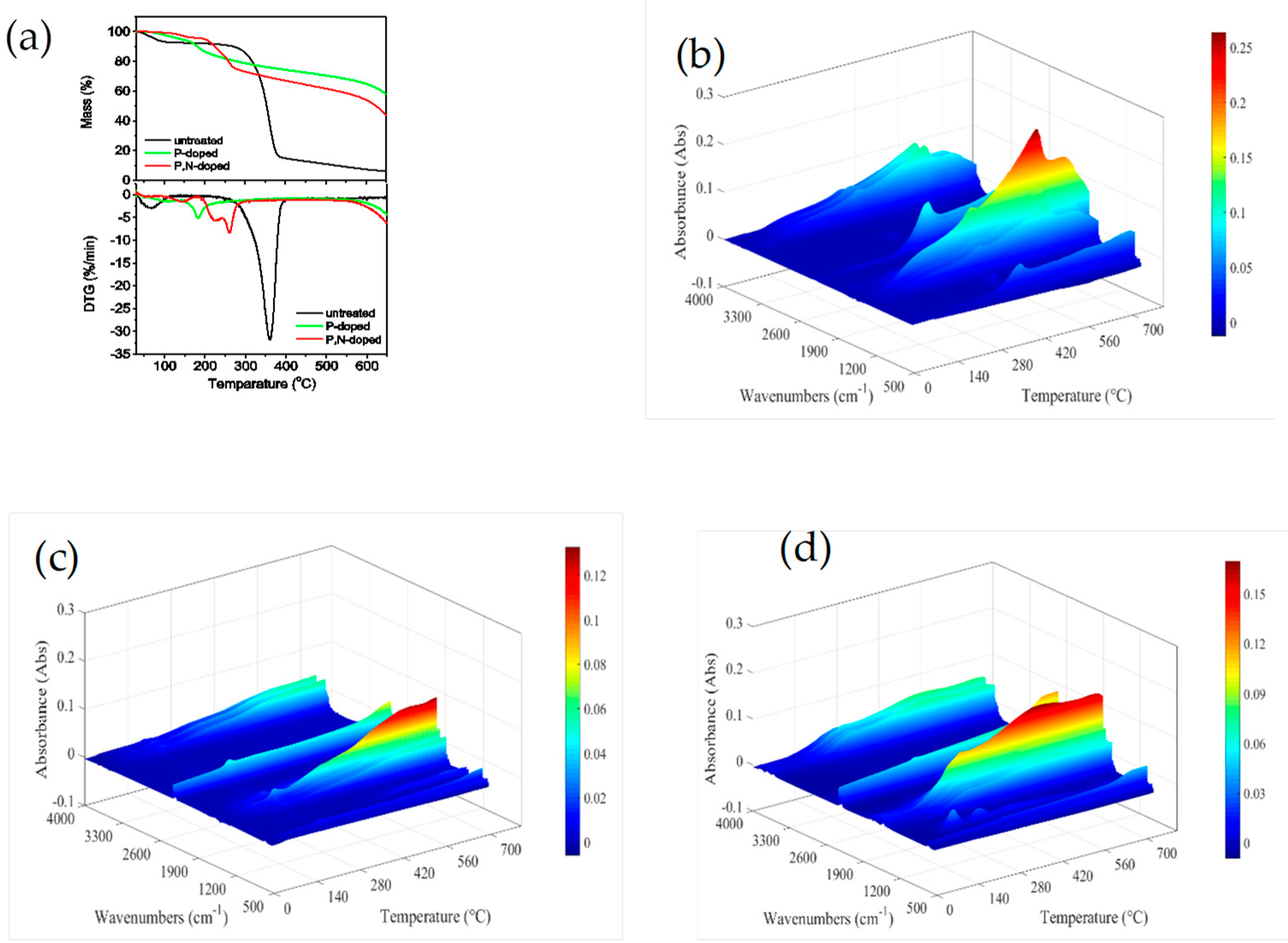
3.3. Thermal Property
3.3.1. Thermal Property of P,N-Doped Cellulose Fibrils
3.3.2. Thermal Property of HDPE Composites
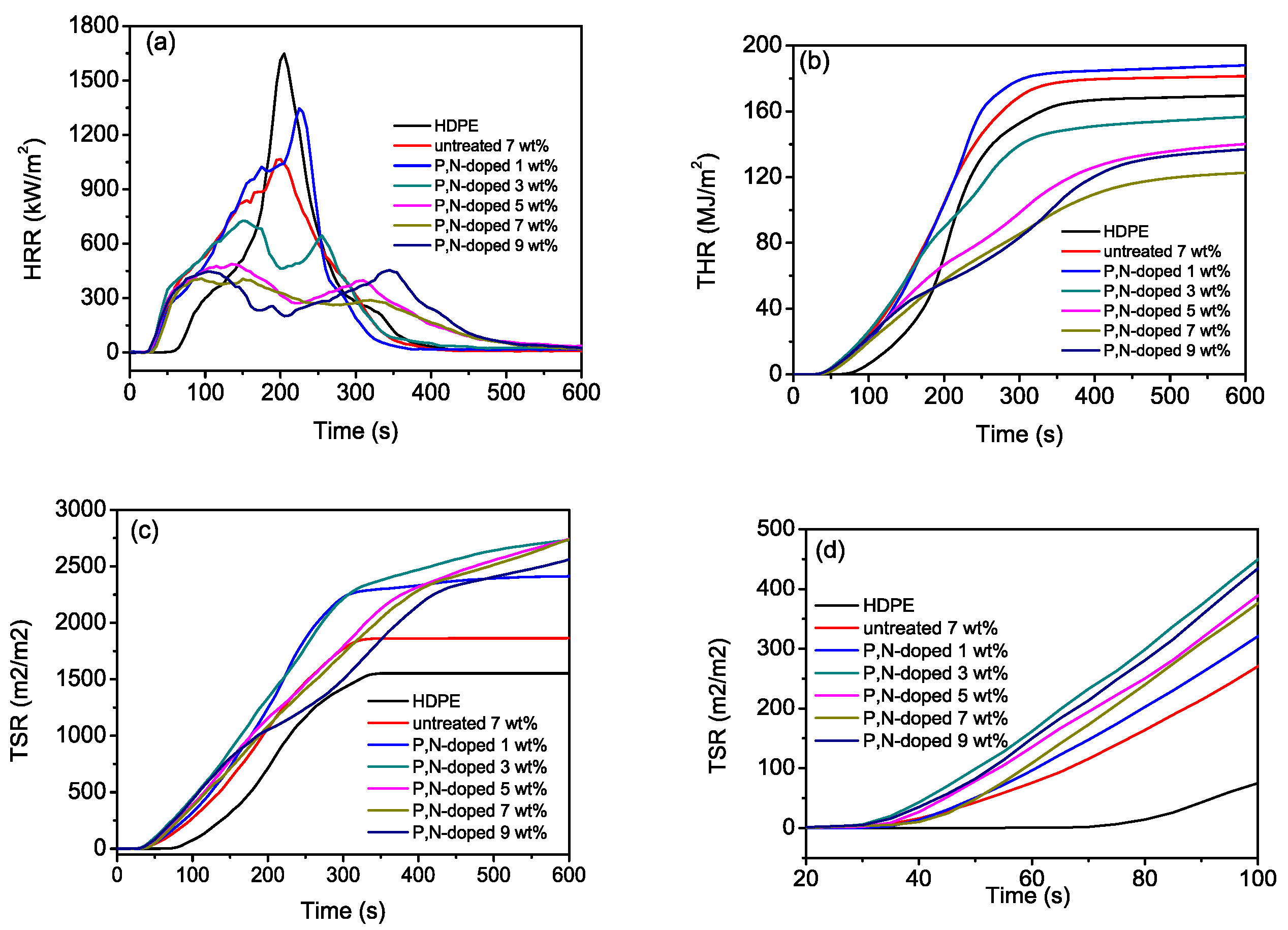
3.4. Flame Retardancy of HDPE Composites
MLR-Mass Loss Rate
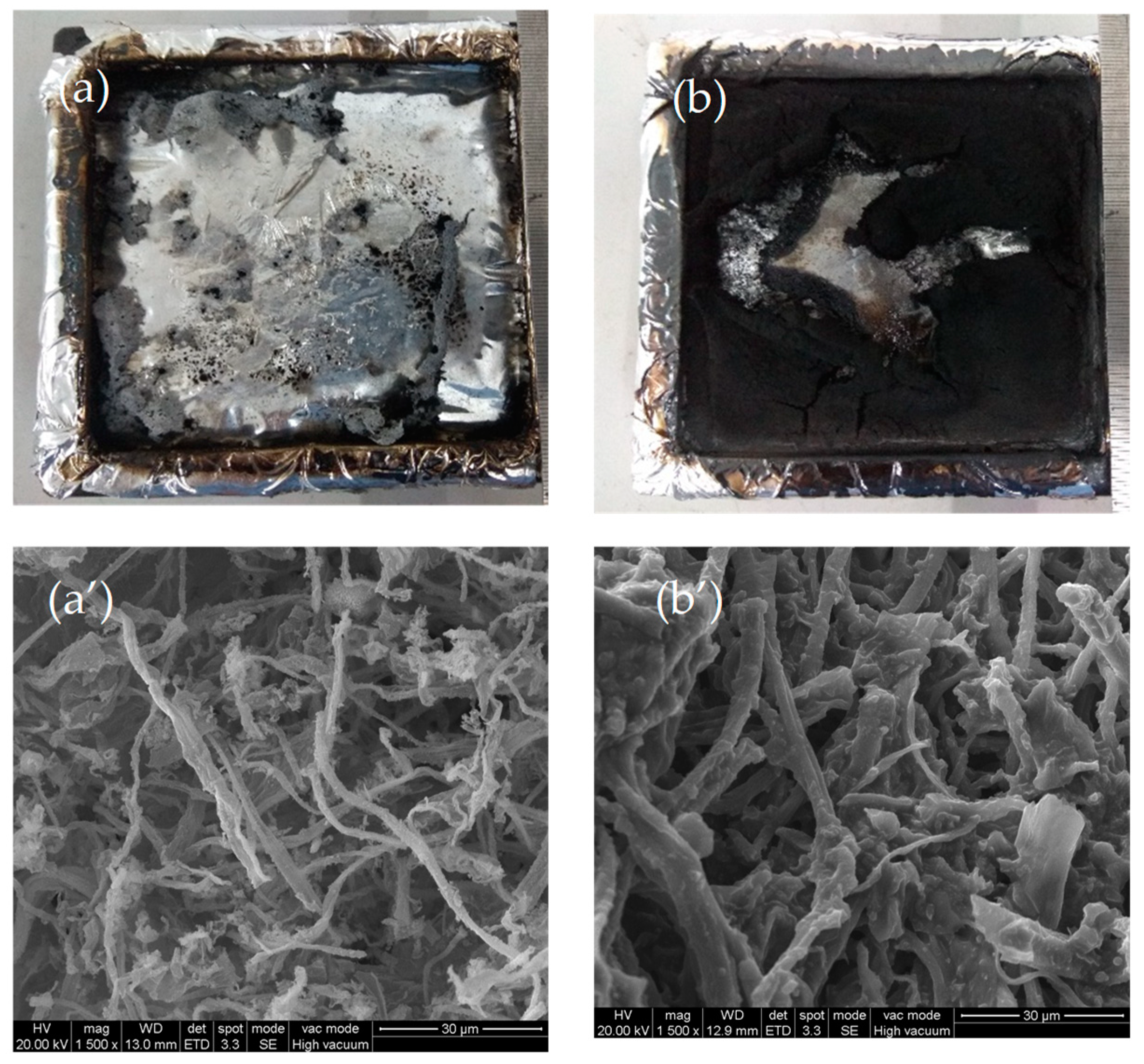
3.5. Morphology and Structure of Char Residues
3.6. Mechanism for Flame Retardancy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Habibi, Y.; Ei-Zawawy, W.K.; Ibrahim, M.M.; Dufresne, A. Processing and characterization of reinforced polyethylene composites made with lignocellulosic fibers from Egyptian agro-industrial residues. Compos. Sci. Technol. 2008, 68, 1877–1885. [Google Scholar] [CrossRef] [Green Version]
- Schartel, B.; Braun, U.; Schwarz, U.; Reinemann, S. Fire retardancy of polypropylene/flax blends. Polymer 2003, 44, 6241–6250. [Google Scholar] [CrossRef]
- Li, B.; He, J.M. Investigation of mechanical property, flame retardancy and thermal degradation of LLDPE wood-fibre composites. Polym. Degrad. Stab. 2004, 83, 241–246. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, S.; Wang, F.; Yan, Y.; Li, J.; Zhang, W. Effect of microencapsulated ammonium polyphosphate on flame retardancy and mechanical properties of wood flour/polypropylene composites. Polym. Compos. 2016, 37, 666–673. [Google Scholar] [CrossRef]
- Song, P.A.; Yu, Y.; Wu, Q.; Fu, S. Facile fabrication of HDPE-g-MA/nanodiamond nanocomposites via one-step reactive blending. Nanoscale Res. Lett. 2012, 7, 355. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.; Mei, C.; Du, J.; Li, G. Synergistic effect of nano silicon dioxide and ammonium polyphosphate on flame retardancy of wood fiber-polyethylene composites. Compos. Part A 2014, 66, 128–134. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Gaan, S.; Sun, G.; Hutches, K.; Engelhard, M.H. Effect of nitrogen additives on flame retardant action of tributyl phosphate: Phosphorus–nitrogen synergism. Polym. Degrad. Stabil. 2008, 93, 99–108. [Google Scholar] [CrossRef]
