ECM Mimetic Electrospun Porous Poly (L-lactic acid) (PLLA) Scaffolds as Potential Substrates for Cardiac Tissue Engineering
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
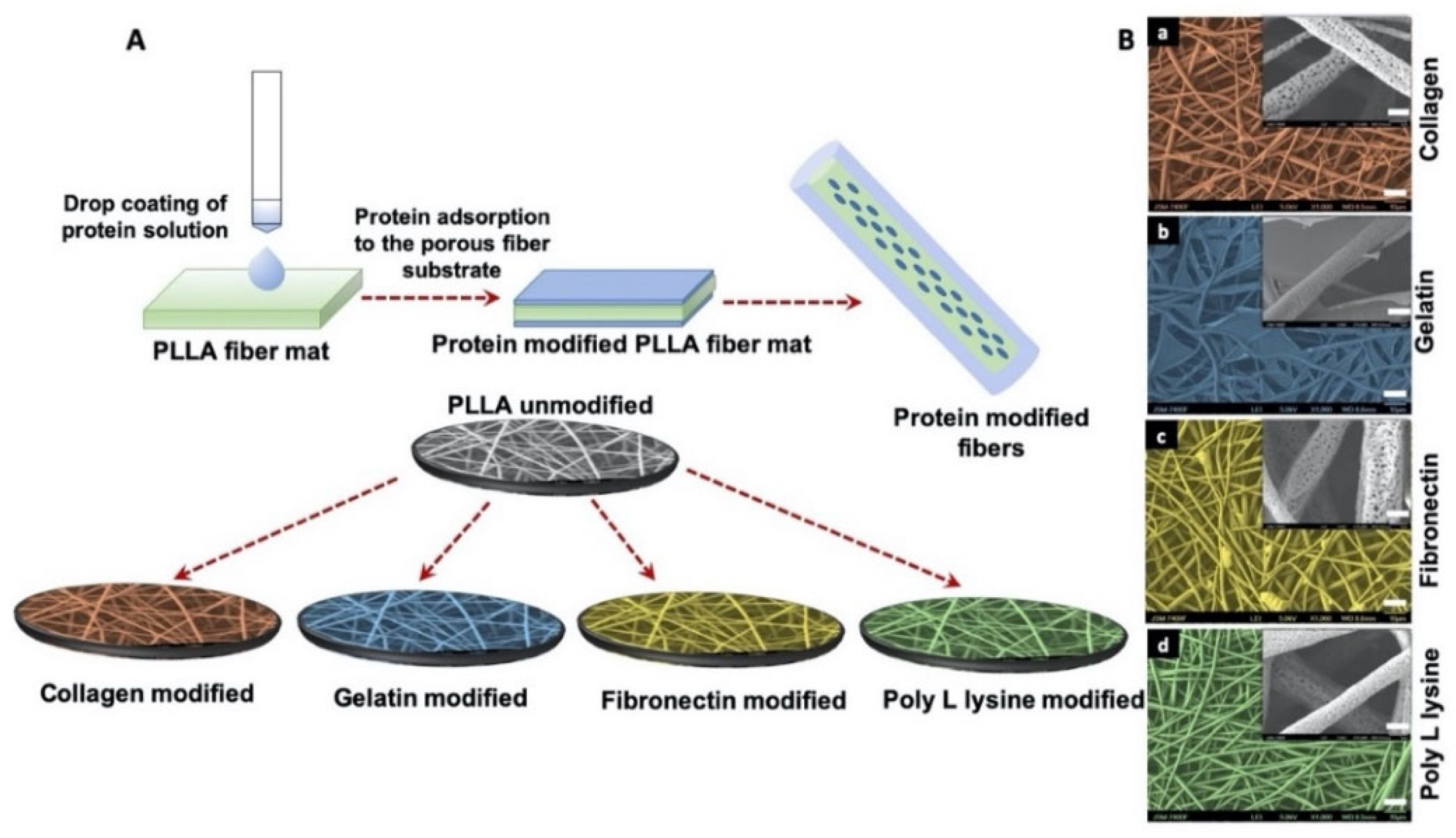
2.2.1. Fabrication of Electrospun Porous PLLA Scaffolds
2.2.2. Surface Functionalization of Electrospun PLLA Scaffolds
2.2.3. Characterization of Electrospun PLLA Fiber Scaffolds
2.2.4. In Vitro Fiber Degradation
2.2.5. Cell Culture
2.2.6. Cell-PLLA Scaffold Preparation for SEM Observation
2.2.7. Ki67 Proliferation and Proteome Profiling Assay
2.2.8. Statistical Analysis
3. Results
3.1. Fabrication of Porous Fiber Scaffolds
3.2. Morphology of Surface Functionalization Porous Scaffolds with ECM Mimetic Proteins
3.3. Physiochemical Characterization
3.4. Surface Wettability and Zeta Potential
3.5. In Vitro Degradation and Weight Loss
3.6. Cell Adhesion and Proliferation
3.7. Cytocompatibility of Scaffolds
3.8. Reactive Oxygen Species Generation
3.9. Cell Proliferation on Scaffolds
3.10. Proteome Profiling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baig, M.K.; Mahon, N.; McKenna, W.J.; Caforio, A.L.; Bonow, R.O.; Francis, G.S.; Gheorghiade, M. The pathophysiology of advanced heart failure. Heart Lung 1999, 28, 87–101. [Google Scholar] [CrossRef]
- Kuraitis, D.; Giordano, C.; Ruel, M.; Musaro, A.; Suuronen, E.J. Exploiting extracellular matrix-stem cell interactions: A review of natural materials for therapeutic muscle regeneration. Biomaterials 2012, 33, 428–443. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Liu, Y.; Guo, C.; Gong, Y.; Yang, S.; Ma, M.; Li, Z.; Gao, W.Q.; He, Z. Generation, characterization and potential therapeutic applications of cardiomyocytes from various stem cells. Stem Cells Dev. 2012, 21, 2095–2110. [Google Scholar] [CrossRef]
- Karam, J.P.; Muscari, C.; Montero-Menei, C.N. Combining adult stem cells and polymeric devices for tissue engineering in infarcted myocardium. Biomaterials 2012, 33, 5683–5695. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Castells-Sala, C.; Semino, C.E. Biomaterials for stem cell culture and seeding for the generation and delivery of cardiac myocytes. Curr. Opin. Organ Transpl. 2012, 17, 681–687. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun nanofibers: New concepts, materials and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Kitsara, M.; Agbulut, O.; Kontziampasis, D.; Chen, Y.; Menasche, P. Fibers for hearts: A critical review on electrospinning for cardiac tissue engineering. Acta Biomater. 2017, 48, 20–40. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, Y.; Pan, J.; Liu, Y. Macro-Alignment of electrospun fibers for vascular tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 508–516. [Google Scholar] [CrossRef]
- Bigg, D.M. Polylactide copolymers: Effect of copolymer ratio and end capping on their properties. Adv. Polym. Technol. 2005, 24, 69–82. [Google Scholar] [CrossRef]
