Novel Superadsorbent Highly Porous Hydrogel Based on Arabic Gum and Acrylamide Grafts for Fast and Efficient Methylene Blue Removal
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Techniques
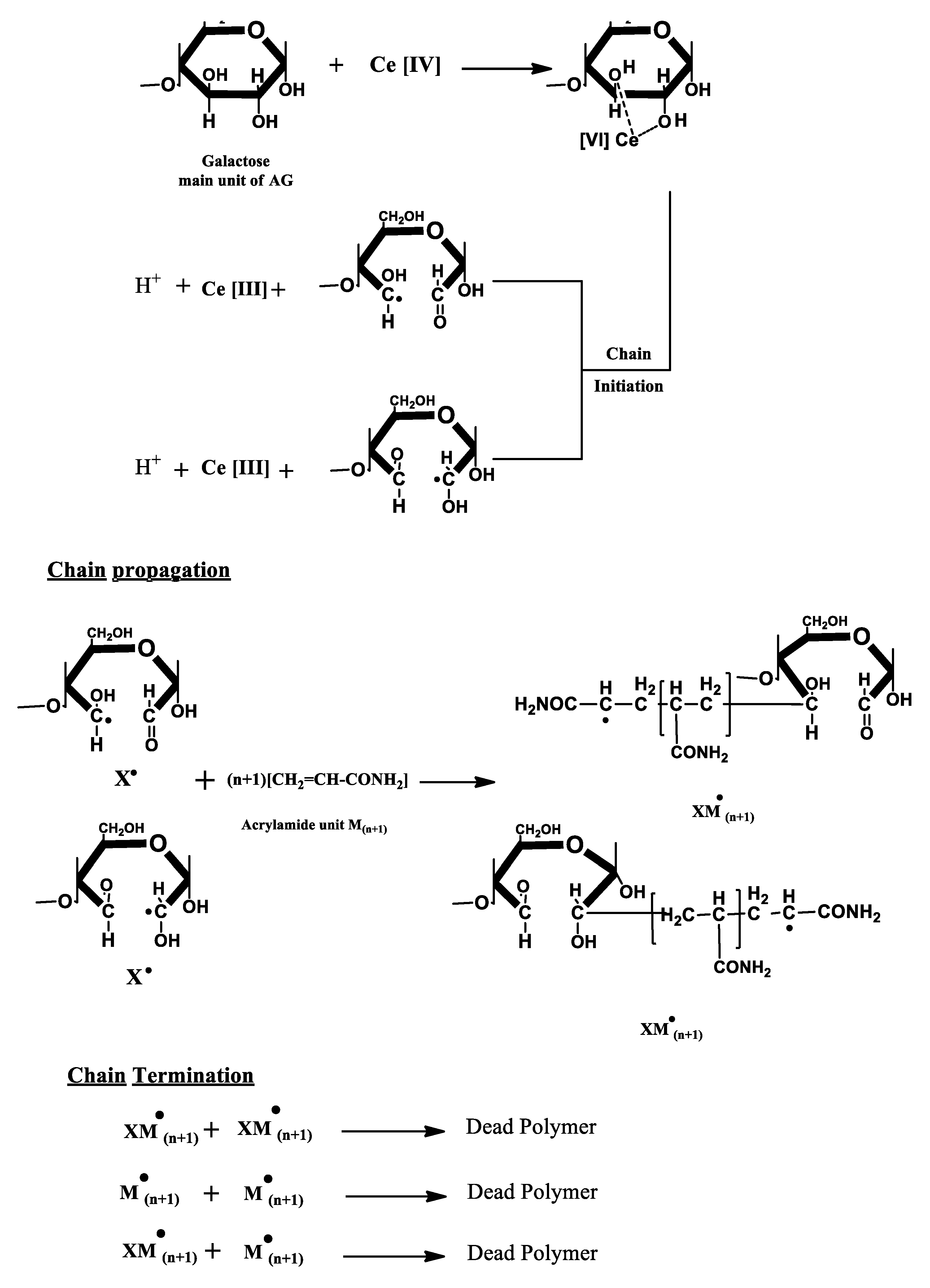
2.2.1. Preparation of Polyacrylamide Grafted Arabic Gum
2.2.2. Partially Hydrolyzation of Graft Copolymer to Introduce Carboxylate Groups
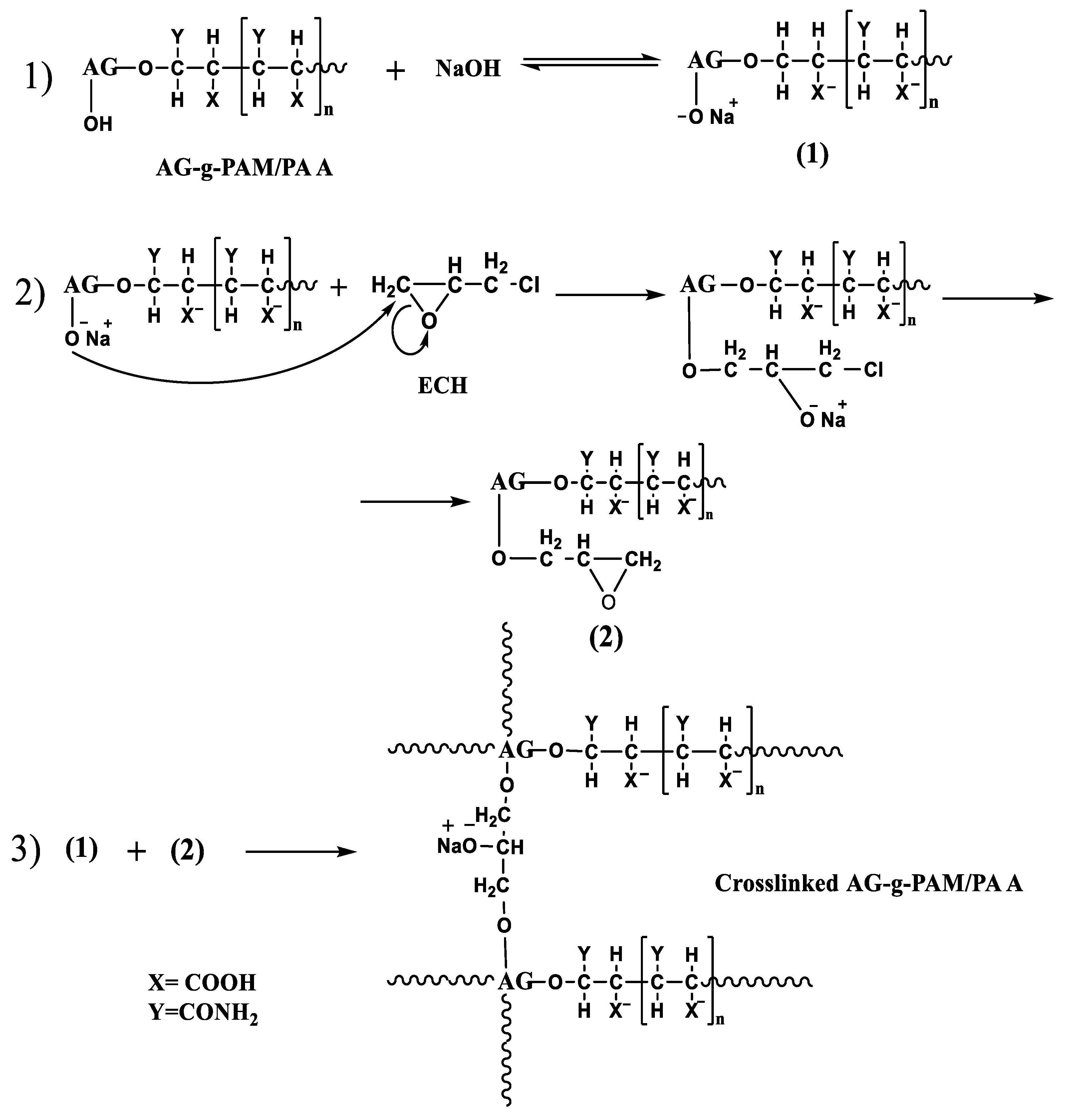
2.2.3. Preparation of the Super-Adsorbent AG-g-PAM/PAA Hydrogel
2.3. Characterization of Samples
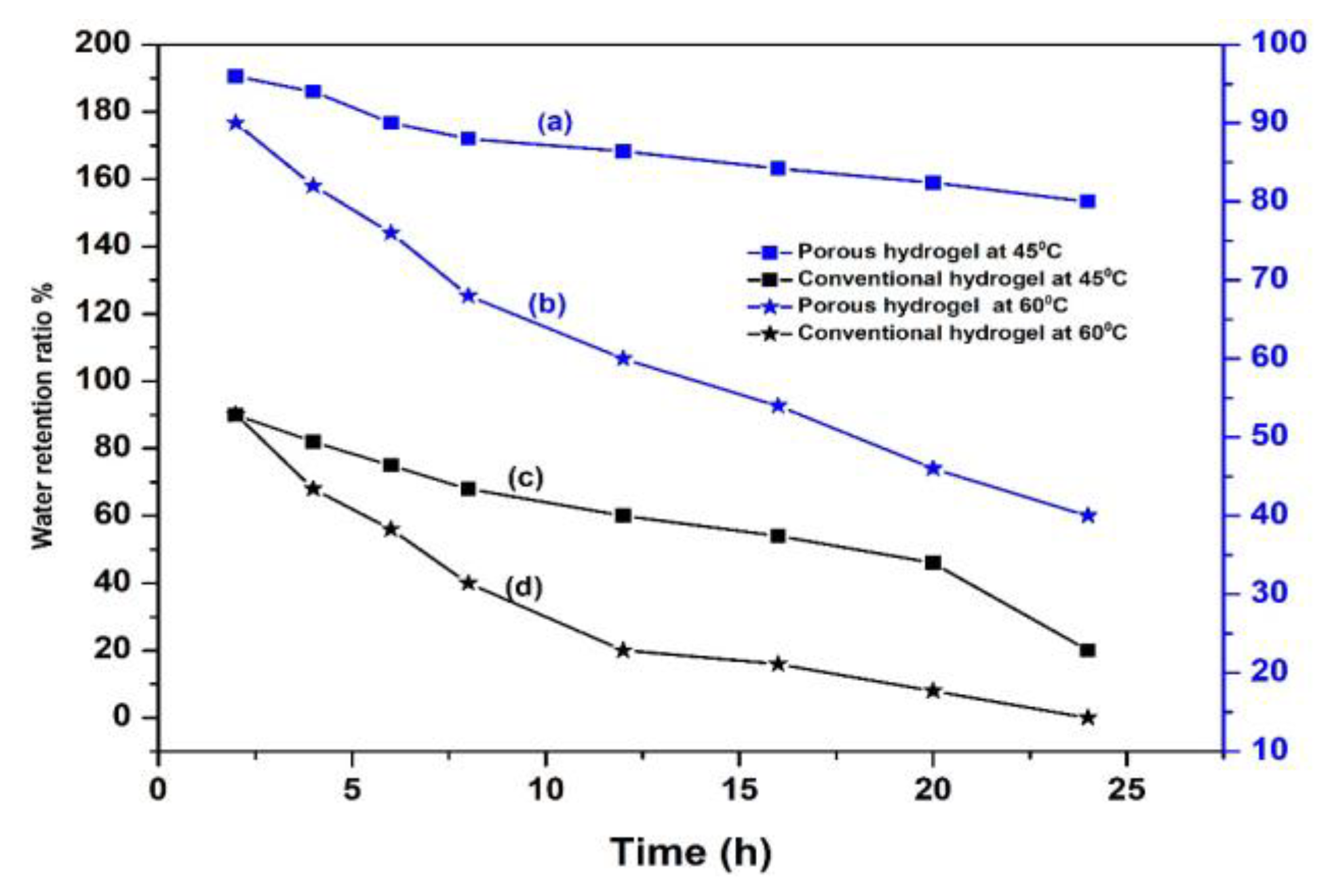
2.4. Water Absorption and Retention Capacities
2.5. Adsorption of MB Using Superadsorbent AG-g-PAM/PAA Hydrogel
2.6. The Reusability of the AG-g-PAM/PAA Hydrogel
3. Results and Discussion
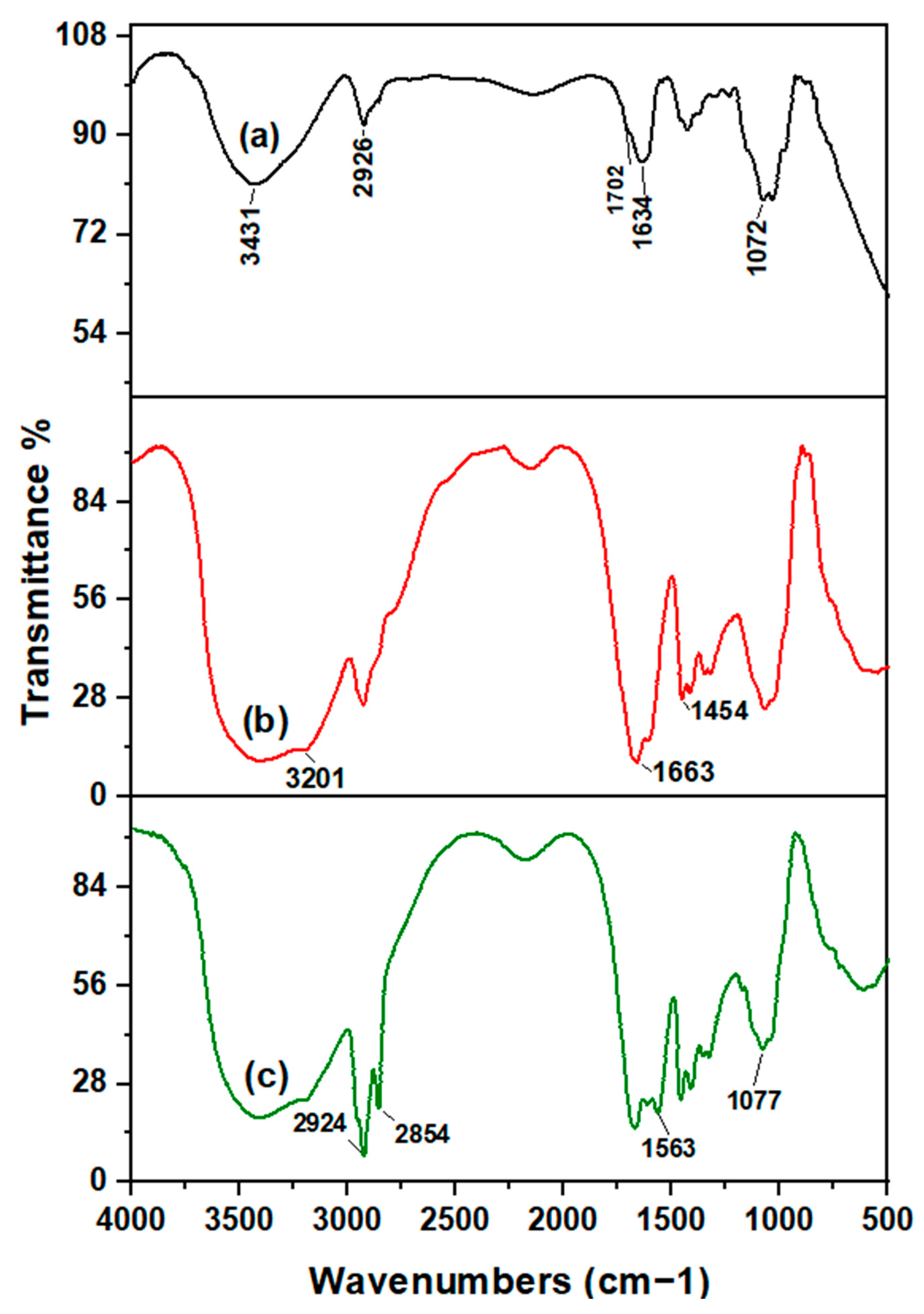
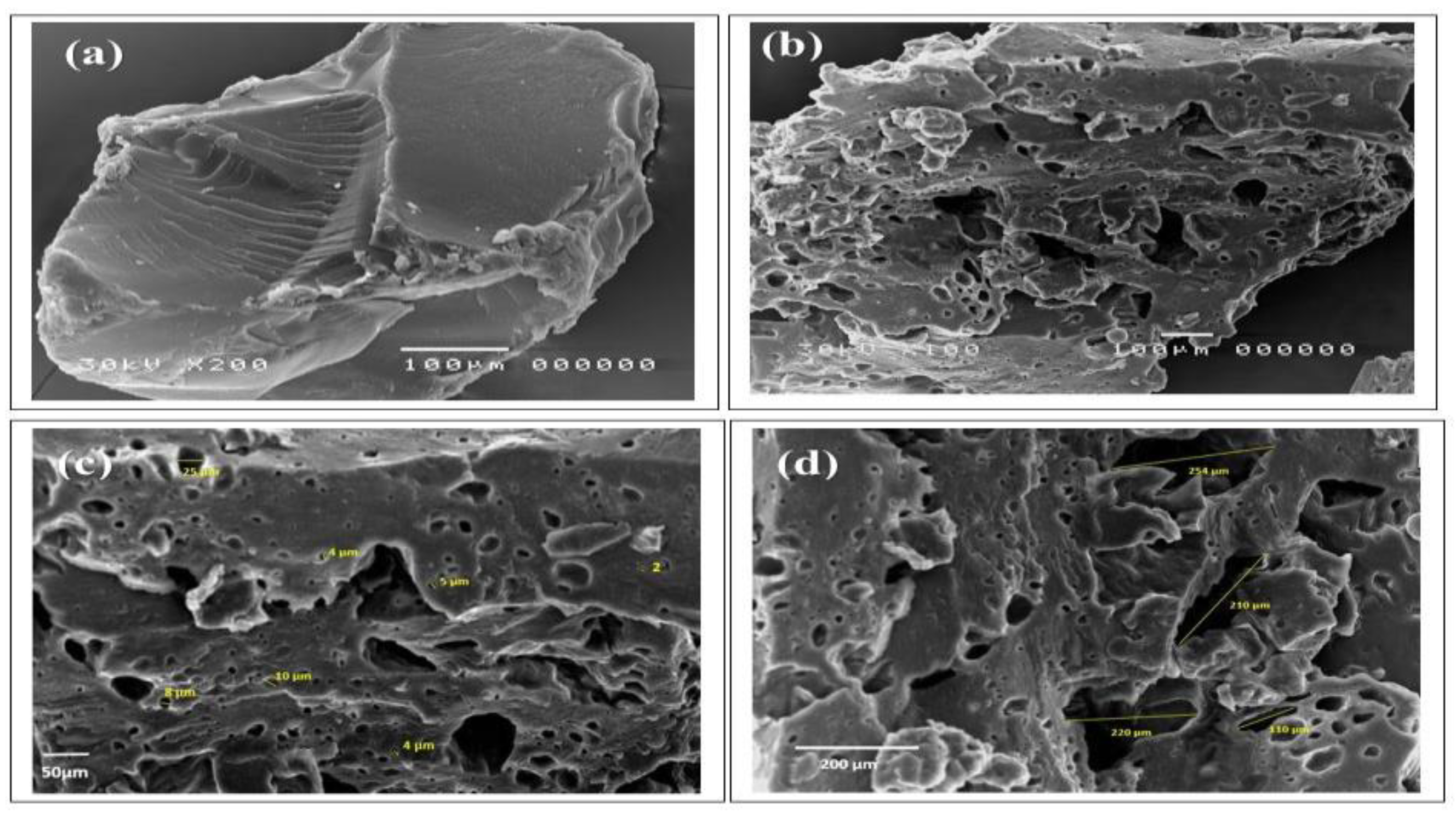
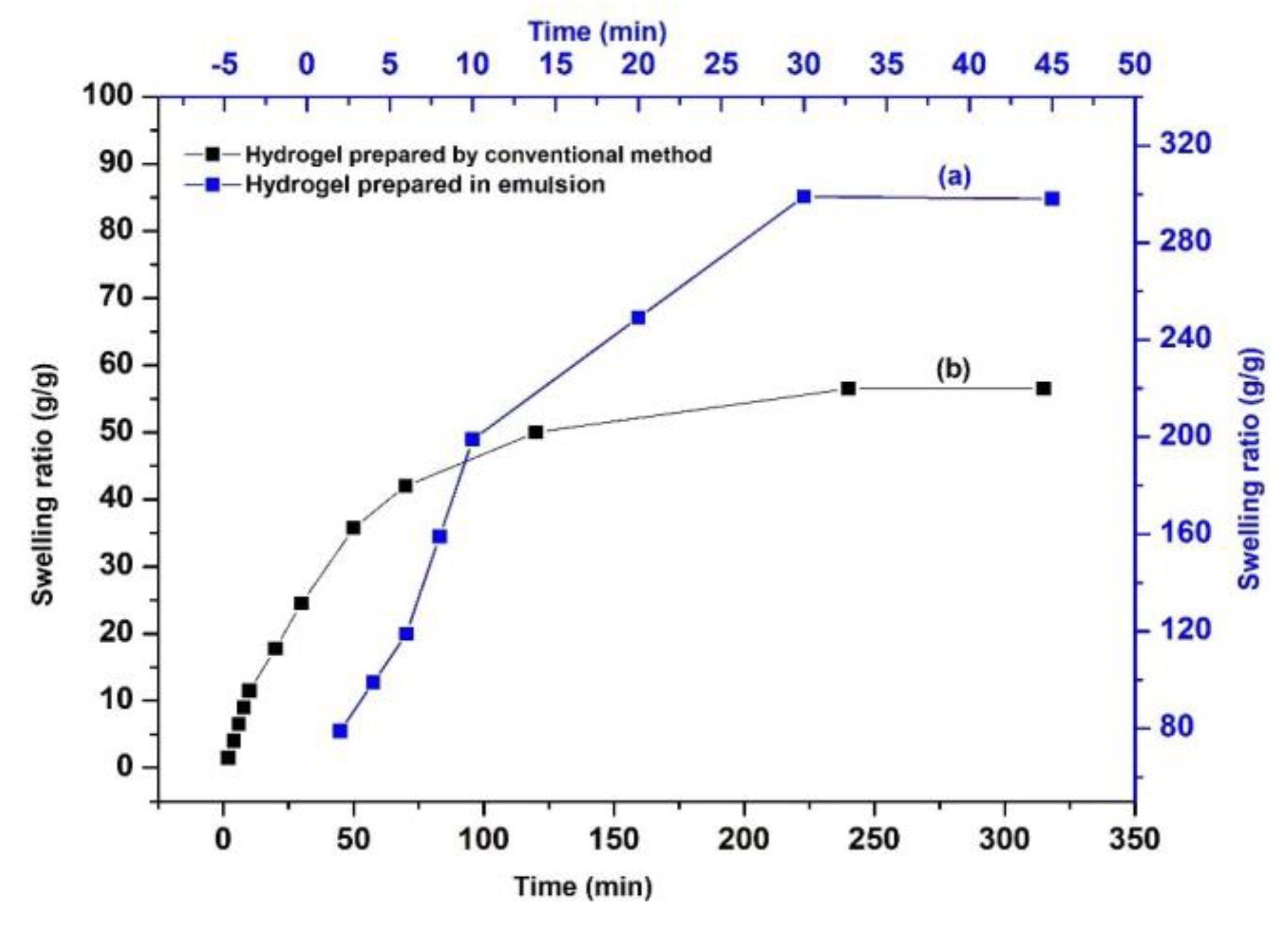
3.1. Preparation and Characterization of AG-g-PAM/PAA Hydrogel
3.2. Influence of MB Initial Concentrations
3.3. Influence of pH on MB Adsorption
3.4. Influence of Contact Time
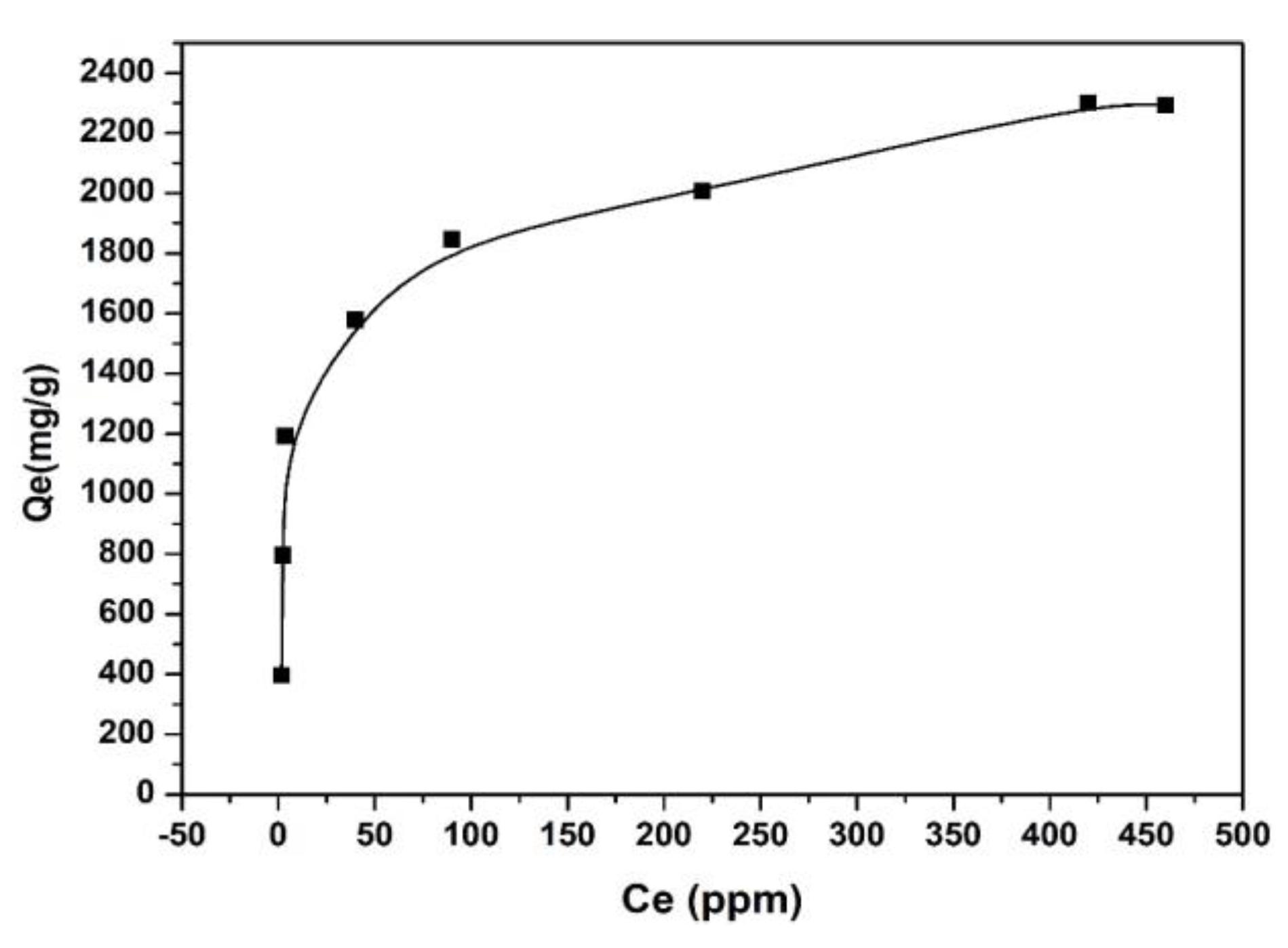
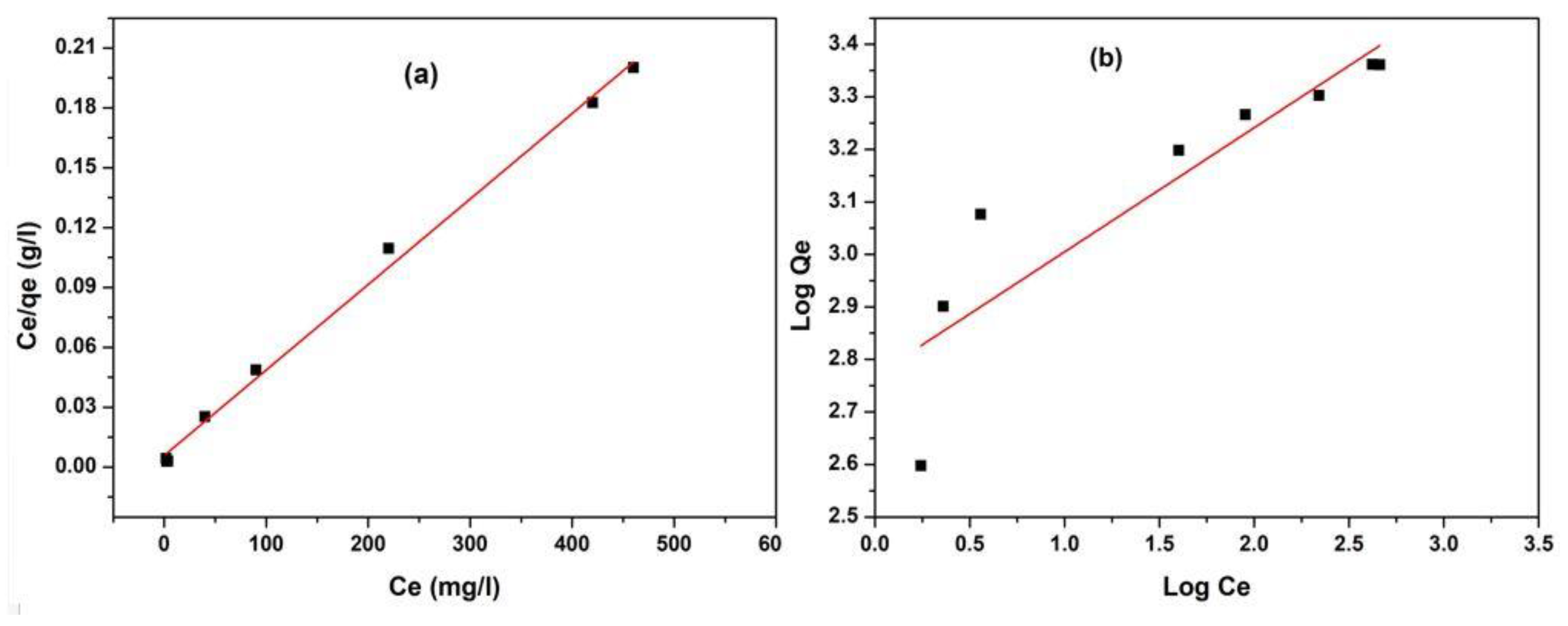
3.5. Equilibrium Adsorption Investigations
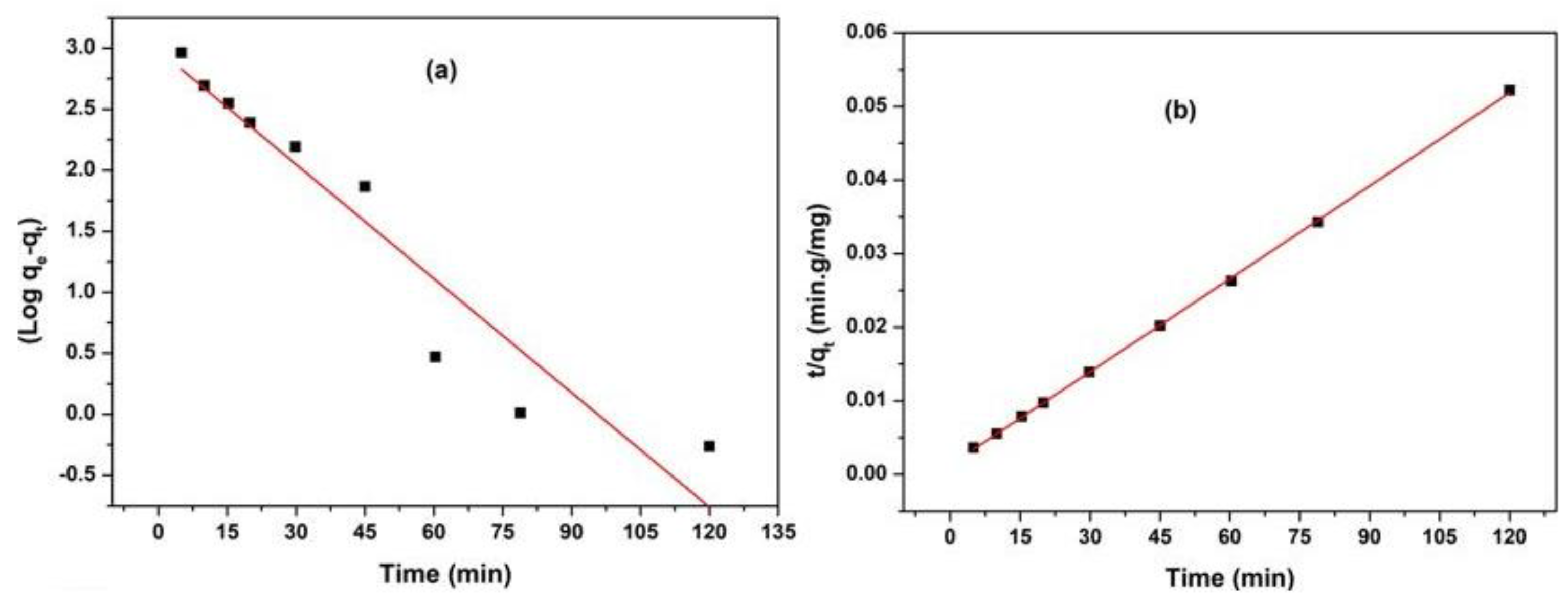
3.6. Adsorption Kinetics
3.7. The Reusability of the AG-g-PAM/PAA Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yaseen, D.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar]
