Investigation on Foamed PP/Nano-CaCO3 Composites in a Combined in-Mold Decoration and Microcellular Injection Molding Process
Abstract
1. Introduction
2. Experimental
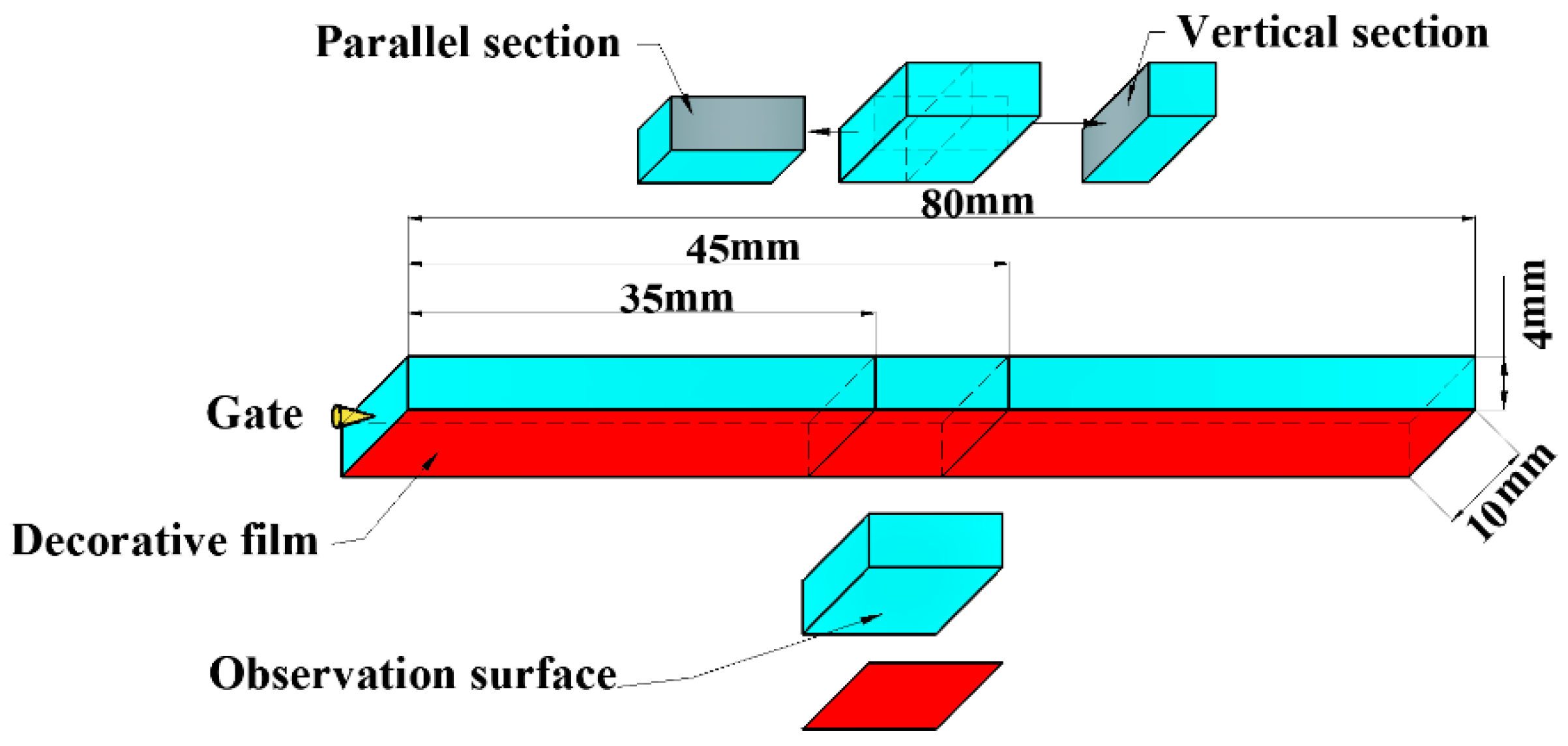
2.1. Combined In-Mold Decoration and Microcellular Injection Molding Process
2.2. Preparation of Composites
2.3. Foaming Process
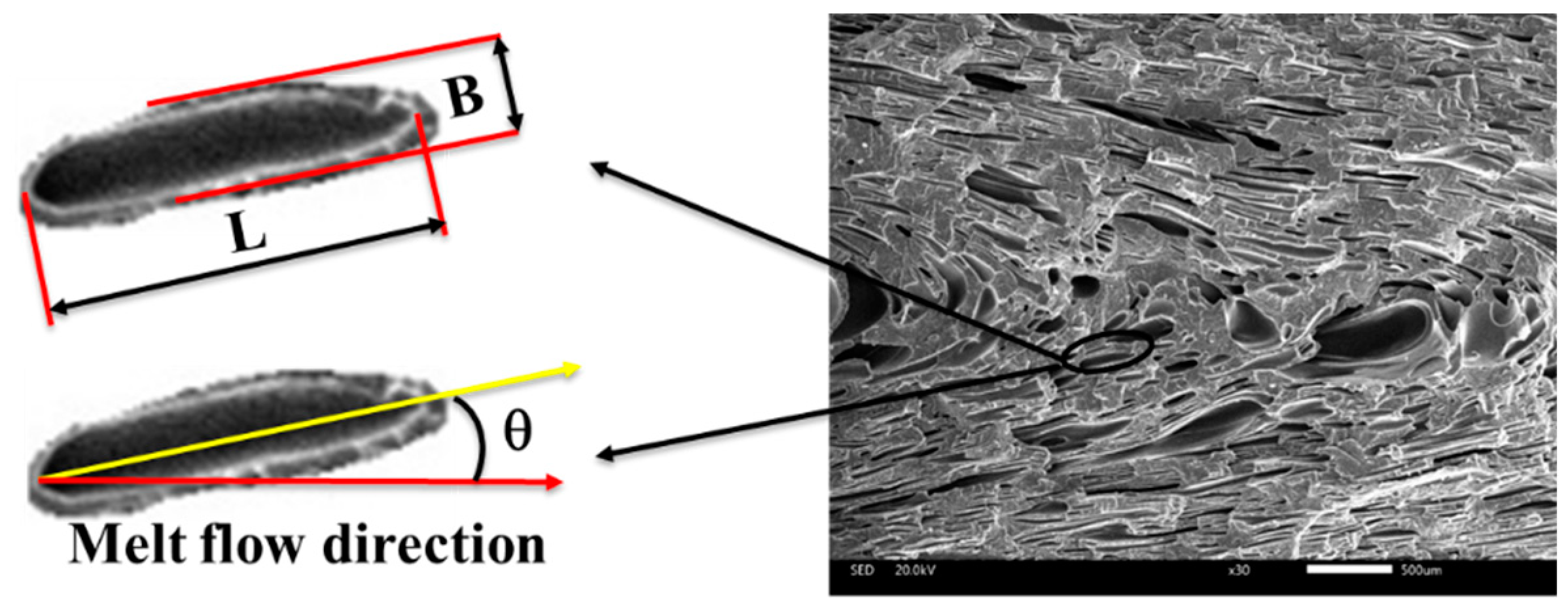
2.4. Characterizations
3. Results and Discussion
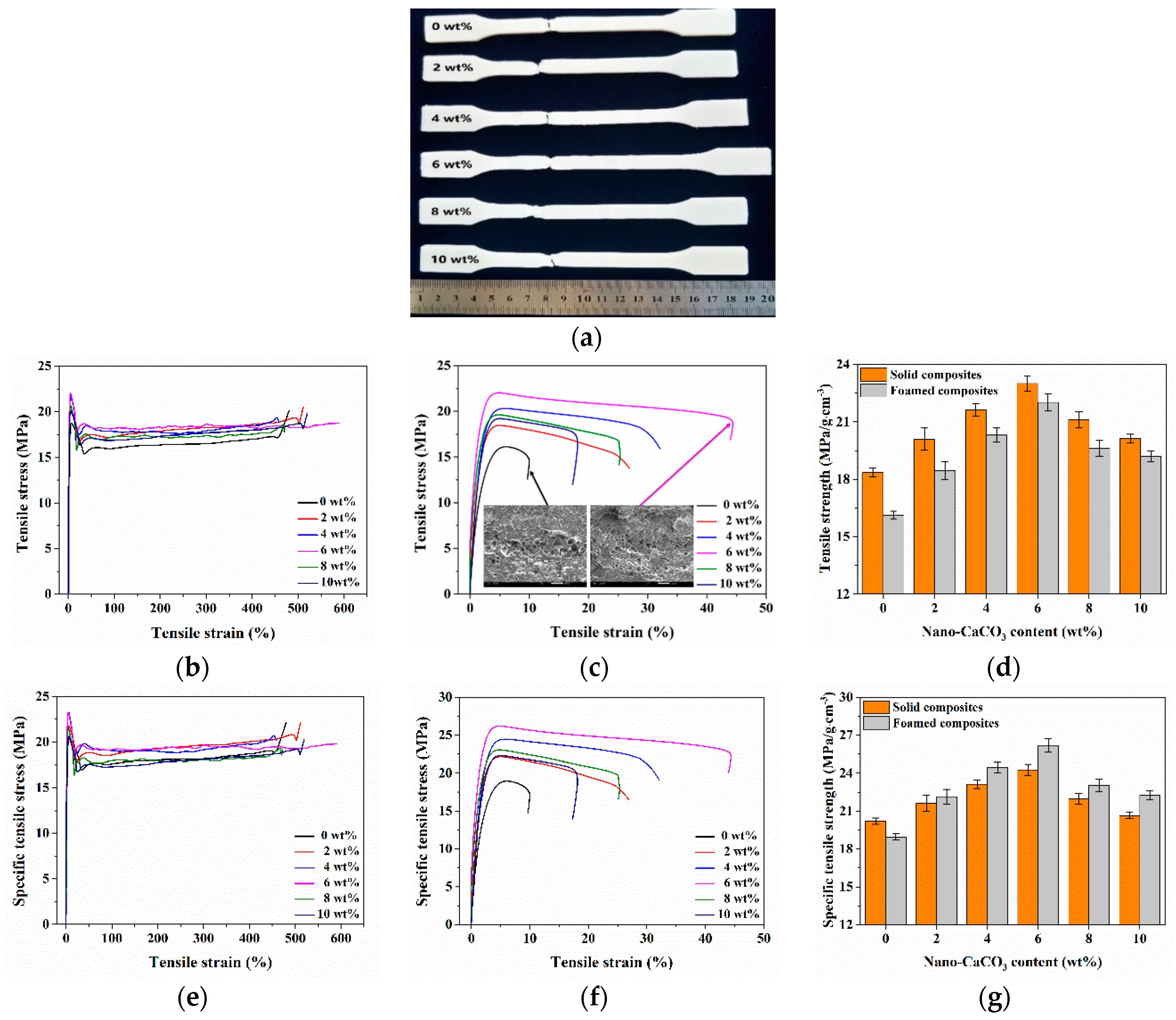
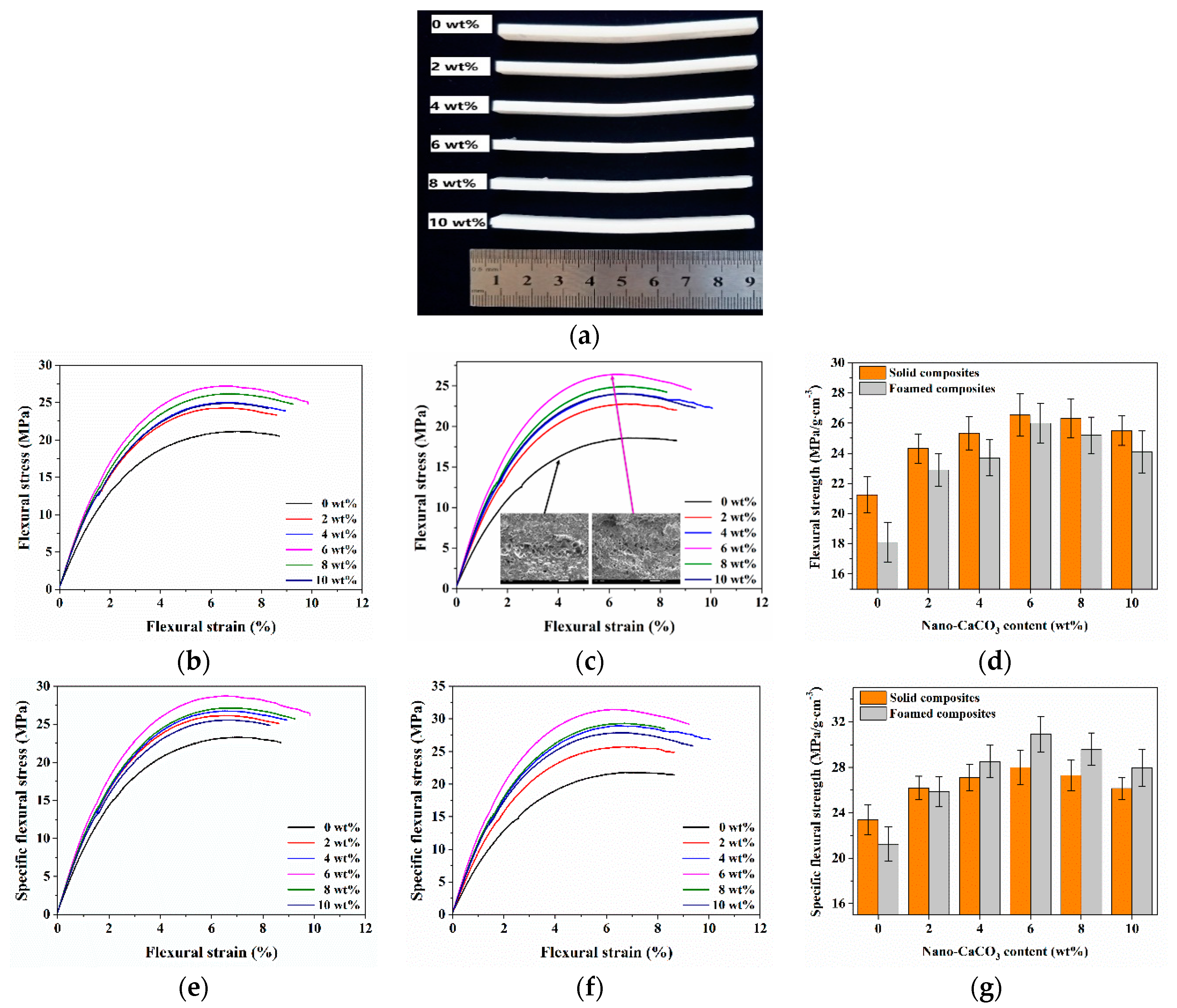
3.1. Mechanical Properties
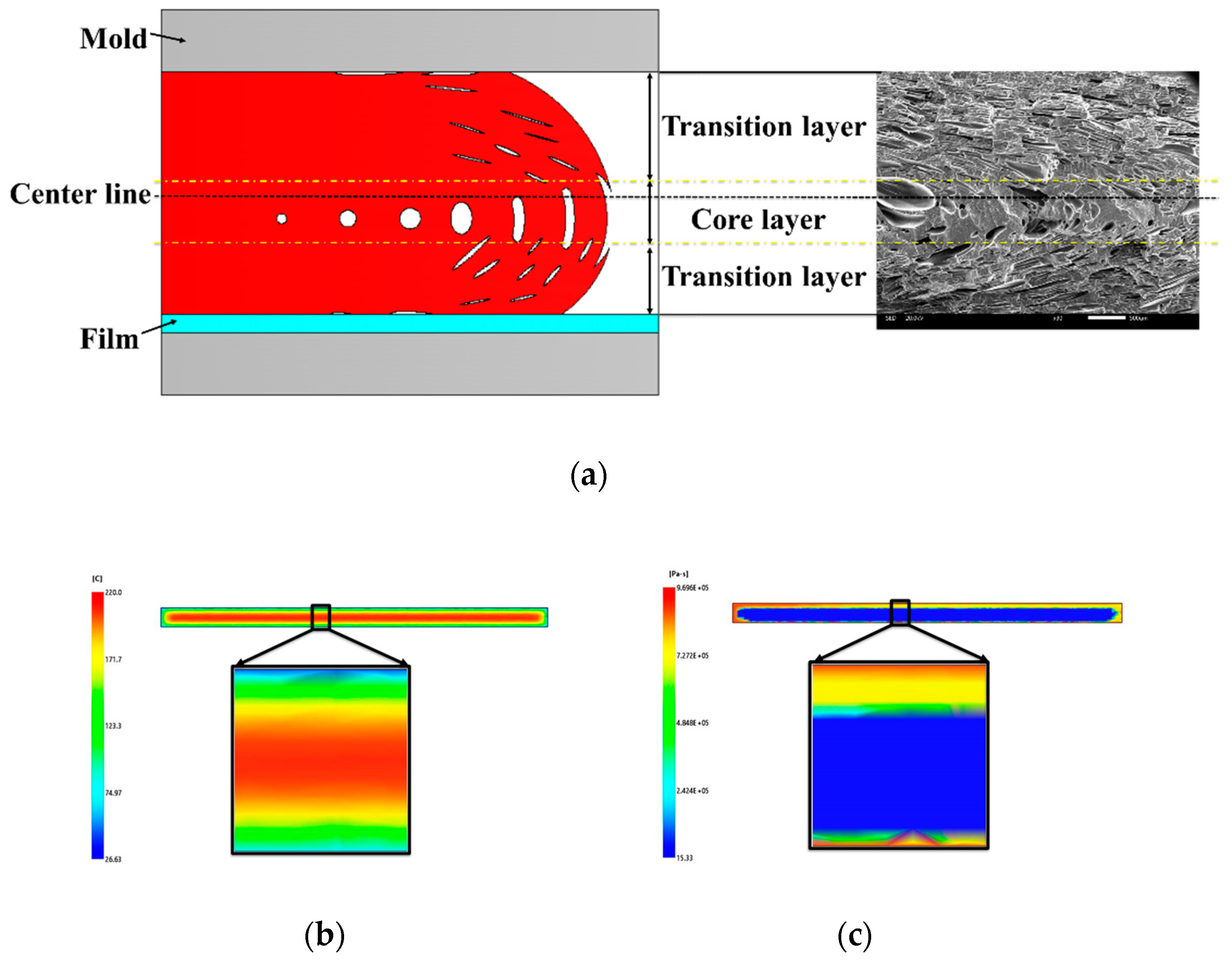
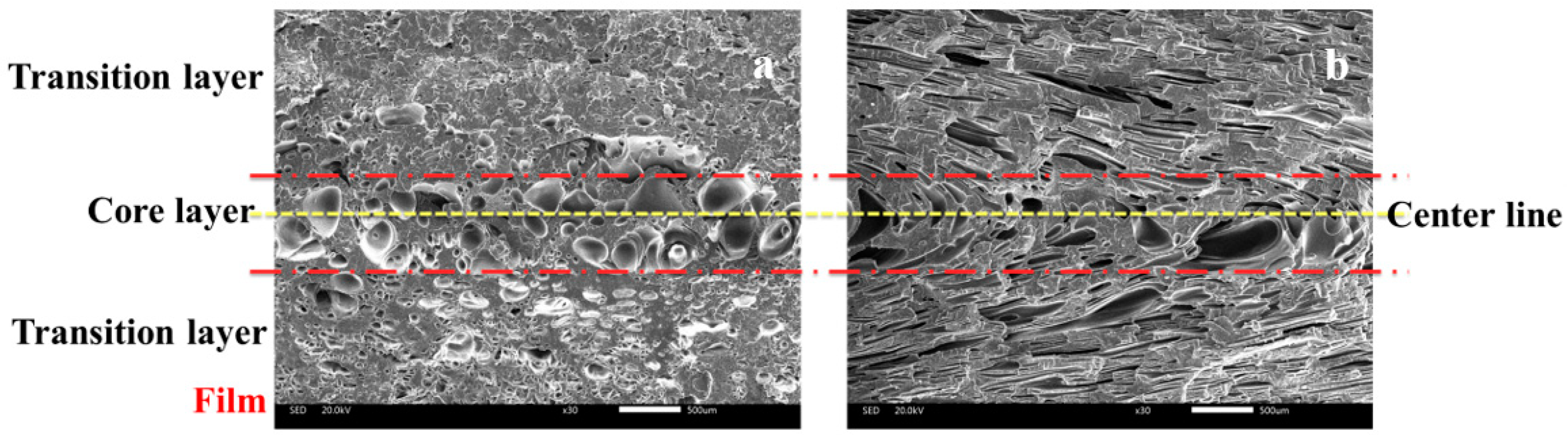
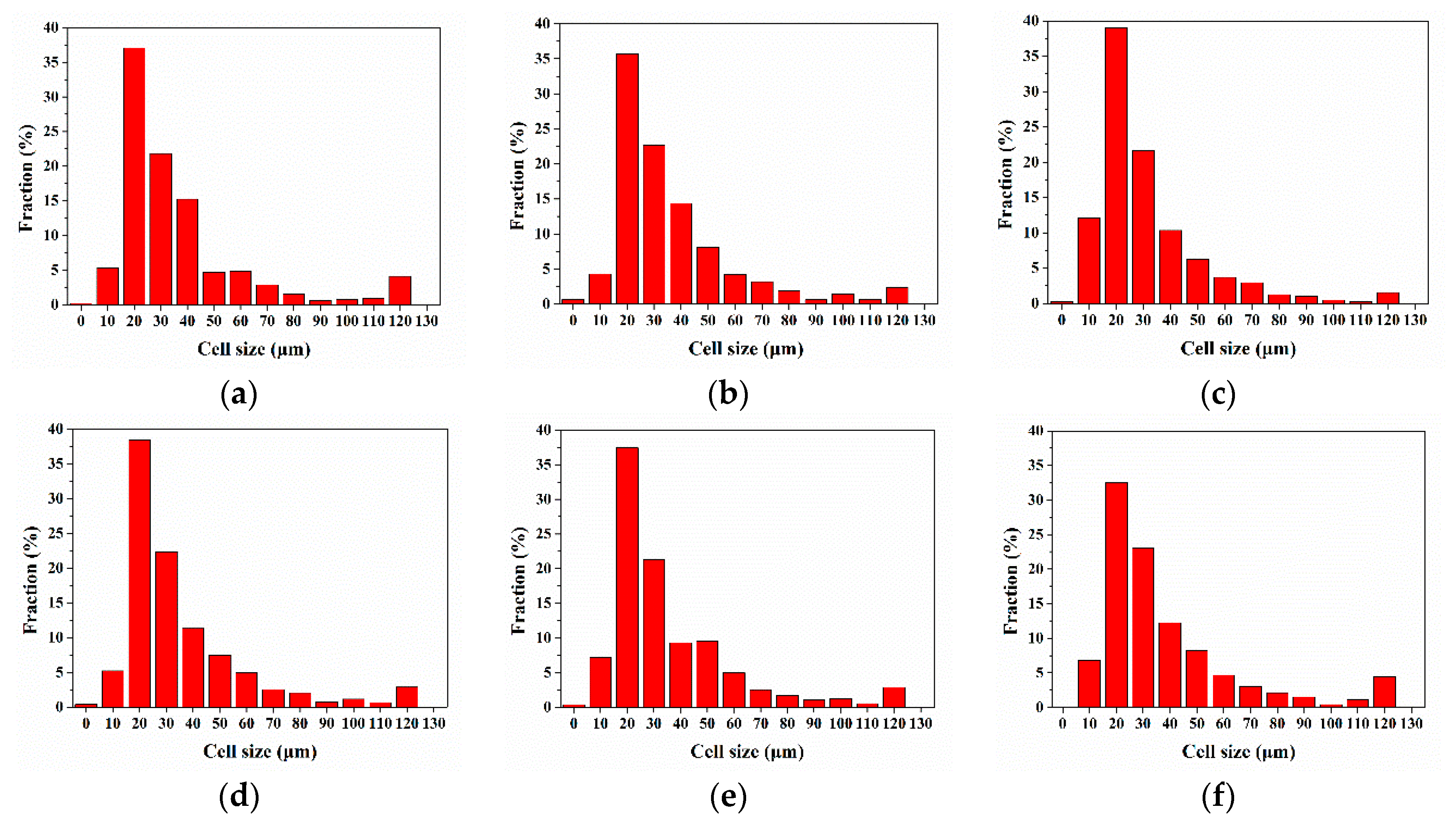
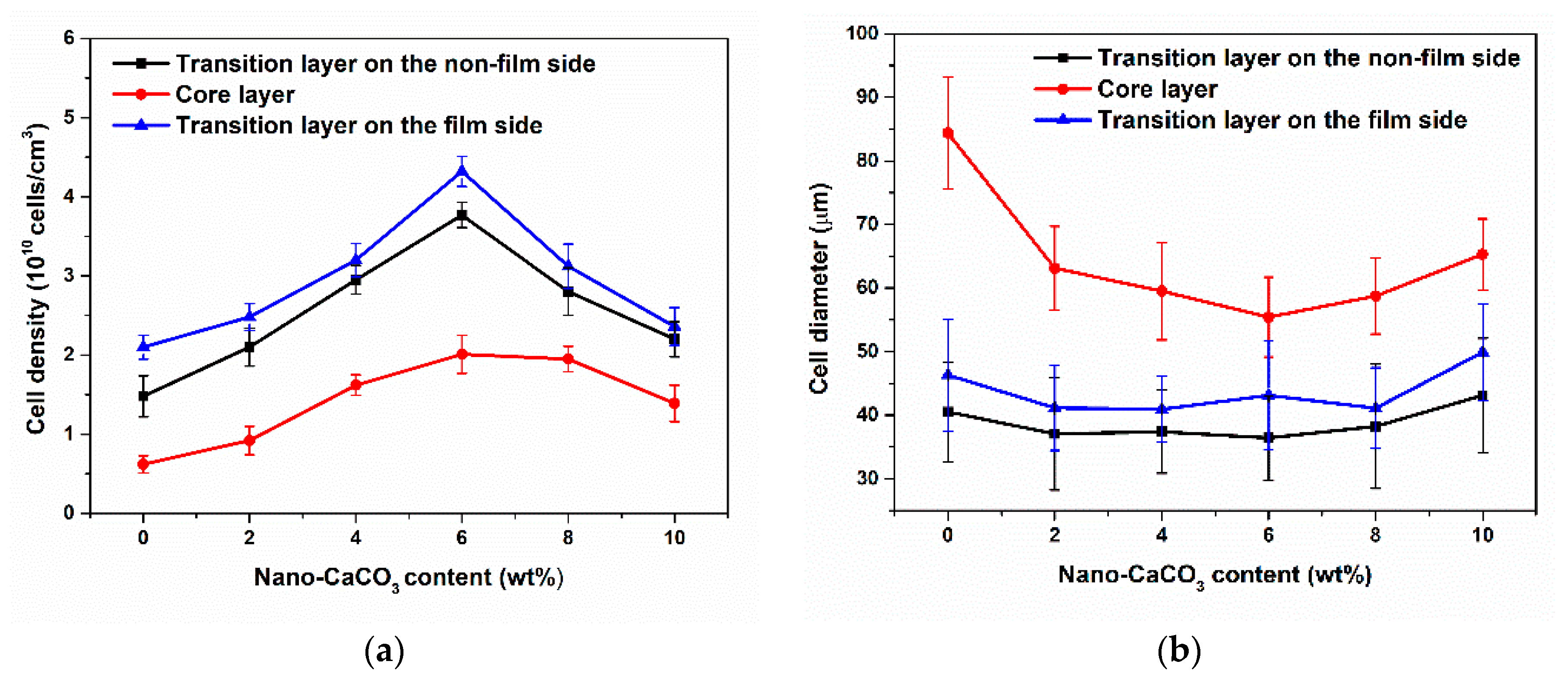
3.2. Cellular Structure
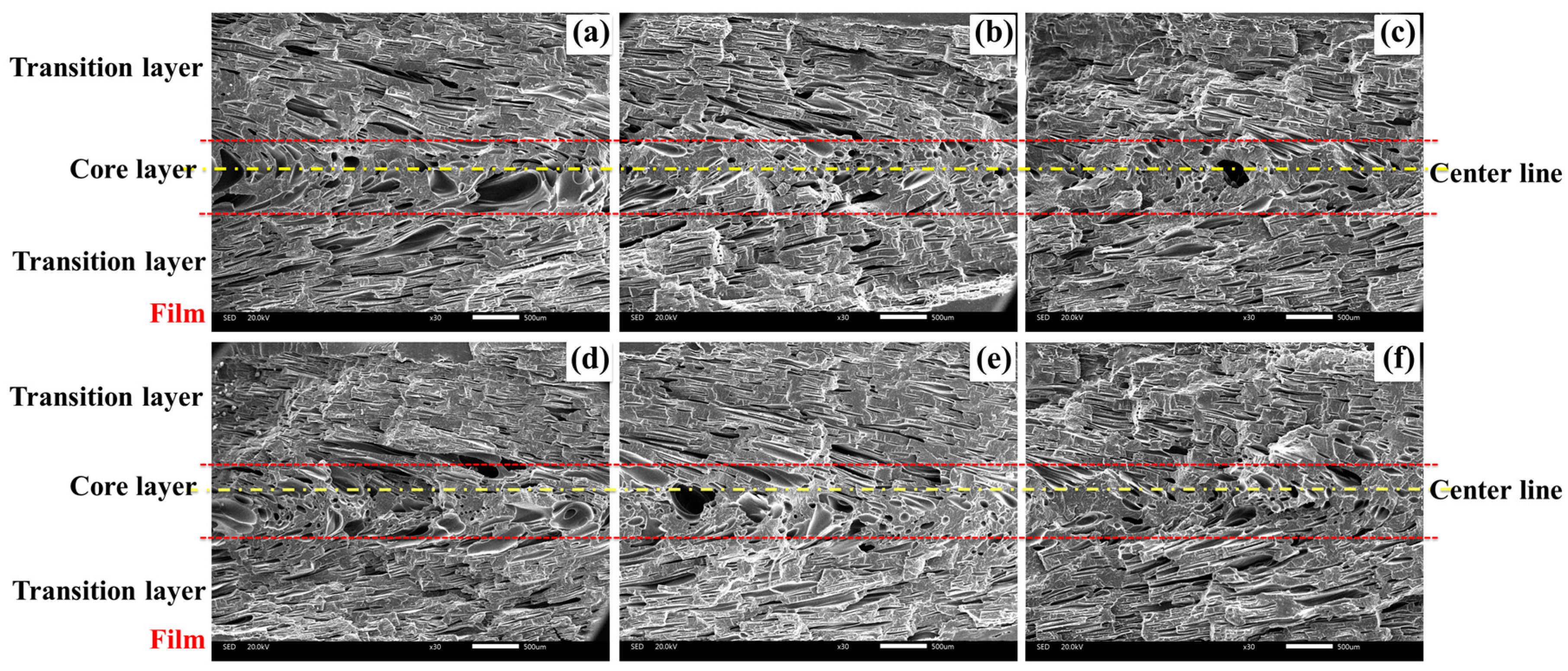
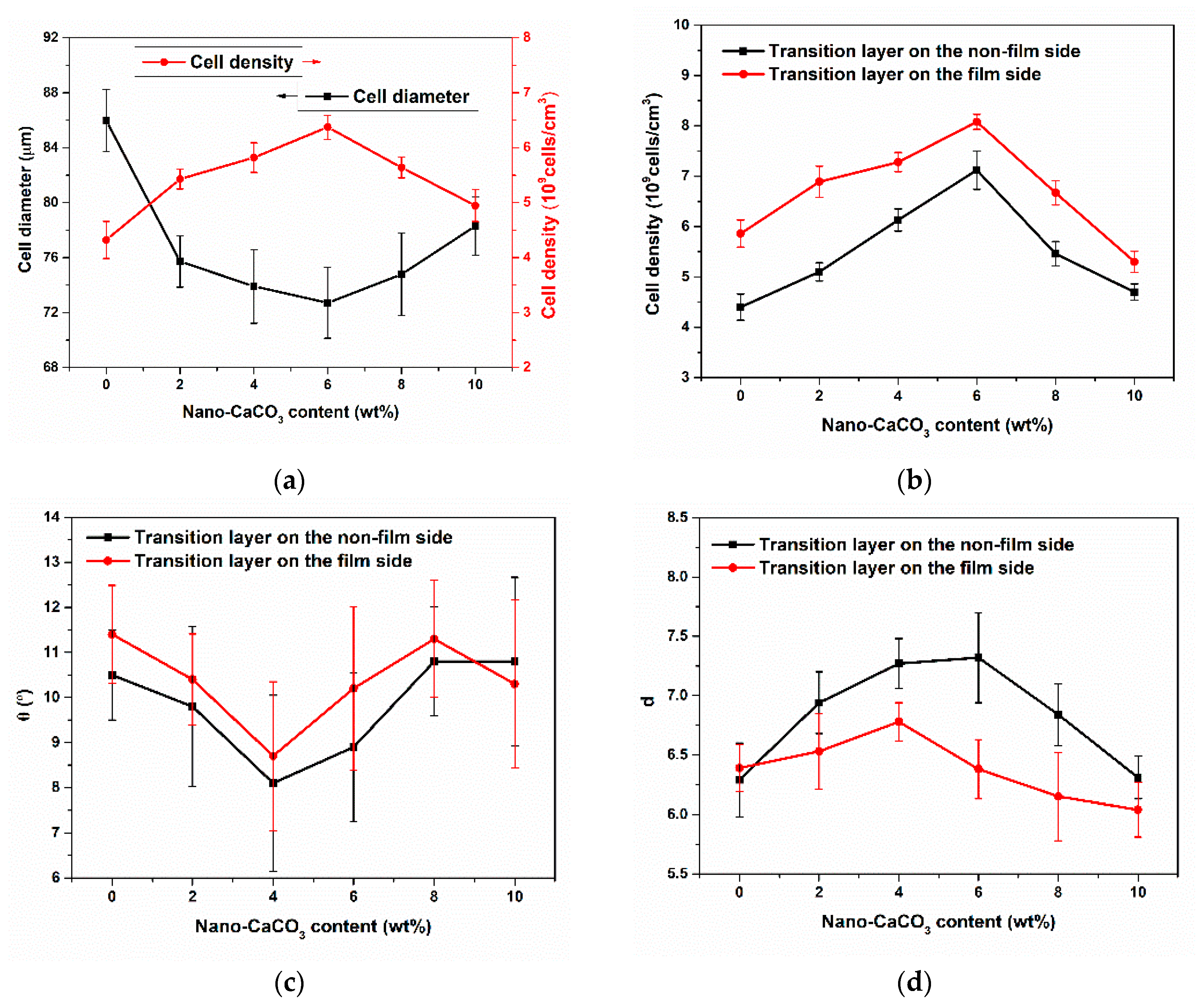
3.2.1. Cellular Structure in the Vertical Section
3.2.2. Cellular Structure in the Parallel Section
