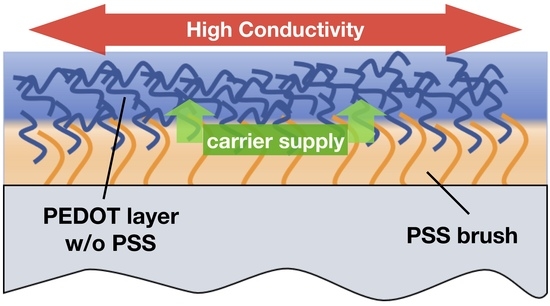
A New Composite Structure of PEDOT/PSS: Macro-Separated Layers by a Polyelectrolyte Brush
Abstract
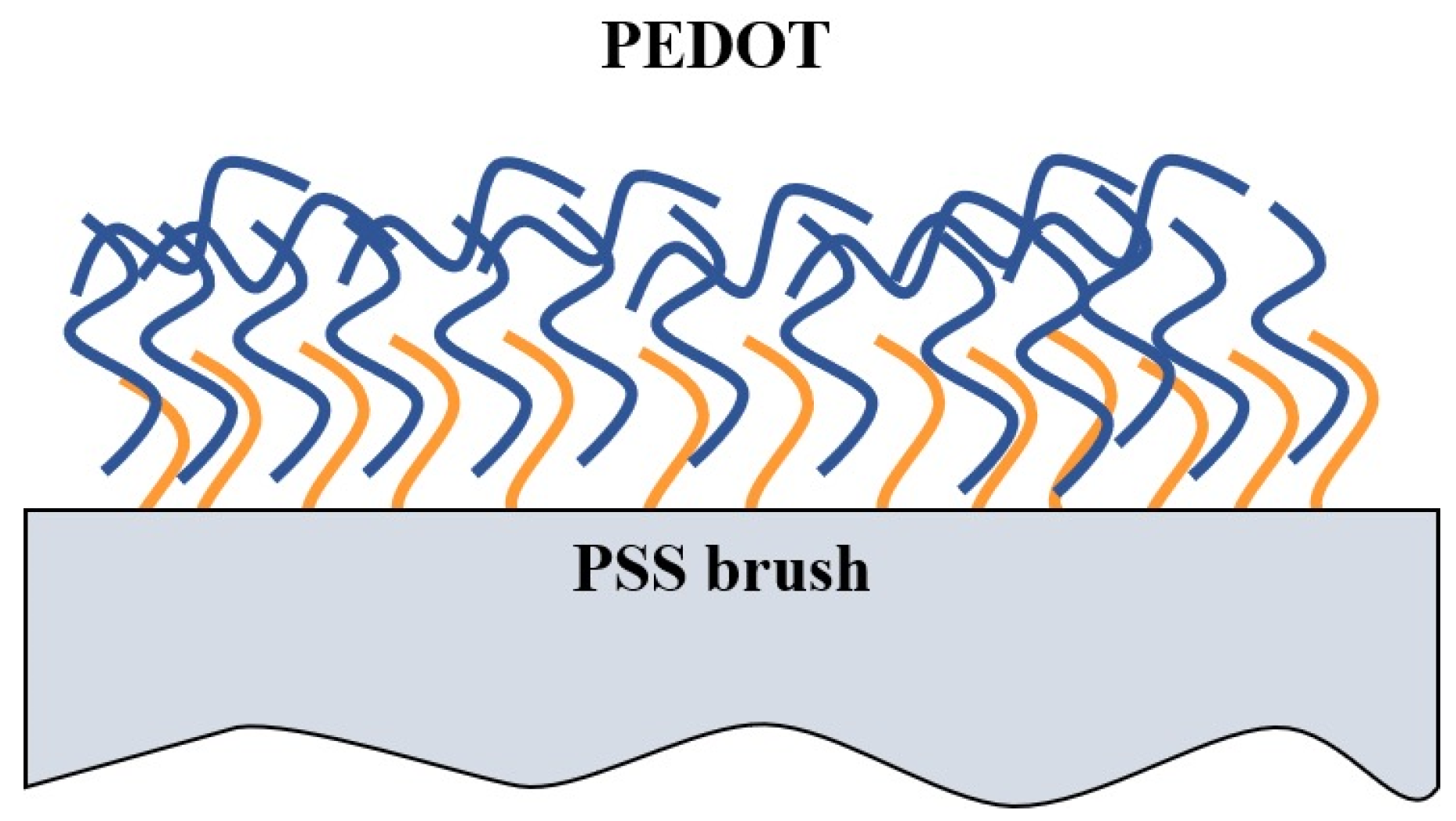
1. Introduction
2. Materials and Methods
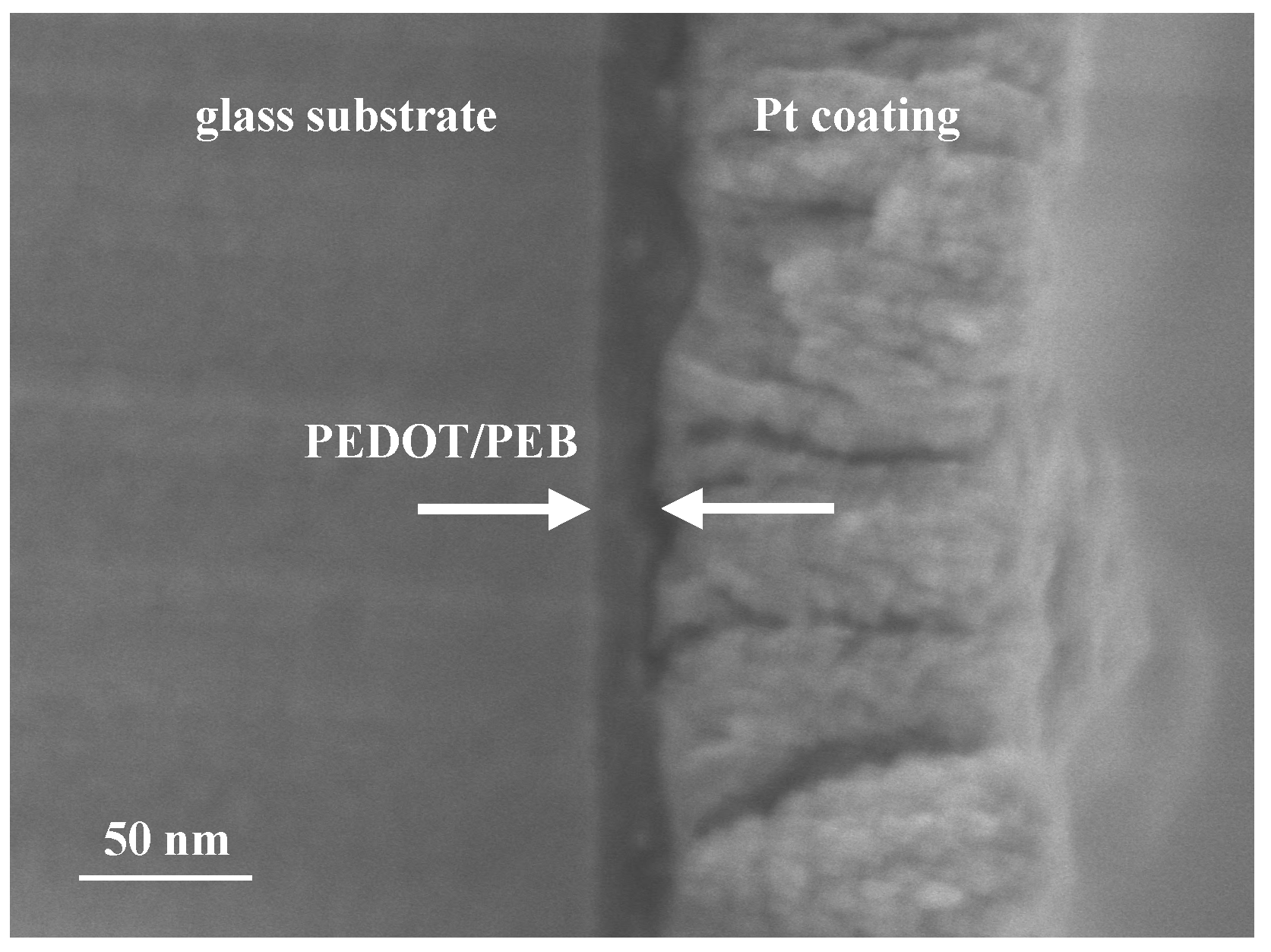
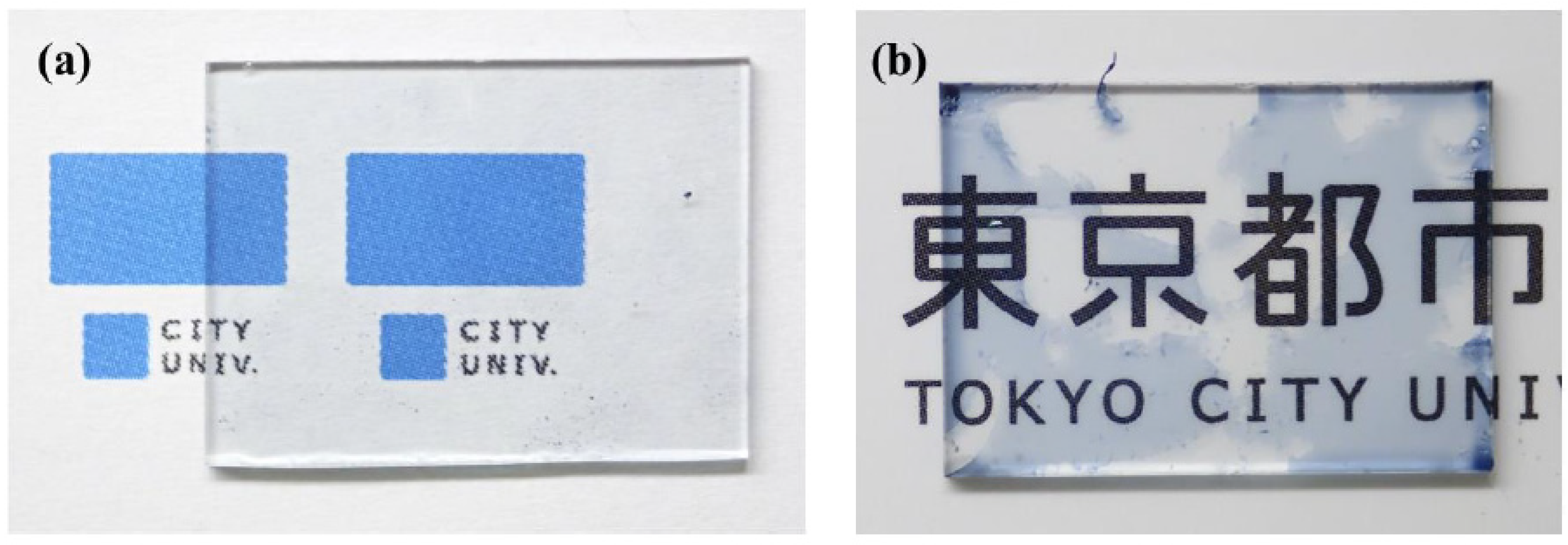
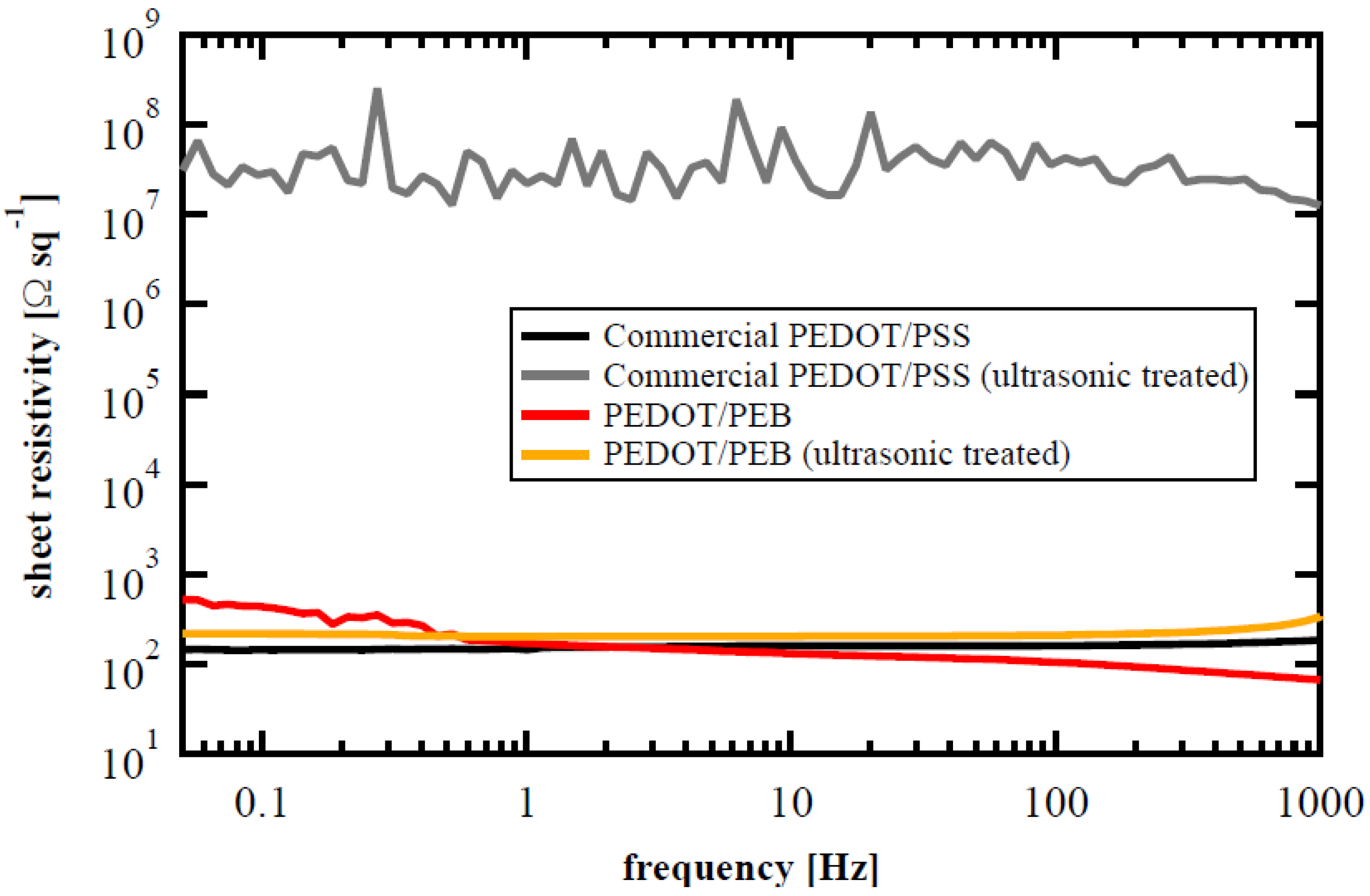
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Granqvist, C.G.; Hultåker, A. Transparent and conducting ITO films: new developments and applications. Thin Solid Films 2002, 411, 1–5. [Google Scholar] [CrossRef]
- Waltman, R.J.; Bargon, J. Electrically conducting polymers: A review of the electropolymerization reaction, of the effects of chemical structure on polymer film properties, and of applications towards technology. Can. J. Chem. 1986, 64, 76–95. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, R.C. Advances in conductive polymers. Eur. Polym. J. 1998, 34, 1053–1060. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Research progress on a novel conductive polymer-poly(3,4-ethylenedioxythiophene) (PEDOT). J. Phys. Conf. Ser. 2009, 152. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, J.; Kim, J.H. Direct synthesis of highly conductive poly(3,4-ethylenedioxythiophene):poly(4-styrenesulfonate) (PEDOT:PSS)/graphene composites and their applications in energy harvesting systems. Nano Res. 2014, 7, 717–730. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088. [Google Scholar] [CrossRef]
- Takano, T.; Masunaga, H.; Fujiwara, A.; Okuzaki, H.; Sasaki, T. PEDOT nanocrystal in highly conductive PEDOT:PSS polymer films. Macromolecules 2012, 45, 3859–3865. [Google Scholar] [CrossRef]
- Jönsson, S.K.M.; Birgerson, J.; Crispin, X.; Greczynski, G.; Osikowicz, W.; Denier van der Gon, A.W.; Salaneck, W.R.; Fahlman, M. The effects of solvents on the morphology and sheet resistance in poly(3,4-ethylenedioxythiophene)-polystyrenesulfonic acid (PEDOT-PSS) films. Synth. Met. 2003, 139, 1–10. [Google Scholar] [CrossRef]
- Crispin, X.; Jakobsson, F.L.E.; Crispin, A.; Grim, P.C.M.; Andersson, P.; Volodin, A.; Van Haesendonck, C.; Van Der Auweraer, M.; Salaneck, W.R.; Berggren, M. The origin of the high conductivity of poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT-PSS) plastic electrodes. Chem. Mater. 2006, 18, 4354–4360. [Google Scholar] [CrossRef]