- Jung, J.S.; Kim, S.H. Application of smectite for textile dyeing and fastness improvement. RSC Adv. 2019, 9, 36631–36639. [Google Scholar]
- Yu, C.; Wang, F.; Zhang, C.; Fu, S.; Lucia, L.A. The synthesis and absorption dynamics of a lignin-based hydrogel for remediation of cationic dye-contaminated effluent. React. Funct. Polym. 2016, 106, 137–142. [Google Scholar]
- Benaïssa, H. Effect of temperature on methylene blue sorption from aqueous solutions by almond peel: Experimental studies and modeling. In Proceedings of the Thirteenth International Water Technology Conference (IWTC13), Hurghada, Egypt, 12–15 March 2009; pp. 377–393. [Google Scholar]
- Chequer, F.D.; de Oliveira, G.A.R.; Ferraz, E.A.; Cardoso, J.C.; Zanoni, M.B.; de Oliveira, D.P. Textile dyes: Dyeing process and environmental impact. Eco-Friendly Text. Dye Finish. 2013, 6, 151–176. [Google Scholar]
- Mani, S.; Chowdhary, P.; Bharagava, R.N. Textile wastewater dyes: Toxicity profile and treatment approaches. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Sigapore, 2019; pp. 219–244. [Google Scholar]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar]
- Mallakpour, S.; Rashidimoghadam, S. Carbon Nanotubes for Dyes Removal. In Composite Nanoadsorbents; Elsevier: Oxford, UK, 2019; pp. 211–243. [Google Scholar]
- Thakur, K.; Kandasubramanian, B. Graphene and graphene oxide-based composites for removal of organic pollutants: A review. J. Chem. Eng. Data 2019, 64, 833–867. [Google Scholar]