3.3. Surface Quality
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Collias, D.I.; Baird, D.G.; Borggreve, R.J.M. Impact toughening of polycarbonate by microcellular foaming. Polymer 1994, 35, 3978–3983. [Google Scholar] [CrossRef]
- Park, C.B.; Baldwin, D.F.; Suh, N.P. Effect of the pressure drop rate on cell nucleation in continuous processing of microcellular polymers. Polym. Eng. Sci. 2010, 35, 432–440. [Google Scholar] [CrossRef]
- Zeng, C.; Hossieny, N.; Zhang, C.; Wang, B.; Walsh, S.M. Morphology and tensile properties of PMMA carbon nanotubes nanocomposites and nanocomposites foams. Compos. Sci. Technol. 2013, 82, 29–37. [Google Scholar] [CrossRef]
- Cha, S.W.; Yoon, J.D. The Relationship of Mold Temperatures and Swirl Marks on the Surface of Microcellular Plastics. Polym. Plast. Technol. 2005, 44, 795–803. [Google Scholar] [CrossRef]
- Li, S.; Zhao, G.Q.; Dong, G.W.; Wang, J.C. Study on reducing sink mark depth of a microcellular injection molded part with many reinforcing ribs. J. Cell. Plast. 2015, 52, 479–502. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G.Q.; Dong, G.W.; Li, S.; Wang, G.L. Bubble morphological evolution and surface defect formation mechanism in the microcellular foam injection molding process. RSC Adv. 2015, 5, 70032–70050. [Google Scholar] [CrossRef]
- Xiao, C.L.; Huang, H.X.; Yang, X. Development and application of rapid thermal cycling molding with electric heating for improving surface quality of microcellular injection molded parts. Appl. Therm. Eng. 2016, 100, 478–489. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G.; Wang, G.; Dong, G.; Wu, H. Investigation on bubble morphological evolution and plastic part surface quality of microcellular injection molding process based on a multiphase-solid coupled heat transfer model. Int. J. Heat Mass Tranfer 2017, 104, 1246–1258. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, G.; Wang, G. Research on the reduction of sink mark and warpage of the molded part in rapid heat cycle molding process. Mater. Des. 2013, 47, 779–792. [Google Scholar] [CrossRef]
- Chen, S.; Lin, Y.; Chien, R.; Li, H. Variable Mold Temperature to Improve Surface Quality of Microcellular Injection Molded Parts Using Induction Heating Technology. Adv. Polym. Technol. 2010, 27, 224–232. [Google Scholar] [CrossRef]
- Chen, S.C.; Hsu, P.S.; Hwang, S.S. The effects of gas counter pressure and mold temperature variation on the surface quality and morphology of the microcellular polystyrene foams. J. Appl. Polym. Sci. 2013, 127, 4769–4776. [Google Scholar] [CrossRef]
- Li, S.; Zhao, G.Q.; Wang, G.L.; Guan, Y.J.; Wang, X.X. Influence of relative low gas counter pressure on melt foaming behavior and surface quality of molded parts in microcellular injection molding process. J. Cell. Plast. 2014, 50, 415–435. [Google Scholar] [CrossRef]
- Chen, S.C.; Liao, W.H.; Chien, R.D. Structure and mechanical properties of polystyrene foams made through microcellular injection molding via control mechanisms of gas counter pressure and mold temperature. Int. Commun. Heat Mass 2012, 39, 1125–1131. [Google Scholar] [CrossRef]
- Suhartono, E.; Chen, S.C.; Chang, Y.H.; Chang, J.A.; Lee, K.H. Improvement on the surface quality of microcellular injection molded parts using microcellular co-injection molding with the material combinations of PP and PP-GF. Int. J. Plast. Technol. 2017, 21, 239–251. [Google Scholar] [CrossRef]
- Turng, L.S.; Kharbas, H. Development of a hybrid solid-microcellular co-injection molding process. Int. Polym. Proc. 2004, 19, 77–86. [Google Scholar] [CrossRef]
