Fabrication of Carboxylmethyl Chitosan Nanocarrier via Self-Assembly for Efficient Delivery of Phenylethyl Resorcinol in B16 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
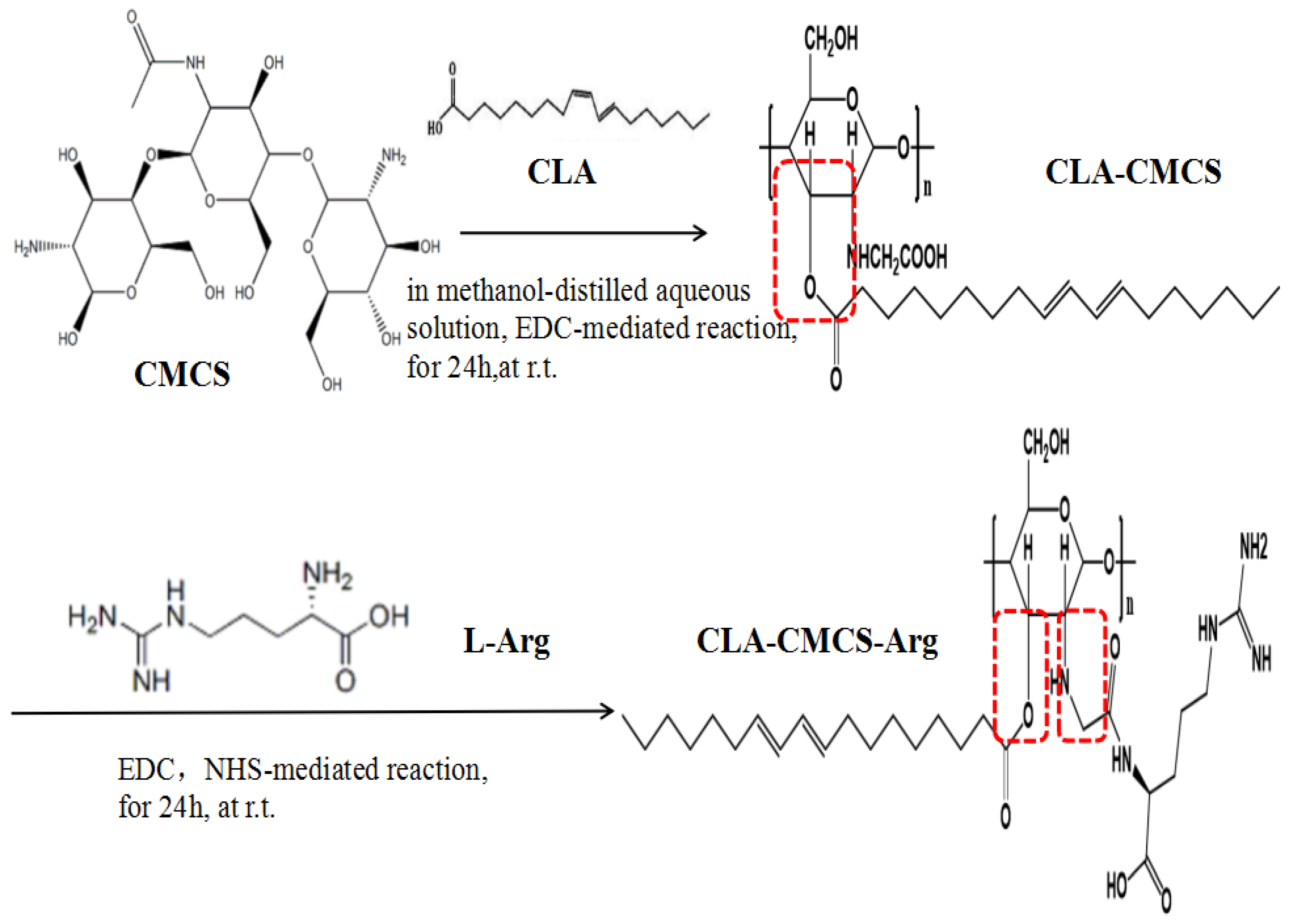
2.2. Synthesis of CLA-CMCS-Arg Conjugates
2.3. Preparation and Characterization of CCA-NPs and PR-Loaded CCA-NPs
2.4. Cell Experiment
2.5. Detecting the Content of Melanin
2.6. Statistical Analysis
3. Results and Discussion
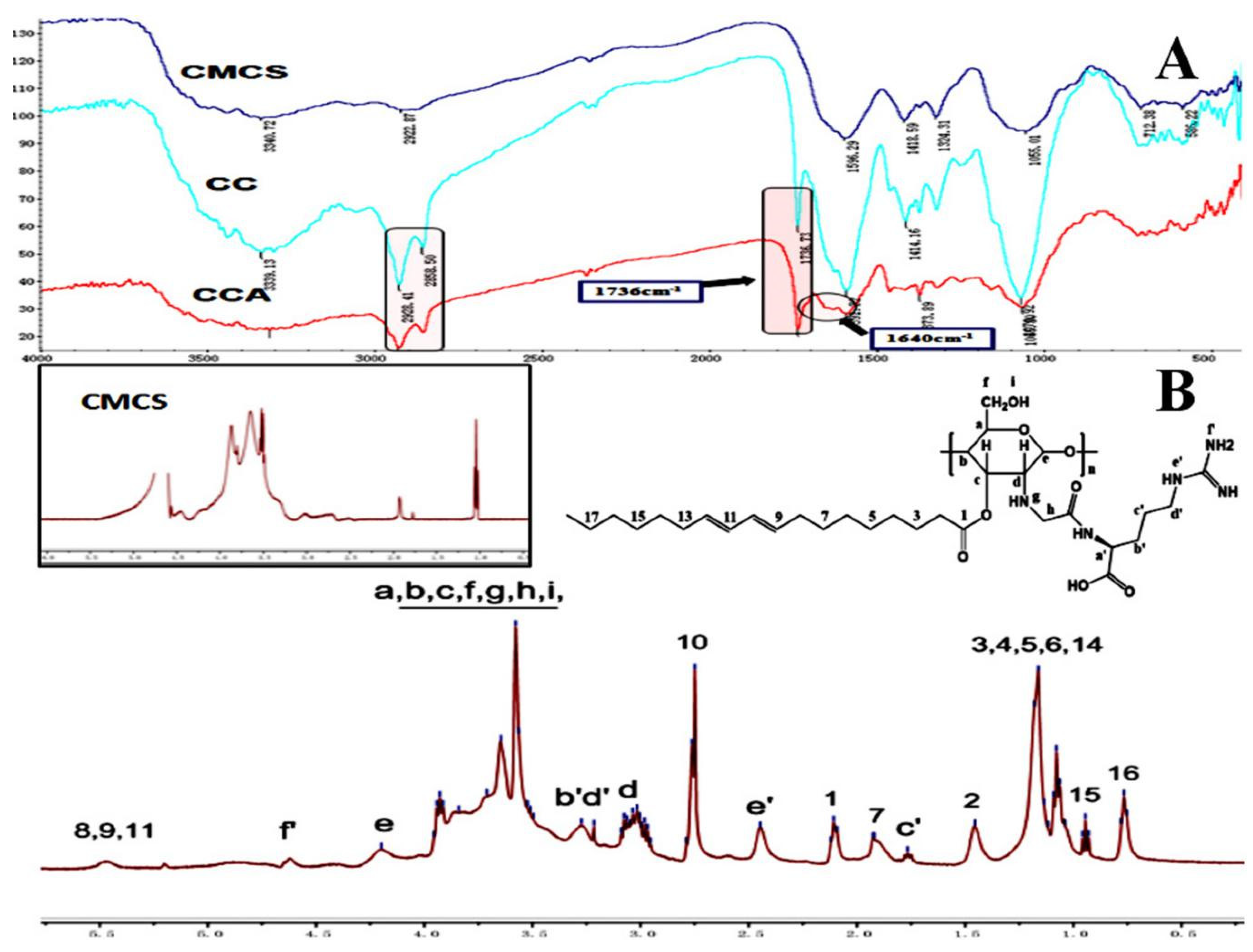
3.1. Characterization of CCA Sample
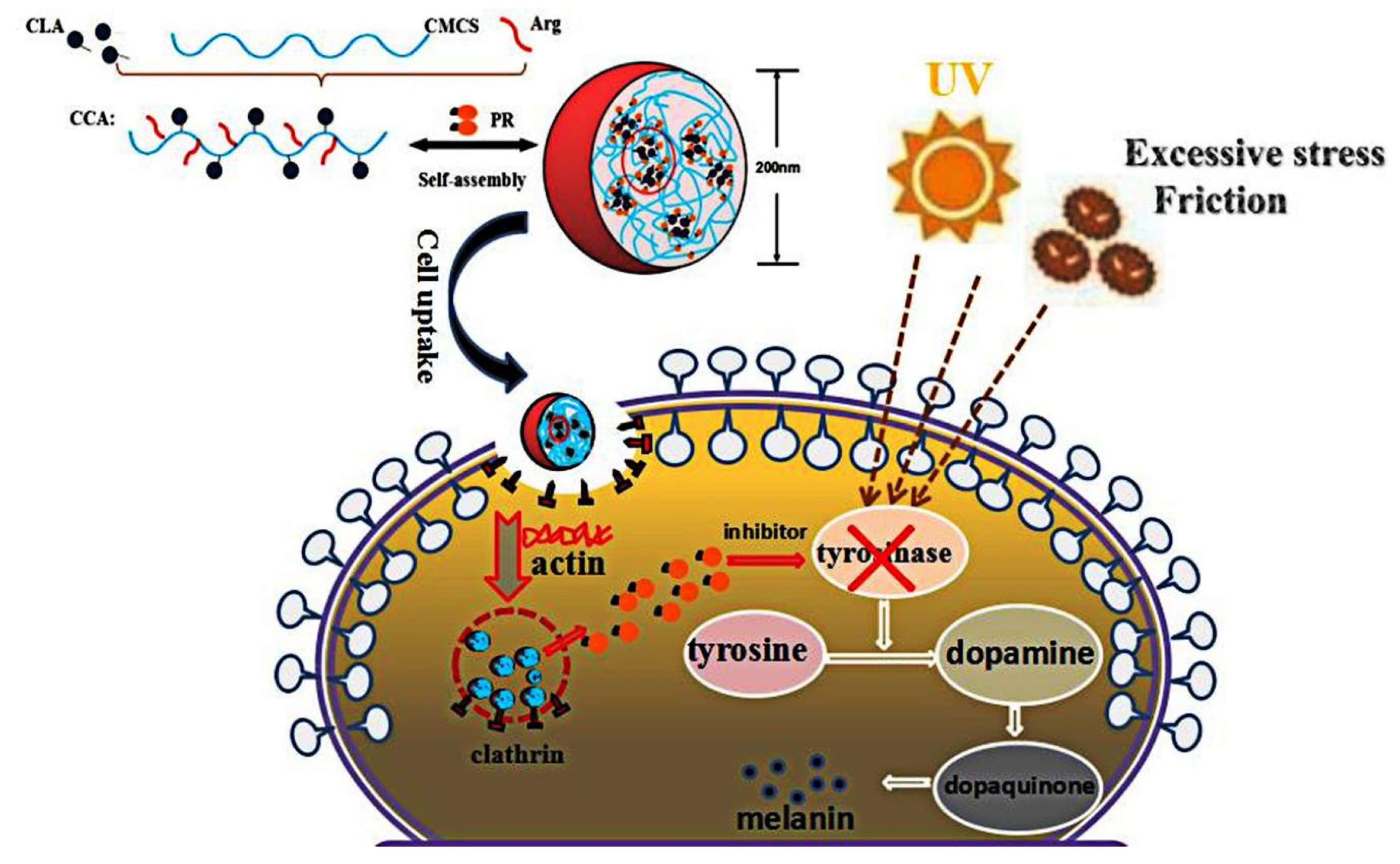
3.2. Formation and Characteristics of Self-Aggregated Nanoparticles
3.3. Cytotoxicity and Cellular Uptake of CCA-NPs
3.4. B16 Cells Death Induced by PR:CCA-NPs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Widlund, H.R.; Feige, E.; Lin, J.Y.; Fisher, D.E. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell 2007, 128, 853–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanlayavattanakul, M.; Lourith, N. Skin hyperpigmentation treatment using herbs: A review of clinical evidences. J. Cosmet. Laser Ther. Off. Publ. Eur. Soc. Laser Dermatol. 2017, 20, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Rendon, M.I.; Gaviria, J.I. Review of skin-lightening agents. Dermatol. Surg 2010, 31, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.W.; Al-Suwayeh, S.A.; Fang, C.-L.; Lin, C.-F.; Chen, C.-C.; Fang, J.-Y. The co-drug of conjugated hydroquinone and azelaic acid to enhance topical skin targeting and decrease penetration through the skin. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E V 2012, 81, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Panyosak, A.; Manosroi, J.; Rojanasakul, Y.; Manosroi, A. Safety assessment of azelaic acid and its derivatives entrapped in nanovesicles. Hum. Exp. Toxicol. 2009, 28, 387–392. [Google Scholar] [CrossRef]