- Rezazadeh, H.; Moghadam, P.N.; Ehsanimehr, S.; Fareghi, A.R. Synthesis of a new magnetic nanocomposite hydrogel based on poly(vinyl acetate-co-maleic anhydride)/melamine for efficient dye removal. J. Elastom. Plast. 2019. [Google Scholar] [CrossRef]
- Samaddar, P.; Kumar, S.; Kim, K.-H. Polymer Hydrogels and Their Applications toward Sorptive Removal of Potential Aqueous Pollutants. Polym. Rev. 2019, 59, 418–464. [Google Scholar]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar]
- Sun, J.; Schmidt, B.V.; Wang, X.; Shalom, M. Self-standing carbon nitride-based hydrogels with high photocatalytic activity. ACS Appl. Mater. Interfaces 2017, 9, 2029–2034. [Google Scholar]
- Li, D.; Li, Q.; Bai, N.; Dong, H.; Mao, D. One-step synthesis of cationic hydrogel for efficient dye adsorption and its second use for emulsified oil separation. ACS Sustain. Chem. Eng. 2017, 5, 5598–5607. [Google Scholar]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-based hydrogels: Present status and applications. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 411–420. [Google Scholar]
- Pakdel, P.M.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar]
- Khan, M.; Lo, I.M. A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: Recent progress, challenges, and perspectives. Water Res. 2016, 106, 259–271. [Google Scholar] [PubMed]
- Sharma, R.K.; Kumar, R.; Singh, A.P. Metal ions and organic dyes sorption applications of cellulose grafted with binary vinyl monomers. Sep. Purif. Technol. 2019, 209, 684–697. [Google Scholar]