- Hou, J.J.; Zhao, G.Q.; Wang, G.L.; Dong, G.W.; Xu, J.J. A novel gas-assisted microcellular injection molding method for preparing lightweight foams with superior surface appearance and enhanced mechanical performance. Mater. Des. 2017, 127, 115–125. [Google Scholar] [CrossRef]
- Chen, H.L.; Chien, R.D.; Chen, S.C. Using thermally insulated polymer film for mold temperature control to improve surface quality of microcellular injection molded parts ☆. Int. Commun. Heat Mass 2008, 35, 991–994. [Google Scholar] [CrossRef]
- Lee, J.; Turng, L.S. Improving surface quality of microcellular injection molded parts through mold surface temperature manipulation with thin film insulation. Polym. Eng. Sci. 2010, 50, 1281–1289. [Google Scholar] [CrossRef]
- Lee, J.J.; Turng, L.S.; Dougherty, E.; Gorton, P. A novel method for improving the surface quality of microcellular injection molded parts. Polymer 2011, 52, 1436–1446. [Google Scholar] [CrossRef]
- Huang, H.; Wang, J. Improving polypropylene microcellular foaming through blending and the addition of nano-calcium carbonate. J. Appl. Polym. Sci. 2007, 106, 505–513. [Google Scholar] [CrossRef]
- Ding, J.; Shangguan, J.; Ma, W.; Zhong, Q. Foaming behavior of microcellular foam polypropylene/modified nano calcium carbonate composites. J. Appl. Polym. Sci. 2013, 128, 3639–3651. [Google Scholar] [CrossRef]
- Ding, J.; Ma, W.; Song, F.; Zhong, Q. Effect of nano-Calcium Carbonate on microcellular foaming of polypropylene. J. Mater. Sci. 2013, 48, 2504–2511. [Google Scholar] [CrossRef]
- Mao, H.; He, B.; Guo, W.; Hua, L.; Yang, Q. Effects of Nano-CaCO3 Content on the Crystallization, Mechanical Properties and Cell Structure of PP Nanocomposites in Microcellular Injection Molding. Polymers 2018, 10, 1160. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, K.; Guo, W.; Mao, H.; Yang, Q.; Meng, Z. Investigation on Foamed PP/Nano-CaCO3 Composites in a Combined in-Mold Decoration and Microcellular Injection Molding Process. Polymers 2020, 12, 363. https://doi.org/10.3390/polym12020363
Yan K, Guo W, Mao H, Yang Q, Meng Z. Investigation on Foamed PP/Nano-CaCO3 Composites in a Combined in-Mold Decoration and Microcellular Injection Molding Process. Polymers. 2020; 12(2):363. https://doi.org/10.3390/polym12020363
Chicago/Turabian StyleYan, Kui, Wei Guo, Huajie Mao, Qing Yang, and Zhenghua Meng. 2020. "Investigation on Foamed PP/Nano-CaCO3 Composites in a Combined in-Mold Decoration and Microcellular Injection Molding Process" Polymers 12, no. 2: 363. https://doi.org/10.3390/polym12020363
APA StyleYan, K., Guo, W., Mao, H., Yang, Q., & Meng, Z. (2020). Investigation on Foamed PP/Nano-CaCO3 Composites in a Combined in-Mold Decoration and Microcellular Injection Molding Process. Polymers, 12(2), 363. https://doi.org/10.3390/polym12020363