- Sprott, M.R.; Gallego-Ferrer, G.; Dalby, M.J.; Salmeron-Sanchez, M.; Cantini, M. Functionalization of PLLA with polymer brushes to trigger the assembly of fibronectin into nanonetworks. Adv. Healthc. Mater. 2019, 8, e1801469. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zheng, H.; Liang, S.; Gao, C. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomater. 2016, 37, 131–142. [Google Scholar] [CrossRef]
- Khatri, Z.; Jatoi, A.; Ahmed, F.; Kim, I.-S. Cell adhesion behavior of poly(ɛ-caprolactone)/poly (L-lactic acid) nanofibers scaffold. Mater. Lett. 2016, 171, 178–181. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Liao, S.; Chan, C.K.; Ramakrishna, S. Enhanced osteogenic differentiation with 3D electrospun nanofibrous scaffolds. Nanomedicine 2012, 7, 1561–1575. [Google Scholar] [CrossRef]
- Jacobs, T.; Declercq, H.; De Geyter, N.; Cornelissen, R.; Dubruel, P.; Leys, C.; Beaurain, A.; Payen, E.; Morent, R. Plasma surface modification of polylactic acid to promote interaction with fibroblasts. J. Mater. Sci. Mater. Med. 2013, 24, 469–478. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.W.; Park, K.E.; Park, W.H.; Lee, K.Y. Stress response of fibroblasts adherent to the surface of plasma-treated poly(lactic-co-glycolic acid) nanofiber matrices. Colloids Surf. B Biointerfaces 2010, 77, 90–95. [Google Scholar] [CrossRef]
- Rim, N.G.; Kim, S.J.; Shin, Y.M.; Jun, I.; Lim, D.W.; Park, J.H.; Shin, H. Mussel-Inspired surface modification of poly(L-lactide) electrospun fibers for modulation of osteogenic differentiation of human mesenchymal stem cells. Colloids Surf. B Biointerfaces 2012, 91, 189–197. [Google Scholar] [CrossRef]
- Shin, Y.M.; Shin, H.; Lim, Y.M. Surface modification of electrospun poly(L-lactide-co-ɛ-caprolactone) fibrous meshes with a RGD peptide for the control of adhesion, proliferation and differentiation of the preosteoblastic cells. Macromol. Res. 2010, 18, 472–481. [Google Scholar] [CrossRef]
- Elsdale, T.; Bard, J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef]
- Grinnell, F.; Bennett, M.H. Ultrastructural studies of cell—Collagen interactions. Methods Enzym. 1982, 82 Pt A, 535–544. [Google Scholar] [CrossRef]
- Strom, S.C.; Michalopoulos, G. Collagen as a substrate for cell growth and differentiation. Methods Enzym. 1982, 82 Pt A, 544–555. [Google Scholar] [CrossRef]
- Cutts, J.; Nikkhah, M.; Brafman, D.A. Biomaterial approaches for stem cell-based myocardial tissue engineering. Biomark Insights 2015, 10, 77–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials—Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Elamparithi, A.; Punnoose, A.M.; Paul, S.F.D.; Kuruvilla, S. Gelatin electrospun nanofibrous matrices for cardiac tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 20–27. [Google Scholar] [CrossRef]