- Gürdağ, G.; Güçlü, G.; Özgümüş, S. Graft copolymerization of acrylic acid onto cellulose: Effects of pretreatments and crosslinking agent. J. Appl. Polym. Sci. 2001, 80, 2267–2272. [Google Scholar]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci 2014, 4, 25–31. [Google Scholar]
- Ghorai, S.; Sarkar, A.; Raoufi, M.; Panda, A.B.; Schönherr, H.; Pal, S. Enhanced removal of methylene blue and methyl violet dyes from aqueous solution using a nanocomposite of hydrolyzed polyacrylamide grafted xanthan gum and incorporated nanosilica. ACS Appl. Mater. Interfaces 2014, 6, 4766–4777. [Google Scholar]
- Hosseinzadeh, H.; Ramin, S. Fabrication of starch-graft-poly (acrylamide)/graphene oxide/hydroxyapatite nanocomposite hydrogel adsorbent for removal of malachite green dye from aqueous solution. Int. J. Biol. Macromol. 2018, 106, 101–115. [Google Scholar]
- Dai, H.; Zhang, Y.; Ma, L.; Zhang, H.; Huang, H. Synthesis and response of pineapple peel carboxymethyl cellulose-g-poly (acrylic acid-co-acrylamide)/graphene oxide hydrogels. Carbohydr. Polym. 2019, 215, 366–376. [Google Scholar]
- Hu, X.-S.; Liang, R.; Sun, G. Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. J. Mater. Chem. A 2018, 6, 17612–17624. [Google Scholar]
- Zhou, Y.; Fu, S.; Liu, H.; Yang, S.; Zhan, H. Removal of methylene blue dyes from wastewater using cellulose-based superadsorbent hydrogels. Polym. Eng. Sci. 2011, 51, 2417–2424. [Google Scholar]