- Shi, X.H.; Xu, Y.J.; Long, J.W.; Zhao, Q.; Ding, X.M.; Chen, L.; Wang, Y.Z. Layer-by-layer assembled flame-retardant architecture toward high-performance carbon fiber composite. Chem. Eng. J. 2018, 353, 550–558. [Google Scholar] [CrossRef]
- Wang, S.; Xin, F.; Chen, Y.; Qian, L.; Chen, Y. Phosphorus-nitrogen containing polymer wrapped carbon nanotubes and their flame-retardant effect on epoxy resin. Polym. Degrad. Stabil. 2016, 129, 133–141. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Khelifa, F.; Rose, G.; Brohez, S.; Delvosalle, C.; Dubois, P. Cellulose/phosphorus combinations for sustainable fire retarded polylactide. Eur. Polym. J. 2016, 74, 218–228. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Li, C.; Yan, M.; Wu, Y.; Cao, J.; He, S. Fabrication of nano-crystalline cellulose with phosphoric acid and its full application in a modified polyurethane foam. Polym. Degrad. Stabil. 2013, 98, 1940–1944. [Google Scholar] [CrossRef]
- Ghanadpour, M.; Carosio, F.; Larsson, P.T.; Wagberg, L. Phosphorylated cellulose nanofibrils: A renewable nanomaterial for the preparation of intrinsically flame-retardant materials. Biomacromolecules 2015, 16, 3399–3410. [Google Scholar] [CrossRef]
- Feng, J.; Sun, Y.; Song, P.; Lei, W.; Wu, Q.; Liu, L.; Yu, Y.; Wang, H. Fire-resistant, strong, and green polymer nanocomposites based on poly(lactic acid) and core-shell nanofibrous flame retardants. ACS Sustain. Chem. Eng. 2017, 5, 7894–7904. [Google Scholar] [CrossRef]