- Thomas, J.P.; Zhao, L.; McGillivray, D.; Leung, K.T. High-efficiency hybrid solar cells by nanostructural modification in PEDOT:PSS with co-solvent addition. J. Mater. Chem. A 2014, 2, 2383–2389. [Google Scholar] [CrossRef]
- Lee, I.; Kim, G.W.; Yang, M.; Kim, T.S. Simultaneously Enhancing the Cohesion and Electrical Conductivity of PEDOT:PSS Conductive Polymer Films using DMSO Additives. ACS Appl. Mater. Interfaces 2016, 8, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, E.; Won, Y.; Lee, H.; Suh, K. The preparation and characteristics of conductive poly(3,4-ethylenedioxythiophene) thin film by vapor-phase polymerization. Synth. Met. 2003, 139, 485–489. [Google Scholar] [CrossRef]
- Mueller, M.; Fabretto, M.; Evans, D.; Hojati-Talemi, P.; Gruber, C.; Murphy, P. Vacuum vapour phase polymerization of high conductivity PEDOT: Role of PEG-PPG-PEG, the origin of water, and choice of oxidant. Polymer (Guildf) 2012, 53, 2146–2151. [Google Scholar] [CrossRef]
- Fujima, T.; Uchiyama, K.; Yasumoro, K.; Ito, T.; Tabata, E. A PSS-Free PEDOT Conductive Film Supported by a Hierarchical Nanoporous Layer Glass. Macromol. Mater. Eng. 2018, 303, 1800183. [Google Scholar] [CrossRef]