- Kumar, A.; Li, S.; Cheng, C.-M.; Lee, D. Recent developments in phase inversion emulsification. Ind. Eng. Chem. Res. 2015, 54, 8375–8396. [Google Scholar]
- Susanto, H.; Ulbricht, M. Photografted thin polymer hydrogel layers on PES ultrafiltration membranes: Characterization, stability, and influence on separation performance. Langmuir 2007, 23, 7818–7830. [Google Scholar]
- Qian, L.; Zhang, H. Porogen incorporation and phase inversion. Porous Polym. 2011, 79–117. [Google Scholar] [CrossRef]
- Reinwald, Y.; Shakesheff, K.; Howdle, S. Biomedical devices. Porous Polym. 2011, 323, 357. [Google Scholar]
- Qi, L. Synthesis of inorganic nanostructures in reverse micelles. Encycl. Surf. Colloid Sci. 2006, 2, 6183–6207. [Google Scholar]
- Elbedwehy, A.M.; Abou-Elanwar, A.M.; Ezzat, A.O.; Atta, A.M. Super Effective Removal of Toxic Metals Water Pollutants Using Multi Functionalized Polyacrylonitrile and Arabic Gum Grafts. Polymers 2019, 11, 1938. [Google Scholar]
- Tripathy, T.; Singh, R. High performance flocculating agent based on partially hydrolysed sodium alginate–g–polyacrylamide. Eur. Polym. J. 2000, 36, 1471–1476. [Google Scholar]
- Abdel-Bary, E.; Elbedwehy, A. Graft copolymerization of polyacrylic acid onto Acacia gum using erythrosine–thiourea as a visible light photoinitiator: Application for dye removal. Polym. Bull. 2018, 75, 3325–3340. [Google Scholar]