- Yin, W.; Chen, L.; Lu, F.; Song, P.; Dai, J.; Meng, L. Mechanically robust, flame-retardant poly(lactic acid) biocomposites via combining cellulose nanofibers and ammonium polyphosphate. ACS Omega 2018, 3, 5615–5626. [Google Scholar] [CrossRef]
- Zhang, F.; Qiu, W.; Yang, L.; Endo, T.; Hirotsu, T. Mechanochemical preparation and properties of a cellulose–polyethylene composite. J. Mater. Chem. 2002, 12, 24–26. [Google Scholar]
- Araujo, J.R.; Mano, B.; Teixeira, G.M.; Spinace, M.A.S.; De Paolia, M.-A. Biomicrofibrilar composites of high density polyethylene reinforced with curauá fibers: Mechanical, interfacial and morphological properties. Compos. Sci. Technol. 2010, 70, 1637–1644. [Google Scholar] [CrossRef]
- Diallo, A.K.; Jahier, C.; Drolet, R.; Tolnai, B.; Montplaisir, D. Cellulose filaments reinforced low-density polyethylene. Polym. Compos. 2019, 40, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Azouz, K.B.; Ramires, E.C.; Fonteyne, W.V.D.; Kissi, N.E.; Dufresne, A. Simple method for the melt extrusion of a cellulose nanocrystal reinforced hydrophobic polymer. ACS Macr. Lett. 2012, 1, 236–240. [Google Scholar] [CrossRef]
- Mariano, M.; Kissi, N.E.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. B-Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, X.; Zhang, J.; Guo, D.; Kong, F.; Zhang, N. Extraction of cellulose nano-crystals from old corrugated container fiber using phosphoric acid and enzymatic hydrolysis followed by sonication. Carbohydr. Polym. 2015, 125, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Granja, P.L.; Pouységu, L.; Pétraud, M.; De Jeso, B.; Baquey, C.; Barbosa, M.A. Cellulose phosphates as biomaterials. I. Synthesis and characterization of highly phosphorylated cellulose gels. J. Appl. Polym. Sci. 2010, 82, 3341–3353. [Google Scholar] [CrossRef]