- Fujima, T.; Futakuchi, E.; Tomita, T.; Orai, Y.; Sunaoshi, T. Hierarchical Nanoporous Glass with Antireflectivity and Superhydrophilicity by One-Pot Etching. Langmuir 2014, 30, 14494–14497. [Google Scholar] [CrossRef]
- Tabata, E.; Ito, T.; Ushioda, Y.; Fujima, T. Fingerprint Blurring on a Hierarchical Nanoporous Layer Glass. Coatings 2019, 9, 653. [Google Scholar] [CrossRef]
- Ballauff, M.; Borisov, O. Polyelectrolyte brushes. Curr. Opin. Colloid Interface Sci. 2006, 11, 316–323. [Google Scholar] [CrossRef]
- Tran, Y.; Auroy, P. Synthesis of poly(styrene sulfonate) brushes. J. Am. Chem. Soc. 2001, 123, 3644–3654. [Google Scholar] [CrossRef]
- Kino, F.; Tomita, T.; Toshiro, O.; Takagi, K.; Fujima, T. Nano frictional properties of polystyrene sulfonate brushes in water. Kobunshi Ronbunshu 2011, 68, 171–175. [Google Scholar] [CrossRef][Green Version]
- Mulfort, K.L.; Ryu, J.; Zhou, Q. Preparation of surface initiated polystyrenesulfonate films and PEDOT doped by the films. Polymer (Guildf) 2003, 44, 3185–3192. [Google Scholar] [CrossRef]
- Park, B.W.; Yang, L.; Johansson, E.M.J.; Vlachopoulos, N.; Chams, A.; Perruchot, C.; Jouini, M.; Boschloo, G.; Hagfeldt, A. Neutral, polaron, and bipolaron states in pedot prepared by photoelectrochemical polymerization and the effect on charge generation mechanism in the solid-state dye-sensitized solar cell. J. Phys. Chem. C 2013, 117, 22484–22491. [Google Scholar] [CrossRef]