- Raj Sharma, B.; Kumar, V.; Soni, P. Ceric ammonium nitrate-initiated graft copolymerization of acrylamide onto Cassia tora gum. J. Appl. Polym. Sci. 2002, 86, 3250–3255. [Google Scholar]
- Yacob, N.; Hashim, K. Morphological effect on swelling behaviour of hydrogel. In Proceedings of the AIP Conference Proceeding, Kajang, Malaysia, 17 February 2015; pp. 153–159. [Google Scholar]
- Mac Kenna, N.; Morrin, A. Inducing macroporosity in hydrogels using hydrogen peroxide as a blowing agent. Mater. Chem. Front. 2017, 1, 394–401. [Google Scholar]
- Zhang, M.; Cheng, Z.; Zhao, T.; Liu, M.; Hu, M.; Li, J. Synthesis, characterization, and swelling behaviors of salt-sensitive maize bran–poly (acrylic acid) superabsorbent hydrogel. J. Agric. Food Chem. 2014, 62, 8867–8874. [Google Scholar] [PubMed]
- Tang, Y.; He, T.; Liu, Y.; Zhou, B.; Yang, R.; Zhu, L. Sorption behavior of methylene blue and rhodamine B mixed dyes onto chitosan graft poly (acrylic acid-co-2-acrylamide-2-methyl propane sulfonic acid) hydrogel. Adv. Polym. Tech. 2018, 37, 2568–2578. [Google Scholar]
- Wang, L.; Zhang, J.; Wang, A. Removal of methylene blue from aqueous solution using chitosan-g-poly (acrylic acid)/montmorillonite superadsorbent nanocomposite. Colloids Surf. Physicochem. Eng. Asp. 2008, 322, 47–53. [Google Scholar]