- Du, J.; Zhao, G.; Pan, M.; Zhuang, L.; Li, D.; Zhang, R. Crystallization and mechanical properties of reinforced PHBV composites using melt compounding: Effect of CNCs and CNFs. Carbohydr. Polym. 2017, 168, 255–262. [Google Scholar]
- Chen, D.; Liu, D.; Zhang, H.; Chen, Y.; Li, Q. Bamboo pyrolysis using TG-FTIR and a lab-scale reactor: Analysis of pyrolysis behavior, product properties, and carbon and energy yields. Fuel 2015, 148, 79–86. [Google Scholar] [CrossRef]
- Woodcock, C.; Sarko, A. Packing analysis of carbohydrates and polysaccharides. 11. Molecular and crystal structure of native ramie cellulose. Macromolecules 1980, 13, 1183–1187. [Google Scholar] [CrossRef]
- Peng, Y.; Gardner, D.J.; Han, Y.; Kiziltas, A.; Cai, Z.; Tshabalala, M.A. Influence of drying method on the material properties of nanocellulose I: Thermostability and crystallinity. Cellulose 2013, 20, 2379–2392. [Google Scholar] [CrossRef]
- Lipson, H.; Steeple, H. Interpretation of X-ray Powder Diffraction Patterns, 5th ed; Macmillan: New York, NY, USA, 1970. [Google Scholar]
- Jiang, D.; Pan, M.Z.; Cai, X.; Zhao, Y.T. Flame retardancy of rice straw-polyethylene composites affected by in situ polymerization of ammonium polyphosphate/silica. Compos. Part A. 2018, 109, 1–9. [Google Scholar] [CrossRef]
- Rao, W.H.; Xu, H.X.; Xu, Y.J.; Qi, M.; Liao, W.; Xu, S.; Wang, Y.Z. Persistently flame-retardant flexible polyurethane foams by a novel phosphorus-containing polyol. Chem. Eng. J. 2018, 343, 198–206. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.P.; Cui, J.; Lynd, L.R.; Kuang, L.R.A. Transition from cellulose swelling to cellulose dissolution by O-phosphoric acid: Evidence from enzymatic hydrolysis and supramolecular structure. Biomacromolecules 2006, 7, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhong, L.; Zhang, C.; Zhang, F.; Zhang, G. A novel reactive phosphorous flame retardant for cotton fabrics with durable flame retardancy and high whiteness due to self-buffering. Cellulose 2018, 25, 5479–5497. [Google Scholar] [CrossRef]
- Dobele, G.; Rossinskaja, G.; Telysheva, G.; Meier, D.; Faix, O. Cellulose dehydration and depolymerization reactions during pyrolysis in the presence of phosphoric acid. J. Anal. Appl. Pyrol. 1999, 49, 307–317. [Google Scholar] [CrossRef]
- Yan, Y.W.; Chen, L.; Jian, R.K.; Kong, S.; Wang, Y.Z. Intumescence: An effect way to flame retardance and smoke suppression for polystyrene. Polym. Degrad. Stabil. 2012, 97, 1423–1431. [Google Scholar] [CrossRef]
- Seefeldt, H.; Braun, U.; Wagner, M.H. Residue stabilization in the fire retardancy of wood-plastic composites: Combination of ammonium polyphosphate, expandable graphite, and red phosphorus. Macromol. Chem. Phys. 2012, 213, 2370–2377. [Google Scholar] [CrossRef]
- Nikolaeva, M.; Kärki, T. Reaction-to-fire properties of wood-polypropylene composites containing different fire retardants. Fire Technol. 2015, 51, 53–65. [Google Scholar] [CrossRef]
- Huang, B.; Wang, X.; Fang, H.; Jiang, S.; Hou, H. Mechanically strong sulfonated polybenzimidazole PEMs with enhanced proton conductivity. Mater. Lett. 2019, 234, 354–356. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Wang, Y.; Yan, H.; Zhang, X.; Xu, B. Highly efficient flame retardant, flexible, and strong adhesive intumescent coating on polypropylene using hyperbranched polyamide. Chem. Eng. J. 2017, 324, 237–250. [Google Scholar] [CrossRef]