- Candan, G.; Michiue, H.; Ishikawa, S.; Fujimura, A.; Hayashi, K.; Uneda, A.; Mori, A.; Ohmori, I.; Nishiki, T.-I.; Matsui, H. Combining poly-arginine with the hydrophobic counter-anion 4-(1-pyrenyl)-butyric acid for protein transduction in transdermal delivery. Biomaterials 2012, 33, 6468–6475. [Google Scholar] [CrossRef] [Green Version]
- SHussein-Al-Ali, H.; Arulselvan, P.; Hussein, M.Z.; Fakurazi, S.; Saifullah, B. Evaluate the Cytotoxicity of Kojic Acid Nanocomposites on Melanoma Cells and Normal Cells of the Skin. J. Biomim. Biomater. Biomed. Eng. 2018, 36, 45–55. [Google Scholar] [CrossRef]
- Chang, T.S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Han, D.; Li, M.-X.; Zhang, P.; Liu, C.-G. Development and validation of an ultraviolet-visible spectrophotometric method for determination of phenylethyl resorcinol in new topical nanoemulsions. Int. J. Cosmet. Sci. Int. J. Cosmet. Sci. 2017, 39, 337. [Google Scholar] [CrossRef] [Green Version]
- Bo-Sik, K.; Young-Guk, N.; Jae-Hwan, C.; Inhye, K.; Eunji, L.; Sung-Yeon, K.; Jae-Young, L.; Cheong-Weon, C. The Improvement of Skin Whitening of Phenylethyl Resorcinol by Nanostructured Lipid Carriers. Nanomaterials 2017, 7, 241–253. [Google Scholar]
- Schütz, C.A.; Juillerat-Jeanneret, L.; Mueller, H.; Lynch, I.; Riediker, M. Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine 2013, 8, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.L.; Hu, C.B.; Ni, S.L.; Qian, A.R.; Xia, Q. Preparation and Comparatively Evaluation of Phenylethyl Resorcinol-Loaded Nanocarriers: Nanoemulsions and Nanostructured Lipid Carriers. Adv. Mater. Res. 2015, 1118, 28–36. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Choi, H.; Liu, T.; Nath, K.; Zhou, R.; Chen, I.-W. Peptide nanoparticle with pH-sensing cargo solubility enhances cancer drug efficiency. Nano Today 2017, 13, 15–22. [Google Scholar] [CrossRef]