- Wang, L.; Zhang, J.; Wang, A. Fast removal of methylene blue from aqueous solution by adsorption onto chitosan-g-poly (acrylic acid)/attapulgite composite. Desalination 2011, 266, 33–39. [Google Scholar]
- Zhu, L.; Guan, C.; Zhou, B.; Zhang, Z.; Yang, R.; Tang, Y.; Yang, J. Adsorption of dyes onto sodium alginate graft poly (acrylic acid-co-2-acrylamide-2-methyl propane sulfonic acid)/kaolin hydrogel composite. Polym. Polym. Compos. 2017, 25, 627–634. [Google Scholar]
- Al, E.; Güçlü, G.; İyim, T.B.; Emik, S.; Özgümüş, S. Synthesis and properties of starch-graft-acrylic acid/Na-montmorillonite superabsorbent nanocomposite hydrogels. J. Appl. Polym. Sci. 2008, 109, 16–22. [Google Scholar]
- Fosso-Kankeu, E.; Mittal, H.; Mishra, S.B.; Mishra, A.K. Gum ghatti and acrylic acid based biodegradable hydrogels for the effective adsorption of cationic dyes. J. Ind. Eng. Chem. 2015, 22, 171–178. [Google Scholar]
- Mittal, H.; Maity, A.; Ray, S.S. Gum karaya based hydrogel nanocomposites for the effective removal of cationic dyes from aqueous solutions. Appl. Surf. Sci. 2016, 364, 917–930. [Google Scholar]
- Ma, J.; Liu, Y.; Ali, O.; Wei, Y.; Zhang, S.; Zhang, Y.; Cai, T.; Liu, C.; Luo, S. Fast adsorption of heavy metal ions by waste cotton fabrics based double network hydrogel and influencing factors insight. J. Hazard. Mater. 2018, 344, 1034–1042. [Google Scholar] [PubMed]

| Sample | Volume of 0.1 M of NaOH (V2) cc | V1 a − V2 mL | N.E. (g) |
|---|---|---|---|
| S2 | 43.6 | 0.6 | 3103.6 |
| S4 | 42.6 | 1.6 | 1163.8 |