- Zozoulenko, I.; Singh, A.; Singh, S.K.; Gueskine, V.; Crispin, X.; Berggren, M. Polarons, Bipolarons, And Absorption Spectroscopy of PEDOT. ACS Appl. Polym. Mater. 2019, 1, 83–94. [Google Scholar] [CrossRef]
- Chiu, W.W.; Travaš-Sejdić, J.; Cooney, R.P.; Bowmaker, G.A. Spectroscopic and conductivity studies of doping in chemically synthesized poly(3,4-ethylenedioxythiophene). Synth. Met. 2005, 155, 80–88. [Google Scholar] [CrossRef]
- Moraes, B.R.; Campos, N.S.; Izumi, C.M.S. Surface-enhanced Raman scattering of EDOT and PEDOT on silver and gold nanoparticles. Vib. Spectrosc. 2018, 96, 137–142. [Google Scholar] [CrossRef]
- Guzinski, M.; Jarvis, J.M.; Perez, F.; Pendley, B.D.; Lindner, E.; De Marco, R.; Crespo, G.A.; Acres, R.G.; Walker, R.; Bishop, J. PEDOT(PSS) as Solid Contact for Ion-Selective Electrodes: The Influence of the PEDOT(PSS) Film Thickness on the Equilibration Times. Anal. Chem. 2017, 89, 3508–3516. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ouyang, J. PEDOT:PSS films with significantly enhanced conductivities induced by preferential solvation with cosolvents and their application in polymer photovoltaic cells. J. Mater. Chem. 2011, 21, 4927–4936. [Google Scholar] [CrossRef]