- Zhou, X.; Su, X.; Zhou, C. Preparation of diblock amphiphilic polypeptide nanoparticles for medical applications. Eur. Polym. J. 2018, 100, 132–136. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, S.-R.; Yu, Y.-Y.; Wang, H.; Yang, Y.; Liu, C.-G. Biocompatibility Profile and In Vitro Cellular Uptake of Self-assembled Alginate Nanoparticles. Molecules 2019, 24, 555. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhao, S.-R.; Li, J.-X.; Hong, L.; Raja, M.A.; Yu, L.-J.; Liu, C.-G. Nanoparticles based on phenylalanine ethyl ester-alginate conjugate as vitamin B2 delivery system. J. Biomater. Appl. 2016, 31, 13–22. [Google Scholar] [CrossRef]
- Li, C.; Tho, C.C.; Galaktionova, D.; Chen, X.; Král, P.; Mirsaidov, U. Dynamics of Amphiphilic Block Copolymers in an Aqueous Solution: Direct Imaging of Micelle Formation and Nanoparticle Encapsulation. Nanoscale 2019, 11, 2299–2305. [Google Scholar] [CrossRef]
- Di Santo, R.; Digiacomo, L.; Palchetti, S.; Palmieri, V.; Perini, G.; Pozzi, D.; Papi, M.; Caracciolo, G. Microfluidic manufacturing of surface-functionalized graphene oxide nanoflakes for gene delivery. Nanoscale 2019, 11, 2733–2741. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Sevin, E.; Gosselet, F.; Lima, J.; Pereira, M.C. Receptor-mediated PLGA nanoparticles for Glioblastoma Multiforme treatment. Int. J. Pharm. 2018, 545, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Chanphai, P.; Froehlich, E.; Mandeville, J.S.; Tajmir-Riahi, H.A. Protein conjugation with PAMAM nanoparticles: Microscopic and thermodynamic analysis. Colloids Surf. B Biointerfaces 2016, 150, 168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J. Mesoporous silica nanoparticle-based intelligent drug delivery system for bienzyme-responsive tumour targeting and controlled release. R. Soc. Open Sci. 2018, 5, 170986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, J.; Cui, Y.; Cai, H.; Bian, S.; Xu, Z.; Zhou, L.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. Synergistic chemotherapeutic effect of sorafenib-loaded pullulan-Dox conjugate nanoparticles against murine breast carcinoma. Nanoscale 2017, 9, 2755. [Google Scholar] [CrossRef] [PubMed]