- Duval, B.; Guet, J.M.; Richard, J.R.; Rouzaud, J.N. Coke properties and their microtexture. Part III: First results about relationship between microtexture and reactivity of some cokes. Fuel. Process. Technol. 1988, 20, 163–175. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, F.; Luo, W.; Zhu, S.; Zhao, M.; Natarajan, B.; Dai, J.; Zhou, L.; Ji, X.; Yassar, R.S.; et al. Low temperature carbonization of cellulose nanocrystals for high performance carbon anode of sodium-ion batteries. Nano Energy 2017, 33, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Qian, M.; Song, P.; Huang, G.; Yu, Y.; Fu, S. Fabrication of green lignin-based flame retardants for enhancing the thermal and fire retardancy properties of polypropylene/wood composites. Acs. Sustain. Chem. Eng. 2016, 4, 2422–2431. [Google Scholar] [CrossRef]
- Gu, W.; Sevilla, M.; Magasinski, A.; Fuertes, A.B.; Yushin, G. Sulfur-containing activated carbons with greatly reduced content of bottle neck pores for double-layer capacitors: A case study for pseudocapacitance detection. Energ. Environ. Sci. 2013, 6, 2465–2476. [Google Scholar] [CrossRef] [Green Version]

| Samples | LOI | Average HRR | Peak HRR | at time | TTI | THR | FGR | EHC | SEA | TSR | MLR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | kW/m2 | kW/m2 | s | s | MJ/m2 | kW/m2· s | MJ/kg | m2/kg | m2/m2 | g/s | |
| HDPE | 19.7 (0.15) | 263.78 (3.93) | 1519.21 (183.04) | 200 (7.07) | 53 (5.66) | 170.16 (0.68) | 7.58 (0.65) | 44.63 (0.48) | 414.48 (39.51) | 1621.64 (97.47) | 0.0522 (0.0014) |
| untreated 7 wt % | 20.3 (0.15) | 260.98 (12.89) | 1035.73 (41.75) | 197.50 (3.54) | 24.5 (0.71) | 176.17 (8.70) | 5.24 (0.12) | 43.42 (0.48) | 446.22 (4.20) | 1832.06 (44.07) | 0.0532 (0.0021) |
| P,N-doped 1 wt % | 20.4 (0.21) | 277.90 (4.72) | 1417.39 (100.69) | 222.50 (3.54) | 24.5 (0.71) | 187.59 (3.20) | 6.37 (0.55) | 45.33 (0.71) | 557.87 (37.56) | 2313.94 (151.58) | 0.0544 (0.0003) |