- Horii, T.; Li, Y.; Mori, Y.; Okuzaki, H. Correlation between the hierarchical structure and electrical conductivity of PEDOT/PSS. Polym. J. 2015, 47, 695–699. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasumoro, K.; Fujita, Y.; Arimatsu, H.; Fujima, T. A New Composite Structure of PEDOT/PSS: Macro-Separated Layers by a Polyelectrolyte Brush. Polymers 2020, 12, 456. https://doi.org/10.3390/polym12020456
Yasumoro K, Fujita Y, Arimatsu H, Fujima T. A New Composite Structure of PEDOT/PSS: Macro-Separated Layers by a Polyelectrolyte Brush. Polymers. 2020; 12(2):456. https://doi.org/10.3390/polym12020456
Chicago/Turabian StyleYasumoro, Keita, Yushi Fujita, Hideki Arimatsu, and Takuya Fujima. 2020. "A New Composite Structure of PEDOT/PSS: Macro-Separated Layers by a Polyelectrolyte Brush" Polymers 12, no. 2: 456. https://doi.org/10.3390/polym12020456
APA StyleYasumoro, K., Fujita, Y., Arimatsu, H., & Fujima, T. (2020). A New Composite Structure of PEDOT/PSS: Macro-Separated Layers by a Polyelectrolyte Brush. Polymers, 12(2), 456. https://doi.org/10.3390/polym12020456