- Pushp, P.; Ferreira, F.C.; Cabral, J.M.S.; Gupta, M.K. Improved survival of cardiac cells on surface modified electrospun nanofibers. Polym. Sci. Ser. A 2017, 59, 515–523. [Google Scholar] [CrossRef]
- Jia, L.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Guiding the orientation of smooth muscle cells on random and aligned polyurethane/collagen nanofibers. J. Biomater. Appl. 2014, 29, 364–377. [Google Scholar] [CrossRef]
- Feng, Z.-Q.; Lu, H.-J.; Leach, M.K.; Huang, N.-P.; Wang, Y.-C.; Liu, C.-J.; Gu, Z.-Z. The influence of type-I collagen-coated PLLA aligned nanofibers on growth of blood outgrowth endothelial cells. Biomed. Mater. 2010, 5, 065011. [Google Scholar] [CrossRef]
- Olsen, D.; Yang, C.; Bodo, M.; Chang, R.; Leigh, S.; Baez, J.; Carmichael, D.; Perala, M.; Hamalainen, E.R.; Jarvinen, M.; et al. Recombinant collagen and gelatin for drug delivery. Adv. Drug Deliv. Rev. 2003, 55, 1547–1567. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 379–386. [Google Scholar] [CrossRef]
- Kharaziha, M.; Nikkhah, M.; Shin, S.R.; Annabi, N.; Masoumi, N.; Gaharwar, A.K.; Camci-Unal, G.; Khademhosseini, A. PGS: Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials 2013, 34, 6355–6366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, I.H.; Ayres, E.; Averous, L.; Schlatter, G.; Hebraud, A.; de Paula, A.C.; Viana, P.H.; Goes, A.M.; Orefice, R.L. Differentiation of human adipose-derived stem cells seeded on mineralized electrospun co-axial poly(epsilon-caprolactone) (PCL)/gelatin nanofibers. J. Mater. Sci. Mater. Med. 2014, 25, 1137–1148. [Google Scholar] [CrossRef]
- Kuppan, P.; Sethuraman, S.; Krishnan, U.M. Interaction of human smooth muscle cells with nanofibrous scaffolds: Effect of fiber orientation on cell adhesion, proliferation and functional gene expression. J. Biomed. Mater. Res. A 2015, 103, 2236–2250. [Google Scholar] [CrossRef] [PubMed]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, K.M.; Maxwell, J.T.; Bhutani, S.; Ghosh-Choudhary, S.; Fierro, M.J.; Johnson, T.D.; Christman, K.L.; Taylor, W.R.; Davis, M.E. Fibronectin and cyclic strain improve cardiac progenitor cell regenerative potential In Vitro. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Konstandin, M.H.; Toko, H.; Gastelum, G.M.; Quijada, P.; De La Torre, A.; Quintana, M.; Collins, B.; Din, S.; Avitabile, D.; Volkers, M.; et al. Fibronectin is essential for reparative cardiac progenitor cell response after myocardial infarction. Circ. Res. 2013, 113, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Ngiam, M.; Liao, S.; Patil, A.J.; Cheng, Z.; Yang, F.; Gubler, M.J.; Ramakrishna, S.; Chan, C.K. Fabrication of mineralized polymeric nanofibrous composites for bone graft materials. Tissue Eng. Part A 2009, 15, 535–546. [Google Scholar] [CrossRef]
- Francois, S.; Chakfe, N.; Durand, B.; Laroche, G. A poly(L-lactic acid) nanofibre mesh scaffold for endothelial cells on vascular prostheses. Acta Biomater. 2009, 5, 2418–2428. [Google Scholar] [CrossRef]