| P,N-doped 3 wt % | 23.5 (0.17) | 230.36 (7.86) | 765.05 (55.24) | 145.00 (7.07) | 23 (2.83) | 155.49 (5.31) | 5.29 (0.64) | 38.83 (0.38) | 683.56 (0.85) | 2737.71 (63.94) | 0.0524 (0.0013) |
| P,N-doped 5 wt % | 25.1 (0.15) | 201.97 (15.69) | 533.33 (65.39) | 132.50 (3.54) | 21 (1.41) | 136.82 (9.94) | 4.03 (0.60) | 36.12 (0.61) | 724.37 (22.28) | 2745.81 (237.12) | 0.0494 (0.0030) |
| P,N-doped 7 wt % | 25.7 (0.11) | 185.76 (0.18) | 412.30 (5.96) | 95.00 (0.00) | 25 (1.41) | 124.93 (0.52) | 4.34 (0.06) | 32.55 (0.21) | 776.35 (5.550) | 2981.84 (15.12) | 0.0504 (0.0003) |
| P,N-doped 9 wt % | 25.3 (0.15) | 201.53 (3.82) | 447.11 (9.76) | 217.50 (180.31) | 20 (0.00) | 137.04 (2.60) | 3.10 (2.53) | 34.57 (0.25) | 701.07 (25.75) | 2777.96 (28.79) | 0.0516 (0.0012) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Chen, H.; Zhang, Y.; Zhang, Y.-m.; Kan, W.; Pan, M. Flame Retardancy of High-Density Polyethylene Composites with P,N-Doped Cellulose Fibrils. Polymers 2020, 12, 336. https://doi.org/10.3390/polym12020336
Zhang S, Chen H, Zhang Y, Zhang Y-m, Kan W, Pan M. Flame Retardancy of High-Density Polyethylene Composites with P,N-Doped Cellulose Fibrils. Polymers. 2020; 12(2):336. https://doi.org/10.3390/polym12020336
Chicago/Turabian StyleZhang, Shuai, He Chen, Yin Zhang, Yi-meng Zhang, Weiyan Kan, and Mingzhu Pan. 2020. "Flame Retardancy of High-Density Polyethylene Composites with P,N-Doped Cellulose Fibrils" Polymers 12, no. 2: 336. https://doi.org/10.3390/polym12020336
APA StyleZhang, S., Chen, H., Zhang, Y., Zhang, Y.-m., Kan, W., & Pan, M. (2020). Flame Retardancy of High-Density Polyethylene Composites with P,N-Doped Cellulose Fibrils. Polymers, 12(2), 336. https://doi.org/10.3390/polym12020336