- Ganbold, T.; Baigude, H. Design of Mannose-Functionalized Curdlan Nanoparticles for Macrophage-Targeted siRNA Delivery. ACS Appl. Mater. Interfaces 2018, 10, 14463. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.P.A.; Talman, V.; Torrieri, G.; Liu, D.; Marques, G.a.; Moslova, K.; Liu, Z.; Pinto, J.o.F.; Hirvonen, J.; Ruskoaho, H. Dual-drug delivery using dextran-functionalized nanoparticles targeting cardiac fibroblasts for cellular reprogramming. Adv. Funct. Mater. 2018, 29, 1705134. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, J.; Zhang, Q.; Yang, S.; Jiang, S.; Huang, C. PTX-loaded three-layer PLGA/CS/ALG nanoparticle based on layer-by-layer method for cancer therapy. J. Biomater. Sci. Polym. Ed. 2018, 29, 1–27. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, C.; Rong, X.; Niu, J.; Kong, D. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 2016, 113, 191–202. [Google Scholar] [CrossRef]
- Chen, X.G.; Park, H.-J. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr. Polym 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 2014, 35, 7654–7665. [Google Scholar] [CrossRef]
- Rui, H.; Hua, Z.; Cao, J.; Davoudi, Z.; Wang, Q. Synthesis and In Vitro Characterization of Carboxymethyl Chitosan-CBA-Doxorubicin Conjugate Nanoparticles as pH-Sensitive Drug Delivery Systems. J. Biomed. Nanotechnol. 2017, 13, 1097–1105. [Google Scholar]
- Jia, J.; Zhixiao, J.; Ming, X.; Chenyu, L.; Xiaoqing, Y.; Weiyao, Z.; Si, L.; Dan, W.; Wenping, Z.; Jianqing, C. Microspheres of Carboxymethyl Chitosan, Sodium Alginate and Collagen as a Hemostatic Agent In Vivo. ACS Biomater. Sci. Eng. 2018, 4, 2541–2551. [Google Scholar]
- Wang, J.; Wang, F.; Li, X.; Zhou, Y.; Wang, H.; Zhang, Y. Uniform carboxymethyl chitosan-enveloped Pluronic F68/poly(lactic-co-glycolic acid) nano-vehicles for facilitated oral delivery of gefitinib, a poorly soluble antitumor compound. Colloids Surf. B Biointerfaces 2019, 177, 425. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in vitro / in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug. Deliver. 