| S6 | 41.7 | 2.5 | 744.8 |
| Langmuir Constants | Freundlich Constants | ||||||
|---|---|---|---|---|---|---|---|
| Qexp. | Qm (mg/g) | b (L/mg) | R2 | RL | Kf | 1/n | R2 |
| 2300 | 2331 | 0.0740 | 0.9958 | 0.0095 | 0.5870 | 0.2362 | 0.7855 |
| pH Value | Desorption Ratio (%) |
|---|---|
| 1 | 99 |
| 2.02 | 90.5 |
| 4 | 48.3 |
| 6.08 | 25.03 |
| Cycle Number | Desorption Ratio (%) |
|---|---|
| 1 | 99 |
| 2 | 98 |
| 3 | 98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbedwehy, A.M.; Atta, A.M. Novel Superadsorbent Highly Porous Hydrogel Based on Arabic Gum and Acrylamide Grafts for Fast and Efficient Methylene Blue Removal. Polymers 2020, 12, 338. https://doi.org/10.3390/polym12020338
Elbedwehy AM, Atta AM. Novel Superadsorbent Highly Porous Hydrogel Based on Arabic Gum and Acrylamide Grafts for Fast and Efficient Methylene Blue Removal. Polymers. 2020; 12(2):338. https://doi.org/10.3390/polym12020338
Chicago/Turabian StyleElbedwehy, Ahmed M., and Ayman M. Atta. 2020. "Novel Superadsorbent Highly Porous Hydrogel Based on Arabic Gum and Acrylamide Grafts for Fast and Efficient Methylene Blue Removal" Polymers 12, no. 2: 338. https://doi.org/10.3390/polym12020338
APA StyleElbedwehy, A. M., & Atta, A. M. (2020). Novel Superadsorbent Highly Porous Hydrogel Based on Arabic Gum and Acrylamide Grafts for Fast and Efficient Methylene Blue Removal. Polymers, 12(2), 338. https://doi.org/10.3390/polym12020338