- Hu, J.; Sun, X.; Ma, H.; Xie, C.; Chen, Y.E.; Ma, P.X. Porous nanofibrous PLLA scaffolds for vascular tissue engineering. Biomaterials 2010, 31, 7971–7977. [Google Scholar] [CrossRef] [Green Version]
- Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber assembly by rotary jet-spinning. Nano Lett. 2010, 10, 2257–2261. [Google Scholar] [CrossRef] [Green Version]
- Kenar, H.; Kose, G.T.; Toner, M.; Kaplan, D.L.; Hasirci, V. A 3D aligned microfibrous myocardial tissue construct cultured under transient perfusion. Biomaterials 2011, 32, 5320–5329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, D.; Prentki, P.; Chandler, M. Use of gel retardation to analyze protein-nucleic acid interactions. Microbiol. Rev. 1992, 56, 509. [Google Scholar] [CrossRef] [PubMed]
- Hadasha, W.; Bezuidenhout, D. Poly(lactic acid) as biomaterial for cardiovascular devices and tissue engineering applications. Adv. Polym. Sci. 2017, 282, 51–77. [Google Scholar] [CrossRef]
- Bertuoli, P.T.; Ordoño, J.; Armelin, E.; Pérez-Amodio, S.; Baldissera, A.F.; Ferreira, C.A.; Puiggalí, J.; Engel, E.; del Valle, L.J.; Alemán, C. Electrospun conducting and biocompatible uniaxial and core-shell fibers having poly(lactic acid), poly(ethylene glycol) and polyaniline for cardiac tissue engineering. ACS Omega 2019, 4, 3660–3672. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Shin, Y.; Hohman, M.; Brenner, M.P.; Rutledge, G. Electrospinning: A whipping fluid jet generates submicron polymer fibers. Appl. Phys. Lett. 2001, 78, 1149–1151. [Google Scholar] [CrossRef]
- Hadjizadeh, A.; Savoji, H.; Ajji, A. A facile approach for the mass production of submicro/micro poly (lactic acid) fibrous mats and their cytotoxicity test towards neural stem cells. Biomed. Res. Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (PLA) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef] [Green Version]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Taylor cone and jetting from liquid droplets in electrospinning of nanofibers. J. Appl. Phys. 2001, 90, 4836–4846. [Google Scholar] [CrossRef] [Green Version]
- Megelski, S.; Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Micro-and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.Y.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-Spinning of pure collagen nano-fibres—Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Y.; Zhang, K.; Zhong, Y.; Lin, L.; Zhou, H.; Chen, L.; Zhang, Y. Hydrophilic and hydrophobic poly(L-lactic acid) films by building porous topological surfaces. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Tzoneva, R.; Faucheux, N.; Groth, T. Wettability of substrata controls cell-substrate and cell-cell adhesions. Biochim. Biophys. Acta 2007, 1770, 1538–1547. [Google Scholar] [CrossRef]