2016, 23, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zongrui, T.; Yu, C.; Yanghe, M.; Huaiyu, F.; Peng, L.; Xiaosai, Q.; Shaohua, J. Bioresponsive Materials for Drug Delivery Based on Carboxymethyl Chitosan/Poly(γ-Glutamic Acid) Composite Microparticles. Mar. Drugs 2017, 15, 127. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Zhang, D.; Li, C.; Jia, L.; Liu, G.; Hao, L.; Zheng, D.; Shen, J.; Li, T.; Guo, Y. Self-assembled nanoparticles based on galactosylated O-carboxymethyl chitosan-graft-stearic acid conjugates for delivery of doxorubicin. Int. J. Pharm. 2013, 458, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, X.; Yin, Y.; Xiong, F.; Gong, X.; Zhu, Z.; Lu, B.; Xu, P. In vitro characterization, and in vivo studies of crosslinked lactosaminated carboxymethyl chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1048–1053. [Google Scholar]
- Li, Q.; Liu, C.-G.; Yu, Y. Separation of monodisperse alginate nanoparticles and effect of particle size on transport of vitamin E. Carbohydr. Polym. 2015, 124, 274–279. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, W.; Peng, Y.; Han, B.; Yang, Y. Toll like receptor 4 (TLR4) mediates the stimulating activities of chitosan oligosaccharide on macrophages. Int. Immunopharmacol. 2014, 23, 254–261. [Google Scholar] [CrossRef]
- Putri, R.M.; Allende-Ballestero, C.; Luque, D.; Klem, R.; Rousou, K.-A.; Liu, A.; Traulsen, C.H.-H.; Rurup, W.F.; Koay, M.S.T.; Castón, J.R. Structural Characterization of Native and Modified Encapsulins as Nanoplatforms for In Vitro Catalysis and Cellular Uptake. ACS Nano 2017, 11, 12796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Meng, X.; Chen, X.; Ren, G. The preparation and characterization of a novel amphiphilic oleoyl-carboxymethyl chitosan self-assembled nanoparticles. Carbohydr. Polym. 2011, 83, 130–136. [Google Scholar] [CrossRef]
- Kele, S.; Altunbek, M.; Culha, M. Influence of EDC/NHS coupling chemistry on stability and cytotoxicity of ZnO nanoparticles modified with proteins. Appl. Surf. Sci. 2017, 403, 455–463. [Google Scholar]
- Jie, X.; Si, N.; Huang, Q. Assembly of kafirin/carboxymethyl chitosan nanoparticles to enhance the cellular uptake of curcumin. Food Hydrocoll. 2015, 51, 166–175. [Google Scholar]
- Jiang, Z.; Han, B.; Li, H.; Yang, Y.; Liu, W. Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr. Polym. 2015, 129, 1–8. [Google Scholar] [CrossRef]
- Shi, L.; Tang, C.; Yin, C. Glycyrrhizin-modified O-carboxymethyl chitosan nanoparticles as drug vehicles targeting hepatocellular carcinoma. Biomaterials 2012, 33, 7594–7604. [Google Scholar] [CrossRef]
- Park, C.; Vo, C.L.-N.; Kang, T.; Oh, E.; Lee, B.-J. New method and characterization of self-assembled gelatin–oleic nanoparticles using a desolvation method via carbodiimide/N-hydroxysuccinimide (EDC/NHS) reaction. Eur. J. Pharm. Biopharm. 2015, 89, 365–373. [Google Scholar] [CrossRef]
- Yadav, P.; Bandyopadhyay, A.; Chakraborty, A.; Sarkar, K. Enhancement of anticancer activity and drug delivery of chitosan-curcumin nanoparticle via molecular docking and simulation analysis. Carbohydr. Polym. 2018, 182, 188–198. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Kobayashi, S.; Shichibe, S.; Mix, D.; Baudys, M.; Kim, S.W.; Sunamoto, J. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilization of insulin. J. Control. Release 1998, 54, 313–320. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Artursson, P. Cell cultures as models for drug absorption across the intestinal mucosa. Crit. Rev. Ther. Drug Carr. Syst. 1991, 8, 305–330. [Google Scholar]
- Wen, S.S.; Ujihara, M.; Chong, W.Y.; Voon, S.H.; Chung, L.Y. Size-dependent effect of cystine/citric acid-capped confeito-like gold nanoparticles on cellular uptake and photothermal cancer therapy. Colloids Surf. B Biointerfaces 2017, 161, 365. [Google Scholar]
- Fitzpatrick, T.B.; Becker, S.W.; Lerner, A.B.; Montgomery, H. Tyrosinase in Human Skin: Demonstration of Its Presence and of Its Role in Human Melanin Formation. Science 1950, 112, 223–225. [Google Scholar] [CrossRef]