- Tamada, Y.; Ikada, Y. Effect of preadsorbed proteins on cell adhesion to polymer surfaces. J. Colloid Interface Sci. 1993, 155, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Yu, M.; Zong, X.; Chiu, J.; Fang, D.; Seo, Y.S.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Control of degradation rate and hydrophilicity in electrospun non-woven poly(D,L-lactide) nanofiber scaffolds for biomedical applications. Biomaterials 2003, 24, 4977–4985. [Google Scholar] [CrossRef]
- Yoon, S.D.; Kwon, Y.S.; Lee, K.S. Biodegradation and biocompatibility of poly L-lactic acid implantable mesh. Int. Neurourol. J. 2017, 21, S48–S54. [Google Scholar] [CrossRef] [Green Version]
- Ifkovits, J.L.; Devlin, J.J.; Eng, G.; Martens, T.P.; Vunjak-Novakovic, G.; Burdick, J.A. Biodegradable fibrous scaffolds with tunable properties formed from photo-cross-linkable poly(glycerol sebacate). ACS Appl. Mater. Interfaces 2009, 1, 1878–1886. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; p. 944. [Google Scholar] [CrossRef]
- Kumar, V.; Mohamed, M.S.; Veeranarayanan, S.; Maekawa, T.; Kumar, D.S. Functionalized carbon nanowalls as pro-angiogenic scaffolds for endothelial cell activation. ACS Appl. Bio Mater. 2019, 2, 1119–1130. [Google Scholar] [CrossRef]
- Humeres, C.; Frangogiannis, N.G. Fibroblasts in the infarcted, remodeling and failing heart. JACC Basic Transl. Sci. 2019, 4, 449–467. [Google Scholar] [CrossRef]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular effects and benefits of exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| PROTEINS | TCP | UNMODIFIED | COLLAGEN | GELATIN | FIBRONECTIN | POLY L LYSINE |
|---|---|---|---|---|---|---|
| IL-1 ß | Y | Y | Y | Y | Y | Y |
| PENTRAXIN3 | Y | Y | Y | Y | Y | Y |
| TIMP1 | Y | Y | Y | Y | Y | Y |
| VEGF | Y | Y | Y | Y | Y | Y |
| ACTIVIN A | – | – | Y | – | Y | – |
| PF4 | – | – | Y | – | – | – |
| FGF-B | – | – | – | – | Y | – |
| PDGF AA | – | – | – | – | Y | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniyandi, P.; Palaninathan, V.; Veeranarayanan, S.; Ukai, T.; Maekawa, T.; Hanajiri, T.; Mohamed, M.S. ECM Mimetic Electrospun Porous Poly (L-lactic acid) (PLLA) Scaffolds as Potential Substrates for Cardiac Tissue Engineering. Polymers 2020, 12, 451. https://doi.org/10.3390/polym12020451
Muniyandi P, Palaninathan V, Veeranarayanan S, Ukai T, Maekawa T, Hanajiri T, Mohamed MS. ECM Mimetic Electrospun Porous Poly (L-lactic acid) (PLLA) Scaffolds as Potential Substrates for Cardiac Tissue Engineering. Polymers. 2020; 12(2):451. https://doi.org/10.3390/polym12020451
Chicago/Turabian StyleMuniyandi, Priyadharshni, Vivekanandan Palaninathan, Srivani Veeranarayanan, Tomofumi Ukai, Toru Maekawa, Tatsuro Hanajiri, and Mohamed Sheikh Mohamed. 2020. "ECM Mimetic Electrospun Porous Poly (L-lactic acid) (PLLA) Scaffolds as Potential Substrates for Cardiac Tissue Engineering" Polymers 12, no. 2: 451. https://doi.org/10.3390/polym12020451