| Components Weight (g) | Mean Size (nm) | PDI | Zeta Potential (mV) | EE (%) | |||
|---|---|---|---|---|---|---|---|
| Samples | CLA | CMCS | Arg | ||||
| 1 | 0.25 | 1.00 | 0.25 | 203.4 ± 3.42 | 0.252 | −39.7 ± 0.26 | 69.21 ± 1.96 |
| 2 | 0.50 | 1.00 | 0.25 | 192.4 ± 6.18 | 0.192 | −42.8 ± 1.37 | 71.48 ± 2.69 |
| 3 | 1.00 | 1.00 | 0.25 | 193.1 ± 2.69 | 0.218 | −47.4 ± 2.34 | 81.93 ± 5.01 * |
| 4 | 0.25 | 1.00 | 0.50 | 210.2 ± 3.08 | 0.286 | −36.2 ± 1.52 | 76.71 ± 2.69 * |
| 5 | 0.25 | 1.00 | 1.00 | 229.4 ± 1.83 | 0.230 | −33.9 ± 0.43 | 75.15 ± 3.17 * |
| Concentration (μg/mL) | Inhibition Rate/% | |
|---|---|---|
| 24 h | 48 h | |
| 0 | 0 | 0 |
| 5 | −0.42 ± 0.52 | −1.07 ± 0.26 |
| 10 | 8.41 ± 1.08 | 12.16 ± 1.37 |
| 20 | 24.19 ± 2.69 | 32.54 ± 2.34 |
| 50 | 42.93 ± 3.08 | 52.24 ± 1.52 |
| 100 | 60.94 ± 1.83 | 69.51 ± 0.43 |
| 200 | 65.43 ± 0.98 | 72.92 ± 1.27 |
| 500 | 73.14 ± 2.18 | 79.42 ± 0.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Guo, H.; Liu, C. Fabrication of Carboxylmethyl Chitosan Nanocarrier via Self-Assembly for Efficient Delivery of Phenylethyl Resorcinol in B16 Cells. Polymers 2020, 12, 408. https://doi.org/10.3390/polym12020408
Zhang P, Guo H, Liu C. Fabrication of Carboxylmethyl Chitosan Nanocarrier via Self-Assembly for Efficient Delivery of Phenylethyl Resorcinol in B16 Cells. Polymers. 2020; 12(2):408. https://doi.org/10.3390/polym12020408
Chicago/Turabian StyleZhang, Pei, Huixia Guo, and Chenguang Liu. 2020. "Fabrication of Carboxylmethyl Chitosan Nanocarrier via Self-Assembly for Efficient Delivery of Phenylethyl Resorcinol in B16 Cells" Polymers 12, no. 2: 408. https://doi.org/10.3390/polym12020408
APA StyleZhang, P., Guo, H., & Liu, C. (2020). Fabrication of Carboxylmethyl Chitosan Nanocarrier via Self-Assembly for Efficient Delivery of Phenylethyl Resorcinol in B16 Cells. Polymers, 12(2), 408. https://doi.org/10.3390/polym12020408




